Abstract
In this study, the effect of malting process on the antioxidant compounds and antioxidant capacity of quinoa seeds was studied. The optimal germination conditions were germination temperature of 23 °C, degree of steeping of 36% and germination time of 3 days. Under these conditions, green quinoa malt was obtained and subsequently roasted at different temperatures (100–190 °C). Results showed maximum increases in phenolic compounds, Maillard reaction products and antioxidant activity (DPPH radical scavenging and reducing power) in samples roasted at 145 °C for 30 min, whereas a more intensive thermal treatment (190 °C) diminished the levels of all evaluated variables. Thus, malting with a moderate thermal treatment could be considered as an effective process to enrich antioxidants in quinoa grains for their further use as functional ingredient in the production of gluten-free foods and beverages.
Keywords: Chenopodium quinoa, Malting, Phenolic compounds, Maillard reaction products, Antioxidant activity
Introduction
Quinoa (Chenopodium quinoa) is a dicotyledonous indigenous plant of the Andean region that has gained worldwide interest in the last years, mainly because of its agronomic potential and nutritional properties. Its rusticity and wide adaptability to different agro-ecological conditions have positioned it as a suitable alternative crop for the diversification of future agricultural systems (Abugoch James 2009). On the other hand, quinoa grain has been recognized as highly nutritious food, due to the good quality and high quantity of protein and essential fatty acids, compared with common cereals (Abugoch James 2009; Poonia and Upadhayay 2015). In addition, several works have also remarked the considerable amount of natural antioxidant compounds with health-promoting properties present in quinoa grain, such as phenolic acids, tocopherols, betalains and flavonoids (Tang et al. 2015; Carciochi et al. 2015).
Raw or processed quinoa grains are now gaining interest as ingredients for the production of gluten-free foods and beverages for consumers with coeliac disease. In particular, malted quinoa grains have been evaluated in beer manufacture and bakery products (Mäkinen et al. 2013; de Meo et al. 2011). Malting procedure basically involves steeping, germination and kilning stages, the latter with the aim to stop metabolic processes and ensure stability and storability of the dried product. Malted grains can optionally be roasted to develop desired aroma and flavour. Moreover, the conditions applied in each step of malting could significantly influence the phenolic content as well as the antioxidant activity of the grains (Coghe et al. 2006).
Quinoa malt has been previously produced under different conditions and evaluated with different purposes. Mäkinen et al. (2013) evaluated the suitability of quinoa malt obtained after 5 h of soaking, 24 h of germination and kilned at 45–65 °C in gluten-free baked formulations. Zarnkow et al. (2008) investigated the influence of three malting parameters on some quality attributes, developing an optimal malting procedure with 5 days germination time at 15 °C, 46% degree of steeping and, 74 °C kilning. The making of malt suitable for the production of quinoa beer was evaluated by de Meo et al. (2011) following a program of steeped at 15 °C, 5 days of germination at 15 °C and a kilned step of 22 h at temperatures between 50 and 74 °C. Although the above mentioned works differ in the conditions for obtaining quinoa malt, the effect of malting process on the antioxidant activity and related compounds is an aspect that has not been taken into account. To the best of our knowledge, there are no previous studies assessing the effect of malting conditions on the antioxidant properties of quinoa seed.
The purpose of the present study was to evaluate the effect of the whole malting process on the antioxidant compounds and antioxidant capacity of quinoa seeds in order to obtain processed quinoa ingredients enhanced in antioxidant substances. Therefore, the objectives of present work were to (1) optimise the experimental conditions (steeping, germination time and temperature) for germination of quinoa, and (2) evaluate the effect of roasting temperatures on the antioxidant properties of optimally germinated quinoa seeds.
Materials and methods
Samples and chemicals
Quinoa seeds (Chenopodium quinoa Willd.) were obtained from Buenos Aires province, Argentina, in September 2012. Seeds were cleaned and stored in polyethylene containers at room temperature until their use. Folin–Ciocalteau reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, p-hydroxybenzoic acid, vanillic acid, p-coumaric acid, ferulic acid, quercetin, kaempferol, quinine sulphate and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were supplied by Sigma-Aldrich Chemical Co. (St. Louis, USA). Sodium carbonate, potassium ferricyanide, trichloroacetic acid, aluminium chloride, sodium hydroxide and ferric chloride were supplied by Merck (Darmstadt, Germany). Ethanol, methanol and hydrochloric acid were of analytical grade.
Germinability test: Experimental procedure
Response surface methodology was used to find the optimal germination conditions of quinoa seeds. Three independent variables at three levels namely, germination temperature (X 1; 10, 20 and 30 °C), degree of steeping (X 2; 36, 40 and 44%) and germination time (X 3; 1, 2 and 3 days) were evaluated in a central composite face-centered design. The experimental design conditions used are shown in Table 1. In each run, one hundred steeped seeds with homogeneous size and appearance were distributed into plastic trays (30 × 20 cm) between two layers of filter paper previously moistened with distilled water. Thereafter, the seeds were incubated at constant temperature in a dark chamber (BF series Delta Labo, France) with 80–90% relative humidity. Germinability (expressed as final percentage of germinated seeds) and Vigour Index (parameter that estimates the speed of germination) were selected as responses of the experimental design. The seeds were considered as germinated if the length of the radicle was greater than 2 mm. A daily count of germinated seeds was performed to calculate the Vigour Index (VI), according to the following formula (Devesa et al. 1998):
where a, b, c,…,z represent the number of germinated seeds after 1, 2, 3,…, n days; and S is the total number of seeds used in each trial.
Table 1.
Experimental design conditions and results from germinated quinoa seeds
| Run | Independent variables | Response variables | |||
|---|---|---|---|---|---|
| Temperature (°C) | Degree of steeping (%) | Germination time (days) | Germinability (%) | Vigour index | |
| 1 | 20 | 40 | 2 | 79 | 64.5 |
| 2 | 20 | 40 | 2 | 77 | 62 |
| 3 | 10 | 36 | 1 | 28 | 28 |
| 4 | 30 | 36 | 1 | 48 | 48 |
| 5 | 10 | 44 | 1 | 20 | 20 |
| 6 | 30 | 44 | 1 | 46 | 46 |
| 7 | 10 | 36 | 3 | 67 | 44.2 |
| 8 | 30 | 36 | 3 | 84 | 64.5 |
| 9 | 10 | 44 | 3 | 42 | 29.8 |
| 10 | 30 | 44 | 3 | 59 | 51.5 |
| 11 | 10 | 40 | 2 | 47 | 39 |
| 12 | 30 | 40 | 2 | 64 | 58 |
| 13 | 20 | 36 | 2 | 96 | 77 |
| 14 | 20 | 44 | 2 | 76 | 59.5 |
| 15 | 20 | 40 | 1 | 50 | 50 |
| 16 | 20 | 40 | 3 | 91 | 68.5 |
The experimental results were analysed using analysis of variance ANOVA followed by Tukey post hoc test, with a confidence level of 95%. For each response a quadratic equation model was obtained and displayed by the response surface plot using Statgraphics Centurion XVI (version 16.1.18, Statpoint Technologies Inc., USA). Optimal germination conditions were calculated using numeric optimisation.
Malting procedure
Quinoa seeds (100 g) were soaked in a 2.5% sodium hypochlorite solution (5 min) for surface sterilization and then washed with distilled water to neutral pH. Then, seeds were steeped at room temperature until reach a moisture content of 36% (~2.5 h), drained and distributed in germination trays forming one layer on wet laboratory paper and covered with the same wet paper to maintain the moisture. Trays were incubated at 23 °C in a dark chamber (BF series, Delta Labo, France) during 3 days. After germination, quinoa sprouts were kilned at 50 °C in a mechanical convection oven for 24 h to obtain a green malt of quinoa (hereinafter named as G), which was further processed with an additional step of roasting. Thus, 20 g of quinoa malt were placed in an aluminium Petri dish and roasted in a forced hot-air convection oven (model FD 23, Binder, Germany) at 100, 145 or 190 °C for 30 min (hereinafter named as G + 100 °C, G + 145 °C, G + 190 °C, respectively). These temperatures and times were chosen according to results of our previous study using raw quinoa seeds (Carciochi et al. 2016). After roasting, malted quinoa was allowed to cool to ambient temperature in a desiccator and used for further analysis. Each roasting condition was performed in triplicate.
Extraction of soluble phenolic compounds
Raw and malted quinoa were extracted twice with 80% ethanol according the optimised extraction conditions described in a previous work (Carciochi et al. 2015). After extraction, both supernatants were pooled, filtered (0.45 μm) and stored at −18 °C for further analysis.
Total phenolic (TPC) and flavonoid (TFC) contents
TPC in extracts was determined using Folin–Ciocalteau reagent, following the method described by Singleton et al. (1999). The results were expressed as milligrams of gallic acid equivalents (GAE) per100 g on dry weight basis (dw). TFC was determined by the aluminium chloride colorimetric method as described in a previous work (Carciochi et al. 2015). Results were expressed as milligrams of quercetin equivalents (QE) per 100 g dw.
Antioxidant activity
DPPH radical scavenging activity was measured in the same conditions described in a previous work (Carciochi et al. 2015). A plot of Trolox concentration versus percentage of DPPH radical scavenging activity was used to express the values as micromoles (μmol) of Trolox equivalents (TE) per 100 g dw, as follows: TE = (% DPPH scavenging −1.693)/0.046 (R2 = 0.998).
Reducing power of the extracts was measured using the cyanoferrate method under conditions described by Chandrasekara and Shahidi (2011). Results were expressed as µg of GAE per mL of extract using appropriate standard curves.
HPLC analysis of phenolic compounds
Reversed phase HPLC method for determination of phenolic acids and flavonoids was carried out with the same HPLC system and in the same conditions as described in a previous work (Carciochi et al. 2015). The identification of the phenolic compounds was achieved by comparing retention times and UV spectra of the unknown compounds with standards. Phenolic acids and flavonoids were quantified as aglycones in duplicate using standard calibration curves for each identified phenolic compound in the range of 5–500 μg/mL with correlation coefficients >0.99. Values were expressed as mg per 100 g dw.
Extraction and quantification of Maillard reaction products (MRPs)
Aqueous extraction of MRPs was performed according to a previous work (Carciochi et al. 2016). Analysis of available fluorescence intermediary compounds (FIC) and final MRPs (melanoidins) were performed according to the methods described by Michalska et al. (2008).
FIC formed in advanced Maillard reaction stages were measured using a LS 50B spectrofluorimeter (Perkin Elmer, Massachusetts, USA) operating at λEx = 353 and λEm = 438 nm. Results were expressed in μg of quinine equivalents per gram of sample dw.
Development of melanoidins in samples was estimated as absorbance at 420 nm using an UVmini 1240 spectrophotometer (Shimadzu, France), and results were expressed as arbitrary absorbance units (AU).
Results and discussion
Optimisation of germination conditions
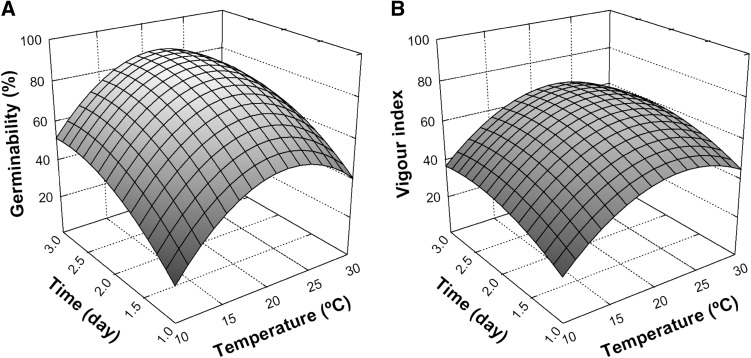
Malting industry needs homogenous and rapid germination, so besides the final percentage of germinated seeds, a parameter that evaluates the seed germination ability in relation with time is important to consider. Thus, Vigour Index (VI) was chosen as an indicator of the germination success in terms of speed, which ranged between 0 and 100. The results of germinability test are shown in Table 1. The percentage of germinated seeds varied from 20 to 96%, while VI values ranged between 20 and 77, showing considerable dependence of the response values from germination conditions. From statistical analysis (Table 2), it was found that germination time, temperature in its linear and quadratic terms and degree of steeping had a significant (p < 0.05) effect on the germinability of quinoa seeds, whilst VI was only significantly influenced by the temperature of germination. For each response, a quadratic equation model was obtained and illustrated by response surface plots in Figs. 1 and 2. The corresponding coefficients of determination (R2) of the models were 0.9856 and 0.9812 for germinability and VI respectively (Table 2), showing that more than 98.12% of the total variation in the response was explained by the models. Moreover, the non-significant value of lack of fit (p > 0.05) showed that the models could be used to predict the responses in this study. The interactive effect of germination temperature and time at fixed degree of steeping (40%) is shown in Fig. 1. Germinability of quinoa seeds (Fig. 1A) mainly increased with the increase of germination time, while temperature also had influence but with lower impact on this response. However, the latter variable was the most important on the Vigour Index (Fig. 1B). An increase in temperature led to a gradual increase in the levels of both responses up to reach a maximum in the region close to 22 °C. After these points, both response values slightly decreased for higher incubation temperatures. Bois et al. (2006) evaluated the rate of germination of ten quinoa cultivars at different temperatures. The authors demonstrated that the speed of germination was markedly increased when germination temperature increased from 2 to 20 °C. Nevertheless, it has been mentioned that temperatures above those considered optimum for germination could cause heat stress on seeds, ultimately leading to loss of vigour and delayed germination (Wahid et al. 2007). This could be the reason for the observed decreases in Germinability and VI values for those trials performed at 30 °C.
Table 2.
Results of the analysis of variance showing the significance of the independent variables and their interactions on Germinability and Vigour Index of quinoa seeds
| Source | Germinability (R2 = 98.56) | Vigour index (R2 = 98.12) | ||||||
|---|---|---|---|---|---|---|---|---|
| DF | SS | F-value | p value | DF | SS | F-value | p value | |
| X 1: Temperature | 1 | 940.9 | 470.45 | 0.0293* | 1 | 1144.9 | 366.4 | 0.0332* |
| X 2: Degree of steeping | 1 | 640.0 | 320.0 | 0.0356* | 1 | 301.4 | 96.4 | 0.0646 |
| X 3: Germination time | 1 | 2280.1 | 1140.0 | 0.0188* | 1 | 442.2 | 141.5 | 0.0534 |
| X 21 | 1 | 1686.6 | 843.3 | 0.0219* | 1 | 859.9 | 275.2 | 0.0383* |
| X 1 .X 2 | 1 | 4.5 | 2.2 | 0.3743 | 1 | 6.8 | 2.2 | 0.3783 |
| X 1 .X 3 | 1 | 18.0 | 9.0 | 0.2048 | 1 | 2.0 | 0.6 | 0.5704 |
| X 22 | 1 | 714.8 | 35.7 | 0.1055 | 1 | 752.7 | 2.4 | 0.3644 |
| X 2 .X 3 | 1 | 200.0 | 100.0 | 0.0635 | 1 | 37.8 | 12.1 | 0.1781 |
| X 23 | 1 | 279.3 | 139.7 | 0.0537 | 1 | 140.9 | 45.1 | 0.0941 |
| Lack of Fit | 5 | 103.6 | 10.4 | 0.2283 | 5 | 673.7 | 4.3 | 0.3447 |
| Pure Error | 1 | 2.0 | 1 | 3.1 | ||||
DF degree of freedom; SS sum of squares
* Significant at p < 0.05
Fig. 1.
Response surface plots showing the influence of temperature and time on Germinability (a) and Vigour index (b) of quinoa seeds
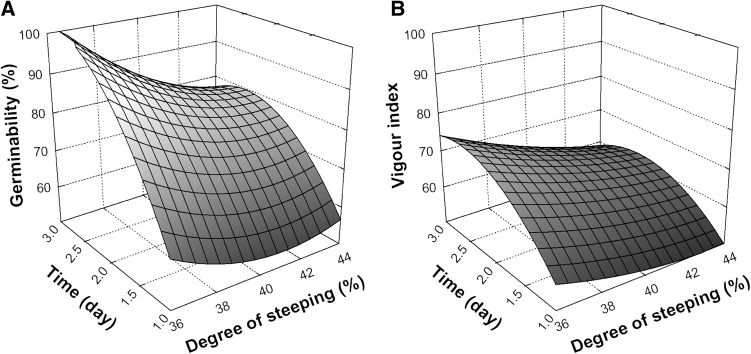
Fig. 2.
Response surface plots showing the influence of degree of steeping and time on Germinability (a) and Vigour index (b) of quinoa seeds
The optimal moisture content for malting quinoa was reported to be 46% (Zarnkow et al. 2008), however in the present study, the increase of moisture content from 36 to 44% led to a gradual decrease in germinability and VI (Fig. 2a and 2b, respectively). Germination process is initiated by the imbibition of water by the dry seed, in which the speed of water uptake was determined by several parameters such as the water content in seeds, the osmoticum, the temperature, and the duration of hydration. Moreover, the initial rate of imbibition was mainly controlled by the seed coat. If this structure has a high permeability, an excessively rapid imbibition may have produced irreversible damage to cell membranes, thus affecting the subsequent germination rate (Bradford 1995). Therefore, the gradual decrease of germination and VI observed in this study could be due to higher steeping times of quinoa seeds.
Numerical optimisation of the experimental data suggested that most suitable germination conditions for quinoa were: germination temperature of 23 °C, degree of steeping of 36% and germination time of 3 days. Germinated quinoa seeds, obtained under the above mentioned conditions, were subsequently kilned/roasted and evaluated considering their antioxidant compounds.
Effect of malting conditions on phenolic compounds
The contents of total phenolic (TPC) and flavonoid (TFC) compounds of raw and malted quinoa extracts are shown in Table 3. In comparison to unprocessed (raw) seeds, green malt showed a significant increase on the levels of both responses, which were further increased during thermal treatment. In this sense, TFC showed a steady increase as roasting temperature increased, whereas for TPC a maximal value (3.6 times more than the initial content) was reached after roasting at 145 °C. A higher processing temperature (190 °C) significantly decreased TPC values in samples, probably due to a degradation of some phenolic compounds. In order to clarify these results, a chromatographic analysis of major phenolic compounds in quinoa was performed. Changes in the levels of p-hydroxybenzoic, vanillic, p-coumaric and ferulic acids, as well as quercetin and kaempferol, were detected between raw and malted samples (Table 4). Results obtained by HPLC confirmed the trend observed by spectrophotometric methods, since sample roasted at 145 °C recorded the highest content of phenolic compounds, whereas samples processed at 190 °C decreased the level of phenolic acids, cinnamic acid derivatives (p-coumaric acid and ferulic acid) being most affected. Regarding flavonoids, quercetin and especially kaempferol increased their contents with temperature increasing from 100 to 190 °C. The observed increase in phenolic compound levels during malting process could be due to the action of endogenous esterases synthesized during germination process, which can lead to the release of phenolic compounds originally bounded in the seed matrix (Maillard et al. 1996) or through de novo synthesis (Krahl et al. 2008). Moreover, roasting process might induce structural changes in plant cell walls provoking as consequence a release of previously-glycosylated/esterified phenolic compounds (Dewanto et al. 2002; Chandrasekara and Shahidi 2011).
Table 3.
Levels of phenolic compounds, DPPH radical scavenging, reducing power and Maillard reaction products in raw and malted quinoa seeds
| TPC (mg GAE/100 g) | TFC (mg QE/100 g) | DPPH scavenging (µmol TE/100 g) | Reducing power (µg GAE/mL) | FIC (µg quinine/g) | Melanoidins (AU 420 nm) | |
|---|---|---|---|---|---|---|
| Raw | 39.29 a | 11.06 a | 13.61 a | 8.73 a | 1.81 a | 0.02 a |
| Green malt (G) | 79.04 b | 17.65 b | 27.39 b | 17.03 b | 22.14 b | 0.11 b |
| G + 100 °C | 90.53 c | 17.82 b | 40.68 c | 26.10 c | 33.88 c | 0.18 c |
| G + 145 °C | 143.29 d | 41.71 c | 70.75 e | 48.98 e | 126.55 e | 0.76 e |
| G + 190 °C | 89.68 c | 48.97 d | 53.04 d | 39.74 d | 96.84 d | 0.48 d |
Mean values within a column with different letter are significantly different (p < 0.05)
Table 4.
Contents of phenolic acids and flavonoids (mg/100 g dw) in extracts of raw and malted quinoa seeds determined by HPLC analysis
| Compound | Raw seed | Green malt (G) | G + 100 °C | G + 145 °C | G + 190 °C |
|---|---|---|---|---|---|
| p-OH-benzoic acid | 0.22 ± 0.02 a | 0.94 ± 0.04 b | 3.08 ± 0.17 c | 7.81 ± 0.72 d | 7.17 ± 0.08 d |
| Vanillic acid | 0.88 ± 0.11 a | 8.54 ± 0.09 c | 8.97 ± 0.29 c | 10.67 ± 0.91 c | 5.80 ± 0.02 b |
| p-Coumaric acid | 0.09 ± 0.05 a | 1.96 ± 0.02 b | 2.32 ± 0.04 b | 7.46 ± 0.04 c | n.d. |
| Ferulic acid | 0.57 ± 0.09 a | 3.61 ± 0.08 b | 5.50 ± 0.07 c | 9.19 ± 0.54 d | n.d. |
| Total phenolic acids | 1.75 ± 0.07 a | 15.0 ± 0.06 c | 19.86 ± 0.03 d | 35.13 ± 0.35 e | 12.97 ± 0.04 b |
| Quercetin | 0.23 ± 0.02 a | 1.36 ± 0.06 b | 1.65 ± 0.01 b | 2.62 ± 0.13 c | 2.75 ± 0.00 c |
| Kaempferol | 0.15 ± 0.04 a | 0.27 ± 0.05 a | 0.27 ± 0.00 a | 1.47 ± 0.13 b | 3.88 ± 0.03 c |
| Total flavonoids | 0.37 ± 0.03 a | 1.63 ± 0.06 b | 1.92 ± 0.01 b | 4.10 ± 0.13 c | 6.63 ± 0.02 d |
| Total phenolic compounds | 2.13 ± 0.06 a | 16.7 ± 0.06 b | 21.78 ± 0.03 d | 39.23 ± 0.27 e | 19.60 ± 0.03 c |
Values (mean ± SD, n = 3) within a row with different letter are significantly different (p < 0.05)
n.d not detected
Concerning thermal stability of phenolic acids, hydroxybenzoic acids showed more stability at 190 °C than hydroxycinnamic acids counterparts. Since it has been mentioned that substituent groups of the ring structure may act as a promoting group of the thermal decarboxylation of phenolic acids (Lindquist and Yang 2011), the acrylic acid side chain seems to be involved in the decarboxylation reaction of cinnamic acid derivatives, in contrast to benzoic acid derivatives which the carboxylic group is directly linked to the benzene ring (Khuwijitjaru et al. 2014).
Effect of malting conditions on MRPs formation
It is well known that advanced and final MRPs have antioxidant activities (Matiacevich and Buera 2006; Chandrasekara and Shahidi 2011), therefore, an estimation of their contents in malted quinoa seeds was carried out. Formation of fluorescence compounds as well as cross-linking products were formed at advanced stages of Maillard reaction, which can be estimated by measuring the maximal fluorescence emission at 340–370 nm excitation wavelengths of samples (Matiacevich and Buera 2006), whereas melanoidins (the final products of Maillard reaction) due to brown colour were commonly estimated as absorbance value at 420 nm. Results of FIC and melanoidin in extracts are shown in Table 3. The FIC level for raw seed extract was 1.81 ± 0.08 µg of quinine equivalents per g of sample dw, which may be due to fluorescent compounds such as tryptophan, tyrosine and phenylalanine, naturally occurring in quinoa grain. Both responses showed a significant increase after green malt production. Their values also increased after thermal treatment at 100 °C. A sharp increase was observed in samples roasted at 145 °C, where the highest response value was recorded in each case. After this point, samples roasted at 190 °C noticeably decreased their levels for both responses. During seed germination, hydrolysis of reserve substances such as starch and proteins take place, producing dextrins/monosaccharides and peptides/free amino acids, respectively; products that are then mobilized from cotyledons to developing embryonic axis (Bau et al. 1997). The carbonyl groups of released reducing sugars and the free amino groups of resulting amino acids or peptides are implicated in the first stage of the complex Maillard reaction (Martins et al. 2000). In addition, it is well known that an increase in temperature leads to an increase in the rate of the Maillard reaction, as it has been demonstrated in different sugar-amino acid model systems (Benzing-Purdie et al. 1985; Martins et al. 2000). Thus, the mentioned increases in MRPs are due to an increase of reactants during germination, whose reactivity increased with an increase in temperature from 100 to 145 °C. Then, the observed decrease in MRPs levels in those samples roasted at 190 °C could be due to thermal degradation of the reactants at the conditions of both high-temperature and low-moisture content in samples (Yahya et al. 2014). For instance, thermal decarboxylation of l-proline to form pyrrolidine arises after a thermal treatment of 10 min at 180 °C, or decomposition of glucose takes place only at temperatures above 150 °C (Nursten 2005). Another probable explanation is that formed melanoidins can undergo thermal degradation to lower molecular weight products and volatiles, as it has been mentioned by Tehrani et al. (2002). Authors have evaluated some degradation products from melanoidins heated at temperatures between 100 and 300 °C, in which formation of volatiles compounds such as pyrazines, pyridines, pyrroles, and furans increased with increasing temperature. For example, pyrroles only started forming at temperatures of 150 °C, and by raising this temperature to 200–220 °C a maximum was reached. Thus, the lower content of melanoidins and FIC observed in malt quinoa samples roasted at 190 °C could be also due to the loss of some volatile compounds.
Effect of malting conditions on the antioxidant activity
The antioxidant activity of extracts was evaluated with two assays extensively used in food matrices: one based on the scavenging of stable free radical (DPPH), and a second one based on the chemical reduction of Fe3+. Results of DPPH radical scavenging activity and reducing power of raw and malted quinoa extracts are presented in Table 3. Both assays were in agreement with the results obtained for phenolic compounds and MRPs. Significant levels (p < 0.05) were observed among tested samples, reaching in both antioxidant assays the highest increase (>5 times with respect to the initial value) in those samples roasted at 145 °C. From these points, a higher roasting temperature (190 °C) significantly reduced the DPPH radical scavenging and reducing power by 25 and 19%, respectively. These results were in agreement with those obtained by Coghe et al. (2006), reporting a maximum increase in the inhibition of DPPH radical for barley malt roasted at 150 °C and a decrease of antiradical activity in samples roasted at 180 °C. The authors have attributed these results to a significant loss of functional groups with antiradical activity as a result of polymerization reactions occurring at high temperatures. On the other hand, it has been mentioned that reducing power in extracts reflected the presence of compounds that were electron donors, which can act as primary and secondary antioxidants (Yen and Chen 1995). Thus, the increased reducing power observed in this study was due to the ability to donate electrons, not only of released phenolic compounds, but also due to the development of reductone-like compounds formed during Maillard reaction (Maillard et al. 1996; Chandrasekara and Shahidi 2011).
Conclusion
The study of the effect of malting process on the antioxidant compounds and antioxidant capacity of quinoa seeds allowed to find the optimal germination conditions: germination temperature of 23 °C, degree of steeping of 36% and germination time of 3 days. Roasting trials performed on green quinoa malt obtained under optimised conditions showed the highest increases of phenolic compounds, Maillard reaction products and antioxidant activity levels in samples roasted at 145 °C for 30 min, whereas a more intensive thermal treatment (190 °C, 30 min) decreased the levels of all evaluated variables. Thus, malting with a moderate thermal treatment could be considered as an effective process to enrich antioxidant compounds in quinoa, which can be subsequently used as a functional ingredient for the production of gluten-free foods and beverages.
Acknowledgements
Authors would like to thank the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) for the parcial support of this project (Postdoctoral fellowship granted to Carciochi).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest. This work does not contain any studies with human or animal subjects.
References
- Abugoch James LE. Quinoa (Chenopodium quinoa Willd.): composition, chemistry, nutritional, and functional properties. Adv Food Nutr Res. 2009;58:1–31. doi: 10.1016/S1043-4526(09)58001-1. [DOI] [PubMed] [Google Scholar]
- Bau HM, Villaume C, Nicolas JP, Méjean L. Effect of germination on chemical composition, biochemical constituents and antinutritional factors of soya bean (Glycine max) seeds. J Sci Food Agric. 1997;73:1–9. doi: 10.1002/(SICI)1097-0010(199701)73:1<1::AID-JSFA694>3.0.CO;2-B. [DOI] [Google Scholar]
- Benzing-Purdie LM, Ripmeester JA, Ratcliffe CI. Effects of temperature on Maillard reaction products. J Agric Food Chem. 1985;33:31–33. doi: 10.1021/jf00061a009. [DOI] [Google Scholar]
- Bois JF, Winkel T, Lhomme JP, Raffaillac JP, Rocheteau A. Response of some Andean cultivars of quinoa Chenopodium quinoa Willd. to temperature: effects on germination, phenology, growth and freezing. Eur J Agron. 2006;25:299–308. doi: 10.1016/j.eja.2006.06.007. [DOI] [Google Scholar]
- Bradford KJ. Water relations in seed germination. In: Kigel J, Galili G, editors. Seed development and germination. 1. New York: Marcell Dekker Inc; 1995. pp. 352–396. [Google Scholar]
- Carciochi RA, Manrique GD, Dimitrov K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J Food Sci Technol. 2015;52:4396–4404. doi: 10.1007/s13197-014-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carciochi RA, Galván D’Alessandro L, Manrique GD. Effect of roasting conditions on the antioxidant compounds of quinoa seeds. Int J Food Sci Tech. 2016;51:1018–1025. doi: 10.1111/ijfs.13061. [DOI] [Google Scholar]
- Chandrasekara N, Shahidi F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J Agric Food Chem. 2011;59:5006–5014. doi: 10.1021/jf2000772. [DOI] [PubMed] [Google Scholar]
- Coghe S, Gheeraert B, Michiels A, Delvaux FR. Development of Maillard reaction related characteristics during malt roasting. J Inst Brew. 2006;112:148–156. doi: 10.1002/j.2050-0416.2006.tb00244.x. [DOI] [Google Scholar]
- de Meo B, Freeman G, Marconi O, Booer C, Perretti G, Fantozzi P. Behaviour of malted cereals and pseudo-cereals for gluten-free beer production. J Inst Brew. 2011;117:541–546. doi: 10.1002/j.2050-0416.2011.tb00502.x. [DOI] [Google Scholar]
- Devesa JA, Ruiz T, Rodríguez P. Seed germination in wild clovers (Trifolium, Leguminosae) from Southwestern Europe (Spain) Plant Byosist. 1998;132:225–232. doi: 10.1080/11263504.1998.10654206. [DOI] [Google Scholar]
- Dewanto V, Wu X, Liu RH. Processed sweet corn has higher antioxidant activity. J Agric Food Chem. 2002;50:4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- Khuwijitjaru P, Plernjit J, Suaylam B, Samuhaseneetoo S, Pongsawatmanit R, Adachi S. Degradation kinetics of some phenolic compounds in subcritical water and radical scavenging activity of their degradation products. Can J Chem Eng. 2014;92:810–815. doi: 10.1002/cjce.21898. [DOI] [Google Scholar]
- Krahl M, Back W, Zarnkow M, Kreisz M. Determination of optimized malting conditions for the enrichment of rutin, vitexin and orientin in common Buckwheat (Fagopyrum esculentum Moench) J Inst Brew. 2008;114:294–299. doi: 10.1002/j.2050-0416.2008.tb00772.x. [DOI] [Google Scholar]
- Lindquist E, Yang Y. Degradation of benzoic acid and its derivatives in subcritical water. J Chromatogr A. 2011;1218:2146–2152. doi: 10.1016/j.chroma.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Maillard MN, Soum MH, Boivin P, Berset C. Antioxidant activity of barley and malt: relationship with phenolic content. LWT—Food Sci Technol. 1996;29:238–244. [Google Scholar]
- Mäkinen OE, Zannini E, Arendt EK. Germination of oat and quinoa and evaluation of the malts as gluten free baking ingredients. Plant Foods Hum Nutr. 2013;68:90–95. doi: 10.1007/s11130-013-0335-3. [DOI] [PubMed] [Google Scholar]
- Martins SIFS, Jongen WMF, van Boekel MAJS. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci Tech. 2000;11:364–373. doi: 10.1016/S0924-2244(01)00022-X. [DOI] [Google Scholar]
- Matiacevich SB, Buera MdP. A critical evaluation of fluorescence as a potential marker for the Maillard reaction. Food Chem. 2006;95:423–430. doi: 10.1016/j.foodchem.2005.01.027. [DOI] [Google Scholar]
- Michalska A, Amigo-Benavent M, Zielinski H, del Castillo MD. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J Cereal Sci. 2008;48:123–132. doi: 10.1016/j.jcs.2007.08.012. [DOI] [Google Scholar]
- Nursten HE. Maillard reaction Chemistry, biochemistry and implications. Cambridge: Royal Society of Chemistry; 2005. [Google Scholar]
- Poonia A, Upadhayay A. Chenopodium album Linn: review of nutritive value and biological properties. J Food Sci Technol. 2015;52:3977–3985. doi: 10.1007/s13197-014-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Tang Y, Li X, Zhang B, Chen PX, Liu R, Tsao R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;166:380–388. doi: 10.1016/j.foodchem.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Tehrani KA, Kersiene M, Adams A, Venskutonis R, De Kimpe N. Thermal degradation studies of glucose/glycine melanoidins. J Agric Food Chem. 2002;50:4062–4068. doi: 10.1021/jf0116247. [DOI] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- Yahya H, Linforth RST, Cook DJ. Flavour generation during commercial barley operations: a time course study. Food Chem. 2014;145:378–387. doi: 10.1016/j.foodchem.2013.08.046. [DOI] [PubMed] [Google Scholar]
- Yen G, Chen H. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- Zarnkow M, Geyer T, Lindemann B, Burberg F, Back W, Arendt EK, Kreisz S, Gastl M. Optimization of the malting conditions of quinoa. Brauwelt. 2008;148:374–379. [Google Scholar]