Abstract
The aim of this study was to compare the oxidative stability of linseed oil using the pressure differential scanning calorimetry (PDSC) and Rancimat methods, and to determine the kinetic parameters of linseed oil oxidation. Five cold pressed linseed oils were oxidized at different temperatures under PDSC (90–140 °C) and Rancimat (70–140 °C) test conditions. The oxidative stability of the linseed oils was calculated based on induction times (PDSCτmax, Rancimat τon), the Arrhenius equation and activated complex theory, frequency factors (Z), the reaction rate coefficient (k) for all temperatures, activation energies (Ea), Q 10 numbers, activation enthalpies (∆H ++), and activation entropies (∆S ++). The PDSC method was more convenient for the determination of the induction time of linseed oils than the Rancimat method. During oxidation measurement by Rancimat method, the linseed oil polymerized, which affected the measurements. The reaction rate coefficient increased with rising temperature during measurement by both methods. The activation energy values of linseed oil oxidation using the PDSC and Rancimat methods ranged from 93.14 to 94.53 and 74.03 to 77.76 kJ mol−1, respectively. The Q 10, ∆H ++, and ∆S ++ values for the analyzed linseed oils were between 2.11–2.13, 90.54–91.30 kJ mol−1, −33.20 to −30.90 J mol K−1 calculated by PDSC measurements, and 2.23–2.32, 71.03–74.76, −59.42 to −49.08 J mol K−1 by Rancimat measurements, respectively.
Keywords: Linseed oil, Oxidative kinetics, Oxidative stability, PDSC, Rancimat method
Introduction
Flax (Linum usitatissimum L.) is a plant belonging to the genus Linum and the family Linaceae. Two varieties of Linum are bred for the production of fibre (Linum usitatissimum L. var.elongatum Vav) and oilseeds (Linum usitatissimum L. var. brevimulticaulis Vav). The variety cultivated for fibre is called flaxseed, whereas oilseed is called linseed or Semen line. World production of linseed has increased over recent years; although it is still small and in 2013 amounted to 2.3 million tonnes, which is only 1.14% of total world oilseed production. Linseed is grown in several geographic areas, but the major world producers are Canada, China, the Russian Federation, Kazakhstan, India, and the European Union (Nykter and Kymäläinen 2006; Popis et al. 2015).
Flax oilseeds are oval and flattened in shape, 4–6 cm long, pale to dark brown, shiny, and containing about 36–40% oil. Typical linseed oil contains a high amount of polyunsaturated fatty acids (PUFA), especially essential α-linolenic acid (ALA, ω3fatty acid) (>50%), whose level is one of the highest of all edible oils (Freeman 1995). Linseed oil, due to its high level of ALA, is considered to have beneficial effects on human health. It is well known that ALA is particularly important for the development of the brain and the retina, and has antiarrhythmic activity which prevents cardiac arrest in patients with ischemic heart disease (Connor 1999). A level of α-linolenic acid as high as this also affects the drying properties of the oil, making it particularly suitable for use in numerous products such as paints, varnishes, wood treatment, and linoleum (Lazzari and Chiantore 1999). On the other hand, and most significantly from the point of view of oil safety in human nutrition, the high amount of polyunsaturated fatty acid in linseed oil causes it to be susceptible to the oxidation process (Choe and Min 2006).
Oxidation is one of the most important processes to take place in oils during storage or heat treatment. Moreover, the oxidative stability of an oil is one of the most important parameters of its quality (Frega et al. 1999). Linseed oil is rapidly oxidized, and the products of oxidation may have adverse effects on the human body. Several methods have been developed to evaluate oil oxidative stability. Due to time factors, which are very important for the industry, primarily accelerated tests are currently used to determined the oxidative stability of oil. Traditionally, the classical accelerated method used for determining oxidative stability in the edible oil industry is Rancimat, which was originally proposed for monitoring rancidity. The Rancimat method is commonly used to evaluate the oxidative stability of edible oils, but various researchers have noticed that the curves for cold-pressed oils have no characteristic course (Pawar et al. 2014). The Rancimat method is based on the conductometric determination of volatile oxidation products formed as a result of oxygen adsorption. The sudden increase in the conductivity of the water is tantamount to the oxidation induction time of the sample. Rancimat apparatus provides many opportunities for the assessment of oxidative stability under different conditions. This facilitates a number of distinct methods, differing in the applied weight of oil (usually 2.5 or 3.0 g), the air flow rate through the sample, and the amount of water in which oxidation products are dissolved (0.06 or 0.05 L). The most commonly used are Rancimat and AOCS methods (Kowalska et al. 2014; Kowalski et al. 2000).
The oxidative process may also be evaluated using thermal-analysis techniques such as pressure differential scanning calorimetry (PDSC). This method is less frequently encountered in the assessment of the stability of vegetable oils than Rancimat. Out of all the thermal-analysis techniques, the isothermal PDSC seems to be more suitable than DSC for studying the oxidation of edible oils (Tan et al. 2001). In a PDSC experiment, the isothermal mode oil and the reference sample are confined under a defined atmosphere in the reaction chamber. As a result of oil oxidation the generated heat is recorded constantly with respect to a reference material in which there are no thermal events. The result of the test is a graph from which time for the maximum of oxidation can be determined directly (Kasote et al. 2013). The time is also known, in the literature, as induction time or maximum induction time (Kowalski et al. 2004; Ciemniewska-Zytkiewicz et al. 2014).
Determination of the oxidative stability of linseed oil using the PDSC and Rancimat methods has been the subject of several studies (Rudnik et al. 2001; Omar et al. 2010; El-Waseif et al. 2013; Raczyk et al. 2015). However, only a limited number of kinetic parameters have been reported for the catalytic reactions of linseed oil. There are no studies on the kinetics of oxidation of linseed oil using PDSC and Rancimat methods, according to our knowledge. Therefore, the aim of this study was to compare the oxidative stability of linseed oil using these two methods.
Materials and methods
Research material
Material for the study consisted of five linseed oils (LO1, LO2, LO3, LO4, LO5). The oils were obtained from five different cultivation areas in Wielkopolska, Poland. The seeds (traditional, Szafir cultivar) were cold pressed using a screw press Farmer 10 (Farmet, Czech Republic), with a nozzle diameter of 8 mm. The temperature inside the press was set at 60 ± 2 °C, and the temperature of the outflowing oil produced from was 38 ± 2 °C. This is standard procedure at this plant for the preparation of cold-pressed oils. The pressed oil is subjected to 24 h of decantation, then filtered and kept in dark, brown glass bottles at refrigeration temperature.
Chemicals
All of the reagents and solvents used for the analysis and sample preparation for GC were of HPLC/GC purity, and were obtained from POCH S.A. (Gliwice, Poland). The Food Industry 37 Component Fatty Acid Methyl Esters (FAME) mix standard was supplied by Restek (Bellefonte, PA, US). Distilled water (0.05 µS) was obtained with an HLP Smart 2000 apparatus (Hydrolab, Poland).
All the chemical analysis of each oil was carried out 1 week after pressing. The first step was determining the physico-chemical quality of the oils. Then, oxidative stability was determined using Rancimat and PDSC tests at different temperatures. The oils were kept in the dark, and in refrigerator conditions, during tests.
Acid, peroxide and anisidine values and the TOTOX
To determine the initial quality of linseed oils, their basic fat values were determined. To evaluate the degree of hydrolytic change, the acid values (AV) were determined according to the AOCS Official Method Cd 3d-63 (2000). Peroxide content was tested based on peroxide values (PV) according to the AOAC Official Method 965.33 (1999). The degree of secondary oxidation products was given in p-anisidine values (AnV) according to the AOCS Official Method Cd 18–90 (2002). Spectrophotometric measurements for AnV were taken with a quartz cuvette with a 10 mm optical path length on a Helios Gamma UV–Vis Spectrophotometer. Based on the AnV and PV, the overall oil oxidation rates were calculated as TOTOX values (TOTOX = 2PV + AnV).
Fatty acid composition analysis
The transesterification of fatty acids into their corresponding FAMEs was prepared by dissolving 0.1 g of oil in 2 mL of 0.5 N methanolic KOH at 70 °C for 7 min. Then, 2 mL of hexane was added into the sample and mixed in a vortexer for 1 min. After separation of the phases, 0.5 mL of the hexane phase was placed into a vial and made up to 1 mL of hexane.
Fatty acids were esterified as methyl esters and analysed by a TRACE 1300 (Thermo Fisher Scientific Inc., Wilmington, DE, US) gas chromatograph equipped with a Restek BPX70 GC capillary column (60 m length × 0.22 mm I.D., 0.25 µm film thickness) and a FID (flame ionization detector) detector. The carrier gas was helium, at a flow rate of 0.75 mL min−1. The temperatures of the oven, injector, and detector were maintained at 200, 240, and 280 °C, respectively. The column temperature was initially kept at 100 °C for 4 min and then increased to 240 °C at rate of 3 °C min−1, where it was maintained for 15 min. Samples of 1 μL were injected by auto-sampler, in the split mode (200:1). The fatty acid composition of the analysed samples was performed by percentage participation of total fatty acid composition, and by comparison with a fatty acid methyl esters standard mix (Restek, Bellefonte, PA).
Pressure differential scanning calorimetric: PDSC
The oxidative stability of the oils was tested by DSC (Q20, TA Instruments) coupled with a high-pressure cell (Q20P). The instrument was calibrated using standard, high purity indium. For the analysis 3–4 mg of oil sample was weighed into an aluminium pan and placed in a PDSC chamber. Oil samples were oxidized under isothermal conditions, in an open aluminium pan, with a 100 mL min−1 oxygen flow rate at six different temperatures (90, 100, 110, 120, 130, 140 °C) and a pressure of 1380–1400 kPa O2.
The experiments were stopped manually after the maximum of exotherm was reached. Diagrams were analysed using TA Universal Analysis 2000 software. The maximum oxidation time, induction time (τmax), was determined based on the maximum rate of oxidation (maximum rate of heat flow) with an accuracy of 0.01.
Oxidative stability by Rancimat method
The oxidative stability of the oils was determined using a Rancimat 743 Metrohm apparatus (Herisau, Switzerland) according to ISO 6886:2009. The Rancimat test was conducted on 2.50 ± 0.01 g of oil samples, which were oxidized at twelve constant temperatures: 70, 75, 80, 85, 90, 95, 100, 105, 110, 120, 130, and 140 °C. Oil samples were blown with an air flow of 20 L h−1. The volatile products formed from the oxidation reaction were soluble in 0.06 L of distilled water. The induction times (τon) were printed automatically by apparatus software with an accuracy of 0.01.
Kinetic parameters
Graphs were plotted on the basis of the results obtained from the analysed linseed oils by Rancimat τon and PDSCτmax at different temperatures and at temperature on the absolute scale, and regression lines with correlation coefficients >0.99 were determined according to the following equations:
| 1 |
| 2 |
where in the first equation T is temperature in °C and in the second equation T is temperature in K, and a, A, b, B are the slop and intercept of the equations, respectively.
Since the oxidation of oil is a reaction of the first order, based on A and B adjustable coefficient from Eq. 2 and Arrhenius equation:
| 3 |
Kinetic parameters: activation energy—Ea, pre-exponential factor—Z, and k-reaction rate coefficient for all temperatures, were calculated.
The activation energy values were calculated according to the Ozawa–Flynn–Wall method, adjusted for isothermal process:
where R is the gas constant and A is the slope of the lnτmax/on = f(1/T) curve.Coefficient Q10 was calculated using the equation:
| 4 |
Enthalpies (H) and entropies (S) of activation were determined by the equation derived from activated complex theory:
| 5 |
where k B is the Boltzmann constant (1.3806586 × 10−23 J K−1) and h is Planck’s constant (6.62607556 × 10−34 J s).
Statistical analysis
All determinations were performed in triplicate for each sample. Relative standard deviation was determined where appropriate for all collected data. Data were subject to analysis of variance (one-way ANOVA). Statistically significant means were subject to analysis by Tuckey’s multiple range test (p < 0.05). Pearson’s linear correlations were calculated at the p < 0.05 level. All statistical analyses were performed using the Statistica 10.0 software (2010, StatSoft, Tulsa, OK, USA).
Results and discussion
Acid, peroxide, anisidine, and TOTOX values
Results of the chemical quality of the tested oils are summarized in Table 1. The acid values were lower than 4 mg KOH g−1 oil, and ranged between 0.21 and 0.53 mg KOH g−1 oil. The investigated oils had low peroxide values (1.56–2.34 meq O2 kg−1), below the value specified in the Codex Alimentarius standard for cold-pressed oils (<15 meq O2 kg−1). Similar results have been presented by other authors (Mińkowski et al. 2011; Kasote et al. 2013). The value of the content of the secondary oxidation product, also known as anisidine, was very low (0.45–0.52), which may be associated to lack of heat treatment during the extraction of oil by cold pressing.
Table 1.
Results of acid, peroxide, anisidine value, and Totox indicator of analysed linseed oils
| Parameter | Linseed oil | |||||
|---|---|---|---|---|---|---|
| LO1 | LO2 | LO3 | LO4 | LO5 | Codex Alimentarius | |
| Acid value (mg KOH g−1) | 0.32 ± 0.02ab | 0.21 ± 0.05a | 0.41 ± 0.02ab | 0.53 ± 0.04b | 0.34 ± 0.10ab | 4 |
| Peroxide value (meq O2 kg−1) | 2.21 ± 0.04ab | 2.10 ± 0.05ab | 1.98 ± 0.03a | 1.56 ± 0.07c | 2.34 ± 0.04b | 15 |
| Anisidine value (absorbation × 100) | 0.43 ± 0.03a | 0.45 ± 0.06a | 0.52 ± 0.08a | 0.65 ± 0.05a | 0.45 ± 0.03a | – |
| TOTOX indicator | 4.84 ± 0.12a | 4.65 ± 0.16a | 4.50 ± 0.12a | 3.77 ± 0.09b | 5.13 ± 0.11a | – |
All values are means of three repetitions with standard deviation
Means within the same row followed by different letters were significantly different at p < 0.05
The tested oils met the recommendations given in the Codex Alimentarius (2005) standard for acid and peroxide values (<4 mg KOH kg−1, <15 meq O2 kg−1), and it may be concluded that the oils were of good quality.
Fatty acid analysis
The composition of the fatty acids of linseed oils are presented in Table 2. All the investigated oils contained 71 to 72% polyunsaturated fatty acids. In this fraction, the oils were characterized to have the highest amount of α-linolenic acid (C18:3) from 55.4 to 56.3%, and linoleic acid (C18:2) from 15.3 to 16.2%. Among the monounsaturated fatty acids, oleic acid had the largest share in the fatty acid composition of linseed oils (C18:1). The content of this acid was at a level of 18%. On the other hand, the content of saturated fatty acid accounted to about 10%. The fatty acid composition of the analysed oil samples was similar to those reported by other researchers (Kymalainen and Sjoberg 2006; Popa et al. 2012; Raczyk et al. 2015; Rudnik et al. 2001; van Ruth et al. 2001; Zambiazi et al. 2007). However, the content of individual fatty acids was different from those reported by Szterk et al. (2010) (65% of α-linolenic acid, 12.2% linoleic acid). The differences in the fatty acids composition may be due to the different quality of the raw material (Nykter and Kymäläinen 2006; Wroniak et al. 2008).
Table 2.
Fatty acid profiles of investigated oil (%)
| Fatty acid | Linseed oil | ||||
|---|---|---|---|---|---|
| LO1 | LO2 | LO3 | LO4 | LO5 | |
| C16:0 | 6.02 ± 0.04 | 6.16 ± 0.02 | 5.82 ± 0.04 | 5.93 ± 0.09 | 5.88 ± 0.05 |
| C16:1 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.04 |
| C18:0 | 3.63 ± 0.05 | 3.73 ± 0.00 | 3.49 ± 0.02 | 3.64 ± 0.01 | 3.54 ± 0.02 |
| C18:1 | 17.76 ± 0.03 | 18.06 ± 0.01 | 17.33 ± 0.03 | 18.09 ± 0.04 | 17.57 ± 0.05 |
| C18:2 | 15.31 ± 0.04 | 15.53 ± 0.04 | 16.16 ± 0.01 | 15.41 ± 0.02 | 16.10 ± 0.04 |
| C18:3 | 56.33 ± 0.08 | 55.35 ± 0.05 | 56.19 ± 0.09 | 55.93 ± 0.05 | 55.90 ± 0.08 |
| C20:0 | 0.11 ± 0.02 | 0.16 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.02 | 0.17 ± 0.04 |
| C20:1 | 0.17 ± 0.02 | 0.21 ± 0.02 | 0.21 ± 0.01 | 0.16 ± 0.00 | 0.19 ± 0.02 |
| C21:0 | 0.19 ± 0.00 | 0.20 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 |
| C24:0 | 0.13 ± 0.01 | 0.16 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.13 ± 0.01 |
| Other* | 0.28 | 0.38 | 0.25 | 0.29 | 0.32 |
| ΣSFA* | 10.08c | 10.41d | 9.79b | 9.94a | 9.90a |
| ΣMUFA* | 18.00ab | 18.33a | 17.61b | 18.32b | 17.83ab |
| ΣPUFA* | 71.64a | 70.88b | 72.35d | 71.34a | 72.00c |
All values are means of three repetitions with standard deviation
Means within the same row followed by different letters were significantly different at p < 0.05
SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids
* Calculated values
Fatty acid composition is one of the most important factors that determine the oxidative stability of the oils. It is known that the rate of C18:2 acid oxidation was 10–40 times higher than that of C18:1, and the rate of C18:3 oxidation was 2–4 times faster than that of C18:2 (Choe and Min 2006).
Oxidative stability analysis
Linseed oil oxidative stability was assessed using PDSC and the Rancimat method; the results are summarized in Table 3. As expected, the induction time of the tested oils decreased with increase in temperature. The induction time of the oils from PDSC ranged between 223.8–258.8, 104.2–115.51, 46.2–51.9, 21.2–24.7, 10.4–11.3, and 4.3–4.8 min at 90, 100, 110, 120, 130, and 140 °C, respectively. The stability of linseed oil as assessed by the PDSC method has only been the subject of a few studies. The induction times of the investigated linseed oils found by PDSC method at 120 °C were similar to those obtained by Raczyk et al. (2015), where OTI values were observed from 19.8 to 25.3 min, which was found to be much lower than those of camelina oils (27.9–32.3 min). In addition, the induction times of linseed oils at 120 °C were also lower than those received by Ciemniewska-Zytkiewicz et al. (2014) for hazelnut (119.25–191.06 min), olive (134.15–180.07 min), and rapeseed oil (82.41–98.46 min).
Table 3.
PDSC and Rancimat induction times for analysed linseed oils
| Temperature (°C) | Linseed oil | ||||
|---|---|---|---|---|---|
| LO1 | LO2 | LO3 | LO4 | LO5 | |
| PDSC τmax (min) | |||||
| 90 | 223.83 ± 0.52 | 255.76 ± 0.24 | 248.38 ± 1.56 | 258.83 ± 0.54 | 243.54 ± 0.85 |
| 100 | 107.30 ± 1.03 | 104.20 ± 0.07 | 111.19 ± 2.23 | 115.51 ± 0.08 | 109.54 ± 1.32 |
| 110 | 51.93 ± 1.20 | 46.19 ± 0.09 | 50.67 ± 2.39 | 51.60 ± 1.73 | 48.93 ± 1.01 |
| 120 | 21.20 ± 1.15 | 23.41 ± 0.25 | 24.57 ± 0.35 | 24.72 ± 0.54 | 24.34 ± 0.94 |
| 130 | 10.48 ± 0.34 | 11.18 ± 1.00 | 11.30 ± 0.20 | 11.06 ± 0.01 | 11.05 ± 0.43 |
| 140 | 4.33 ± 0.56 | 4.72 ± 0.54 | 4.58 ± 0.15 | 4.97 ± 0.32 | 4.84 ± 0.21 |
| Rancimat τon (h) | |||||
| 70 | 33.62 ± 0.22 | 39.04 ± 0.32 | 38.25 ± 0.28 | 36.34 ± 0.24 | 37.03 ± 0.21 |
| 75 | 22.54 ± 0.14 | 26.45 ± 0.16 | 26.54 ± 0.20 | 23.64 ± 0.16 | 24.32 ± 0.25 |
| 80 | 13.97 ± 0.18 | 18.01 ± 0.19 | 18.32 ± 0.15 | 15.61 ± 0.10 | 16.15 ± 0.19 |
| 85 | 8.97 ± 0.21 | 11.80 ± 0.11 | 11.89 ± 0.17 | 10.97 ± 0.13 | 11.23 ± 0.15 |
| 90 | 7.53 ± 0.10 | 9.20 ± 0.15 | 7.68 ± 0.12 | 7.47 ± 0.16 | 7.53 ± 0.14 |
| 95 | 5.21 ± 0.09 | 6.11 ± 0.04 | 6.06 ± 0.08 | 5.07 ± 0.09 | 5.53 ± 0.14 |
| 100 | 3.84 ± 0.11 | 4.48 ± 0.18 | 4.65 ± 0.13 | 3.87 ± 0.12 | 4.02 ± 0.09 |
| 105 | 2.60 ± 0.03 | 2.64 ± 0.12 | 2.69 ± 0.21 | 2.54 ± 0.09 | 2.62 ± 0.08 |
| 110 | 1.43 ± 0.62 | 1.43 ± 0.57 | 1.52 ± 0.54 | 1.47 ± 0.67 | 1.45 ± 0.57 |
| 120 | 0.76 ± 0.12 | 0.72 ± 0.10 | 0.39 ± 0.02 | 0.41 ± 0.05 | 0.52 ± 0.12 |
| 130 | 0.27 ± 0.02 | 0.30 ± 0.01 | 0.24 ± 0.02 | 0.17 ± 0.01 | 0.23 ± 0.01 |
| 140 | 0.15 ± 0.00 | 0.16 ± 0.00 | 0.16 ± 0.00 | 0.14 ± 0.00 | 0.14 ± 0.00 |
All values are means of three repetitions with standard deviation
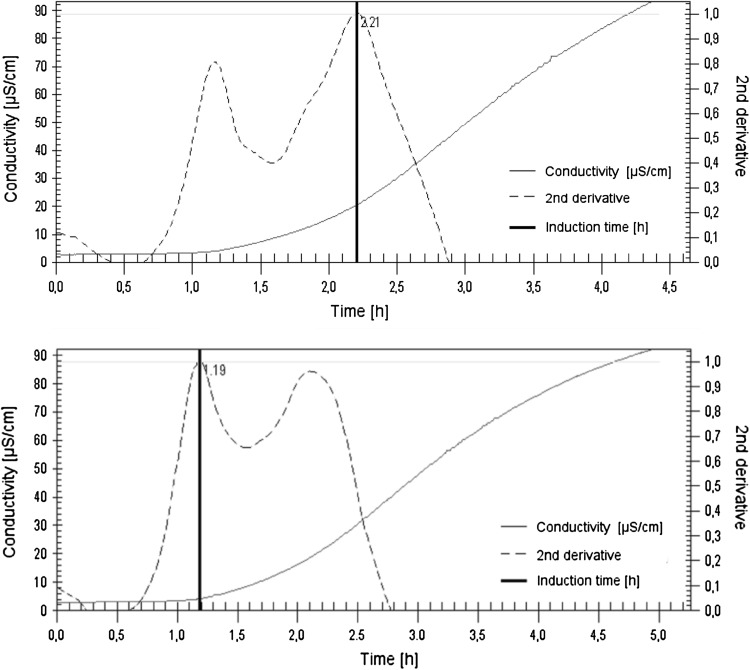
Examination of the oxidative stability of linseed oil by the Rancimat test caused some difficulties, particularly at high temperatures. As a result of the high temperature and the constant supply of air, a layer forms on the surface of oil, which may be connected with the drying process occurring in the linseed oil. The drying mechanism of linseed oil has been described, by Lazzari and Chiantore (1999) among others. Generally, it may be described as oxygen uptake, which caused a polymerization involving crosslinking, the liberation of volatile compounds and the formation of a dried film (Lazzari and Chiantore, 1999). This may have caused limited access of air to the sample. Linseed oils at high temperatures, e.g. 140 °C, changed their density and formed a gel; this could be caused by an increasing amount of the resulting polymerized compounds. Therefore, the Rancimat test was executed additionally at lower temperatures (95, 90, 85, 80, 75, 70 °C); at temperature <70 °C the time of induction could not be determined due to the slow rate of formation of oxidation products. Water evaporation has also been observed, resulting in a decrease in the volume of water in the reaction vessel, the unveiling of the electrodes, and an inability to further determine conductivity. Calculations involved eight induction times, which had been obtained at a temperature below 110 °C, into consideration; above this value, induction times were too short for results to differentiate, and induction times were not repeatable (Fig. 1). As was apparent from the graphs presented in Fig. 1, the lack of reproducibility of the approach was caused by the numerical method used to quantify the induction time from the conductivity measurements (the second derivative of the curve). However, the oxidation curves of various repetitions has the same course. Therefore, the linseed oil oxidation stability could be determined using a fixed increase in conductivity as a different approach or as a potential solution to improve the repeatability of the coefficients estimated from the Rancimat measurements.
Fig. 1.
Two curves of linseed oil (LO3) oxidation determined using Rancimat method at 110 °C
Table 3 indicates that the induction times of the analysed linseed oils were an average of 36.81 at 70 °C to 2.61 h at 105 °C. The oxidative stability of cold-pressed edible oils is commonly assessed by the Rancimat test at 100 °C. Our result showed that the oxidative stability of linseed oils at 100 °C varied, and ranged from 3.84 to 4.65 h. Similar results have been presented earlier for induction times (Raczyk et al. 2015), El-Waseif et al. (2013). On the other hand, the induction times were lower than those reported (6.4 h) by Rudnik et al. (2001). These differences may be due to the use of different speeds of air flow (10 L h−1), or the lower content of polyunsaturated fatty acids in the oil samples. The induction times were higher than those presented (2.8 h) by Omar et al. (2010), where the analysed linseed oil was deprived of natural antioxidants.
The results obtained enable us to conclude that the analysed linseed oils were characterized by low oxidative stability. The tested linseed oils were characterized by lower induction times using the Rancimat test at 100 °C than those presented by Szterk et al. (2010) in their study of cold-pressed rapeseed (7.07 h), camelina (6.12 h), primrose (6.14 h), amaranth (6.14 h), and pumpkin oil (13.63 h). The obtained induction times were also lower than those for hazelnut oil (22.44 h) reported by Ciemniewska-Żytkiewicz et al. (2014).
The low oxidative stability of linseed oil depends on the participation of polyunsaturated fatty acids in fatty acid composition.
Kinetic analysis of Rancimat and PDSC data
The results of our kinetic parameters calculations are listed in Tables 4 and 5.
Table 4.
Calculated values of kinetic parameters of linseed oils oxidation based on PDSCτmax
| Parameter | Calculation based on PDSCτmax | ||||
|---|---|---|---|---|---|
| LO1 | LO2 | LO3 | LO4 | LO5 | |
| Equation 1 | |||||
| −a | 0.0342 | 0.0339 | 0.0342 | 0.0341 | 0.0337 |
| B | 5.4517 | 5.4314 | 5.4729 | 5.4735 | 5.4157 |
| R2 | 0.9988 | 0.9983 | 0.9991 | 0.9998 | 0.9996 |
| Equation 2 | |||||
| A | 5.1309 | 5.0896 | 5.1228 | 5.1132 | 5.0550 |
| −B | 11.7310 | 11.6080 | 11.6820 | 11.6470 | 11.5100 |
| R2 | 0.9957 | 0.9982 | 0.9966 | 0.9986 | 0.9980 |
| *Ea | 94.53 | 93.77 | 94.39 | 94.21 | 93.14 |
| Z† | 5.38 × 1011 | 4.06 × 1011 | 4.81 × 1011 | 4.44 × 1011 | 3.24 × 1011 |
| k† at 140 °C | 0.6010 | 0.5659 | 0.5597 | 0.5445 | 0.7434 |
| k† at 130 °C | 0.3037 | 0.2875 | 0.2831 | 0.2757 | 0.3794 |
| k† at 120 °C | 0.1482 | 0.1411 | 0.1383 | 0.1349 | 0.1871 |
| k† at 110 °C | 0.0697 | 0.0667 | 0.0651 | 0.0636 | 0.0890 |
| k† at 100 °C | 0.0315 | 0.0303 | 0.0294 | 0.0288 | 0.0406 |
| k† at 90 °C | 0.0136 | 0.0132 | 0.0127 | 0.0125 | 0.0178 |
| Q10 | 2.13 | 2.12 | 2.13 | 2.13 | 2.11 |
| *ΔH++ | 91.3 | 90.54 | 91.16 | 90.98 | 91.94 |
| **ΔS++ | −30.9 | −33.2 | −31.8 | −32.4 | −27.3 |
† Z and k from Rancimat in h−1 from PDSC in min−1
* Ea, ΔH in kJ mol−1
** ΔS in J mol K−1
Table 5.
Calculated values of kinetic parameters of linseed oils oxidation based on Rancimat τon
| Parameter | Calculation based on Rancimat τon | ||||
|---|---|---|---|---|---|
| LO1 | LO2 | LO3 | LO4 | LO5 | |
| Equation 1 | |||||
| −a | 0.0309 | 0.0323 | 0.0321 | 0.0325 | 0.0322 |
| B | 3.6516 | 3.8458 | 3.8228 | 3.8112 | 3.8007 |
| R2 | 0.9913 | 0.9964 | 0.9941 | 0.998 | 0.998 |
| Equation 2 | |||||
| A | 4.0181 | 4.1881 | 4.1672 | 4.2206 | 4.1831 |
| −B | 10.206 | 10.604 | 10.553 | 10.747 | 10.629 |
| R2 | 0.994 | 0.9952 | 0.9943 | 0.9991 | 0.999 |
| *Ea | 74.03 | 77.16 | 76.78 | 77.76 | 77.07 |
| Z† | 1.61 x 1010 | 4.02 x 1010 | 3.57 x 1010 | 5.58 x 1010 | 4.26 x 1010 |
| k† at 105 °C | 0.9562 | 0.8822 | 0.8841 | 1.0118 | 0.9620 |
| k† at 100 °C | 0.6975 | 0.6350 | 0.6374 | 0.7264 | 0.6927 |
| k† at 95 °C | 0.5044 | 0.4530 | 0.4554 | 0.5168 | 0.4943 |
| k† at 90 °C | 0.3615 | 0.3201 | 0.3224 | 0.3643 | 0.3495 |
| k† at 85 °C | 0.2567 | 0.2241 | 0.2261 | 0.2543 | 0.2447 |
| k† at 80 °C | 0.1806 | 0.1552 | 0.1569 | 0.1757 | 0.1696 |
| k† at 75 °C | 0.1257 | 0.1064 | 0.1078 | 0.1201 | 0.1164 |
| k† at 70 °C | 0.0866 | 0.0722 | 0.0732 | 0.0812 | 0.0789 |
| Q10 | 1.99 | 2.05 | 2.04 | 2.06 | 2.04 |
| *ΔH++ | 71.03 | 74.16 | 73.78 | 74.76 | 74.07 |
| **ΔS++ | −59.42 | −52.80 | −52.77 | −49.08 | −51.33 |
* Ea, ΔH in kJ mol −1
** ΔS in J molK−1
† Z and k from Rancimat in h−1 from PDSC in min−1
Activation energy (Ea) of oxidation reaction for the analysed linseed oils using the PDSC method ranged from 93.14 to 94.53 kJ mol−1, against between 74.03 and 77.76 kJ mol−1 by Rancimat method. Differences in this parameter might be caused by the fact that these two methods were based on different results of oxidation reactions. In the Rancimat method followed measuring the conductivity of the water, which was changed with the dissolving of the volatile oxidation products, whereas heat production was used as a measure in the PDSC method. These two methods also differ in oxidation process parameters; the PDSC method used pure oxygen and high pressure, while in the Rancimat method the oil sample was oxidized by exposure to air with an appropriate flow rate. Furthermore, different sample sizes were used for each method, and the calculations were based on oil oxidation induction times at different temperatures. Ciemniewska-Żytkiewicz et al. (2014) reached a similar dependence for Ea values obtained using the Rancimat and isothermal PDSC techniques for cold-pressed hazelnut, and rapeseed oils.
The analysed linseed oils were characterized by low activation energy as calculated by the Rancimat measurement compared to the value of this parameter for other edible oils in the literature, where Ostrowska-Ligęza et al. (2010) obtained, for olive oil, an Ea of between 97.5–101.9 kJ mol−1, Ciemniewska-Żytkiewicz et al. (2014) reported activation energy for hazelnut oil (84.7 kJ mol−1). While, Farhoosha et al. (2008) reported activation energy for soybean oil (92.42 kJ mol−1), sunflower oil (90.24 kJ mol−1), and corn oil (88.14 kJ mol−1). The lower amount of energy needed to initiate the oxidation reaction resulted from the fact that linseed oil was characterised to have high amount of polyunsaturated fatty acids – linolenic and linoleic. There have been no studies on the activation energy of linseed oil oxidation based on the Rancimat τon measurement. Litwinienko (2005), using the isothermal DSC method to oxidize linseed oil, reached an Ea equal to 73.1 kJ mol−1, and for α-linolenic acid, which had the highest fatty acid composition of linseed oils—72.9 kJ mol−1. Despite the fact that linseed oil was characterised by low stability, the calculated activation energies from the PDSCτmax were higher than those presented by Ciemniewska-Żytkiewicz et al. (2014) for rapeseed oil (92.67 mol−1), olive oil (92.81 kJ mol−1), and hazelnut oil (89.06 kJ mol−1); this could be due to evaluation of the stability of oils at various temperature ranges. The presented Ea by Kowalski et al. (2004) for different rapeseed oils were higher (101.9 kJ mol−1), and lower (66.0 kJ mol−1) than those calculated for analysed linseed oils. The higher amount of Ea of linseed oil oxidation could be caused by the fact that oil rich in linolenic acid tends to form a skin layer at the point of contact with oxygen, causing a diffusion barrier to oxygen resulting in a high level of residual unsaturation in the film (Stenberg et al. 2005). It can further slow down the oxidation rate and affected activation energy.
The k values for linseed oil oxidation at each temperature are presented in Tables 4 and 5. The rate of oil oxidation depends on many factors (fatty acid composition and triacylglycerol structure, catalysts, inhibitors etc.) (Frankel 2014). Unfortunately, the PDSC and Rancimat methods have no way of separating these effects, and for practical purposes comparative studies of oil oxidation were performed. Reaction rate coefficient increased with rising temperatures in both methods, but in the PDSC method the k values were lower than that of Rancimat method, for example at 100 °C the reaction rate coefficient was between 0.0636—0.0890 min−1, and 0.6350—0.7264 h−1, respectively. The reaction rates obtained at 100 °C for linseed oil using the PDSC and Rancimat methods were higher than the reaction rate coefficients presented by Ciemniewska-Zytkiewicz et al. (2014) for hazelnut, olive and rapeseed oils.
The magnitude of the temperature effect on the oxidation rate of the linseed oils was evidenced by the Q10—the reaction temperature coefficient, specifying how the reaction rate changes with an increase in temperature of 10 °C.
In general, a higher Q10 number implied that a slight temperature change was needed to induce a certain change in the rate of lipid oxidation. The results of Q10 are presented in Tables 4 and 5. Q10 value depends on the range of temperatures for which we compared reaction rate coefficients. Using the PDSC method for L1 oil at temperatures from 130 to 140 °C, the Q10 value was 1.98, and 2.32 at 90–100 °C. Using the Rancimat method for the same oil in the range of 95–105 °C, the Q10 value was 1.90 and in the range of 70–75 °C, 2.09. In addition, this value depends on the method used to assess oxidative stability, e.g., Q10 values for L1 oil in the range of 90–100 °C using the PDSC and Rancimat methods were 2.32 and 1.93, respectively.
The Q10 for the analysed linseed oils using the PDSC method was an average of 2.12 and using the Rancimat method the value of this parameter was an average of 2.03; this indicated that a temperature increase of 10 °C caused more than double the rate of oxidation reaction. The Q10 values calculated by Farhoosha et al. (2008) using the Rancimat measurements for canola, soybean, sunflower, corn, and olive oil were on the level of 2.13, 2.18, 2.15, 2.10, and 2.08, respectively. The Q10 value depends on the type of oil.
From the slopes and intercepts of the lines, ∆H ++ and ∆S ++ were calculated and summarized in Tables 4 and 5.
The ∆H ++ and ∆S ++ values calculated from PDSCτmax differed from those calculated from Rancimat τon. ∆H ++ values based on PDSC measurements, ranged from 90.54 to 91.30 kJ mol−1, while ∆S ++ from −33.20 to −30.90 J mol K−1, whereas those calculated by the Rancimat method were from 71.03 to 74.76 kJ mol−1, and from −59,42 to −49.08 J mol K−1, respectively. Calculations of ∆H ++ and ∆S ++ values provided some information. First of all, the negative values for entropies indicated that the activated complexes were more ordered than the molecules of the reactants. Secondly, the presence of antioxidants caused a greater change in ∆S ++ than ∆H ++ (Cho 1997).
Conclusion
The study thus concluded that the PDSC method appeared more suitable for the assessment of the oxidative stability of linseed oil at high temperatures >105 °C. Moreover, the PDSC method was more convenient for the determination of the induction time of linseed oils than the Rancimat method. During Rancimat method measurement, linseed oil was polymerized due to oxidation, which caused problems of cleaning the reaction vessel. The results of the kinetic oxidation of linseed oil enable to predict the process of oxidation under various conditions. The results might also be helpful in prediction of the oxidation of other oils.
References
- Acid value. AOCS (2000) Official Method Cd 3d-63
- Cho HY. Reaction mechanism and kinetics of antioxidants using Arrhenius equation in soyabean oil oxidation. J Food Sci Nutr. 1997;2:6–10. [Google Scholar]
- Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saf. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Ciemniewska-Żytkiewicz H, Ratusz K, Bryś J, Reder M, Koczoń P. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J Therm Anal Calorim. 2014;118:875–881. doi: 10.1007/s10973-014-3861-9. [DOI] [Google Scholar]
- Codex Alimentarius, FAO/WHO Codex standard for named vegetable oil. Codex Alimentarius. Amendment 2005, 2011
- Connor WE. α-Linolenic acid in health and disease. Am J Clin Nutr. 1999;69:827–828. doi: 10.1093/ajcn/69.5.827. [DOI] [PubMed] [Google Scholar]
- El-Waseif MA, Hashem HA, Abd El-Dayem HH. Using flaxseed oil to prepare therapeutical fat spreads. Ann Argic Sci. 2013;58:5–11. [Google Scholar]
- Farhoosha R, Niazmand R, Rezaei M, Sarabi M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur J Lipid Sci Technol. 2008;110:587–592. doi: 10.1002/ejlt.200800004. [DOI] [Google Scholar]
- Frankel EN. Lipid oxidation. Amsterdam: Elsevier; 2014. [Google Scholar]
- Freeman SC. Structure of flaxseed. In: Cunnane LU, editor. Flaxseed in human nutrition. 3. Champaign: AOCS Press; 1995. pp. 11–21. [Google Scholar]
- Frega N, Mozzon M, Lercker G. Effects of free fatty acids on oxidative stability of vegetable oil. J Am Oil Chem Soc. 1999;76:325–329. doi: 10.1007/s11746-999-0239-4. [DOI] [Google Scholar]
- ISO 6886:2009. Animal and vegetable fats and oils—determination of oxidation stability (accelerated oxidation test). International Organization for Standardization, Geneva, Switzerland
- Kasote DM, Badhe YS, Hegde MV. Effect of mechanical press oil extraction processing on quality of linseed oil. Ind Crops Prod. 2013;42:10–13. doi: 10.1016/j.indcrop.2012.05.015. [DOI] [Google Scholar]
- Kowalska D, Kostecka M, Tarnowska K, Kowalski B. Oxidative stabilities of enzymatically interesterified goose fat and rapeseed oil blend by Rancimat and PDSC. J Therm Anal Calorim. 2014;115:2063–2070. doi: 10.1007/s10973-013-3125-0. [DOI] [Google Scholar]
- Kowalski B, Gruczyńska E, Maciaszek K. Kinetics of rapeseed oil oxidation by pressure differential scanning calorimetry measurements. Eur J Lipid Sci Technol. 2000;106:337–341. doi: 10.1002/(SICI)1438-9312(200005)102:5<337::AID-EJLT337>3.0.CO;2-3. [DOI] [Google Scholar]
- Kowalski B, Ratusz K, Kowalska D, Bekas W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat methods. Eur J Lipid Sci Technol. 2004;106:165–169. doi: 10.1002/ejlt.200300915. [DOI] [Google Scholar]
- Kymalainen HR, Sjoberg AM. Cadium content of linseed and estimated consumer intake. Agr Food Sci. 2006;15:3–11. doi: 10.2137/145960606777245533. [DOI] [Google Scholar]
- Lazzari M, Chiantore O. Drying and oxidative degradation of linseed oil. Polym Degrad Srab. 1999;65:303–313. doi: 10.1016/S0141-3910(99)00020-8. [DOI] [Google Scholar]
- Litwinienko G. Analysis of lipid oxidation by differential scanning calorimetry. In: Kamel-Eldin A, Pokorný A, editors. Analysis of lipid oxidation. Champaign, IL: JAOCS Press; 2005. pp. 152–193. [Google Scholar]
- Mińkowski K, Grzeskiewicz S, Jerzewska M. Ocena wartości odżywczej olejów roślinnych o dużej zawartości kwasów linolenowych na podstawie składu kwasów tłuszczowych, tokoferoli i steroli. Zywn-Nauk Technol Ja. 2011;75:124–135. [Google Scholar]
- Nykter M, Kymäläinen HR. Quality characteristics of edible linseed oil. Agric Food Sci. 2006;15:402–413. doi: 10.2137/145960606780061443. [DOI] [Google Scholar]
- Omar KA, Shan L, Wang YL, Wang X. Stabilizing flaxseed oil with individual antioxidants and rancimat measurements. Eur J Lipid Sci Technol. 2010;112:1003–1011. doi: 10.1002/ejlt.200900264. [DOI] [Google Scholar]
- Ostrowska-Ligeza E, Bekas W, Kowalska D, Lobacz M, Wroniak M, Kowalski B. Kinetics of commercial olive oil oxidation: dynamic differential scanning calorimetry and Rancimat studies. Eur J Lipid Sci Technol. 2010;112:268–274. doi: 10.1002/ejlt.200900064. [DOI] [Google Scholar]
- p-Anisidine value. AOCS (2002) Official method Cd 18–20
- Pawar N, Purohit A, Gandhi K, Arora S, Singh RRB. Effect of operational parameters on determination of oxidative stability measured by rancimat method. Int J Food Prop. 2014;17:2082–2088. doi: 10.1080/10942912.2012.680220. [DOI] [Google Scholar]
- Peroxide value of oils and fats. AOCS (1999) Official method 965.33
- Popa VM, Gruia A, Raba DN, Dumbrava D, Moldovan C, Bordean D, Mateescu C. Fatty acids composition and oil characteristics of linseed (Linum Usitatissimum L.) from Romenia. J Agroaliment Proc Technol. 2012;18:136–140. [Google Scholar]
- Popis E, Ratusz K, Przybysz MA, Krygier K, Sakowska A, Konarska M. World and Polish production of linseed and linseed oil. Sci J Warsaw Univ Life Sci SGGW Probl World Agric. 2015;15:106–116. [Google Scholar]
- Raczyk M, Popis E, Kruszewski B, Ratusz K, Rudzińska M. Physicochemical quality and oxidative stability of linseed and camelina cold-pressed oils from retail outlets. Eur J Lipid Sci Technol. 2015 [Google Scholar]
- Rudnik E, Szczucinska A, Gwardiak H, Szulc A, Winiarska A. Comparative studies of oxidative stability of linseed oil. Thermochim Acta. 2001;370:135–140. doi: 10.1016/S0040-6031(00)00781-4. [DOI] [Google Scholar]
- Stenberg C, Svensson M, Johnsson M. A study of the drying of linseed oils with different fatty acid patterns using RTIR-spectroskopy and chemiluminescence (CL) Ind Crop Prod. 2005;21:263–272. doi: 10.1016/j.indcrop.2004.04.002. [DOI] [Google Scholar]
- Szterk A, Roszko M, Sosińska E, Derewiaka D, Lewicki PP. Chemical composition and oxidative stability of selected plant oils. J Am Oil Chem Soc. 2010;87:637–645. doi: 10.1007/s11746-009-1539-4. [DOI] [Google Scholar]
- Tan CP, Man YC, Selamat J, Yusoff MSA. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J Am Oil Chem Soc. 2001;78:1133–1138. doi: 10.1007/s11746-001-0401-1. [DOI] [Google Scholar]
- van Ruth SM, Shaker ES, Morrissey PA. Influence of methanolic extracts of soyabean seeds and soybean oil on lipid oxidation in linseed oil. Food Chem. 2001;84:177–184. doi: 10.1016/S0308-8146(01)00195-9. [DOI] [Google Scholar]
- Wroniak M, Krygier K, Kaczmarczyk M. Comparison of the quality of cold pressed and virgin rapeseed oils with industrially obtained oils. Pol J Food Nutr Sci. 2008;58:85–89. [Google Scholar]
- Zambiazi RC, Przybylski R, Zambiazi MW, Mendonça CB. Fatty acid composition of vegetable oils and fats. B. Ceppa. Curitiba. 2007;25:111–120. [Google Scholar]