Abstract
This study aimed to assess the nutrient composition, minerals, and stability of betalains pigment from fruit extracts of Basella rubra. The proximate composition included total carbohydrates (1.64 g), protein (51 mg), total lipid (1.38%), moisture (81.76%), niacin (0.5 mg), ascorbic acid (89.33 mg), and total tocopherols (1.27 mg) in 100 g fresh deseeded fruit. Total dietary fibre of 32.52 g and soluble dietary fibre of 12.34 g in 100 g dry deseeded fruits. Fatty acid composition of dry deseeded fruits comprised 57.52% SFA, 20.62% MUFA and 22.28% PUFA. Pigment (betalain) rich fruit extracts showed excellent stability over 20 days of storage under varied pH, temperature, light, acids, salts and sugars. B. rubra fruit was observed as a potential source of nutrients and betalains as a functional food.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2404-8) contains supplementary material, which is available to authorized users.
Keywords: Ascorbic acid, Colour stability, Dietary fibre, Minerals
Introduction
Phytoconstituents derived from plants have benefits for health and well-being and offer exciting commercial opportunities (Mittal et al. 2013). Consumers prefer fruits for their beneficial nutrients and expect value addition associated with its potential bioactivity (Nöthlings et al. 2008). There is a great impetus to identify new biomolecules with promising activity from fruits, because, as natural ingredients they have a proven benefit for human health (Khan et al. 2012; Harsha et al. 2013) and turn out to be new opportunity for food processing industry. Recent studies have shown fruits from less-known and unexplored plants as excellent source of nutrients and biologically active compounds for food and non-food applications (Khan et al. 2011, 2015). Basella rubra L. (Basellaceae), commonly called as Indian or Malabar spinach is found in tropical and sub-tropical areas (Wealth of India 2000). It is a succulent, climbing annual or biennial herb, with circular to ovate leaves and eaten along with tender stems (Palada and Crossman 1999). The tender stem oozes gelatinous or mucilaginous substances, especially when cooked (Palada and Crossman 1999). The lower surfaces of the young leaves (Cyunel 1989), stalks, petioles and fruits are red-violet due to the presence of betalains (Palada and Crossman 1999). The plant has been explored for food and medicinal purposes in both Chinese and Indian traditional medicinal practice to treat constipation and as anti-inflammatory (Toshiyuki et al. 2001). Recent studies have confirmed the nutritional and nutraceutical potential from leaves of Basella species (Kumar et al. 2015a). Similarly, the antioxidant activity along with cytotoxicity of B. rubra fruit extracts against human cervical carcinoma cells was reported to prove the functional attributes of fruit extracts (Kumar et al. 2015b). The betalains extraction from fruits and its use in food formulations have been recently reported (Kumar et al. 2015c, d). However, not much is known about the potential nutritional benefits of B. rubra fruits and their pigment stability.
As betalains are considered as an alternative for synthetic colourants in the food industry due to its positive effects on health (Lin et al. 2010; Khan et al. 2012) and also finds use in pharmaceutical and cosmetic industry as reviewed (Khan 2015). The betalains identification and characterization during ontogeny of B. rubra fruit extracts by HPLC and MS was reported (Kumar et al. 2015d). Interestingly, many plants accumulate betalains, but only two (B. vulgaris and the prickly pear Opuntia ficus-indica) are approved to be used in food (Delgado-Varges and Lopez 2002). Due to the red-violet colour of the fruits of B. rubra, and its numerous health benefits (Lin et al. 2010; Khan et al. 2012, 2015; Kumar et al. 2015b), there is a need to investigate the stability aspects of pigment extracts from fruits of B. rubra that helps to promote it as an ingredient in functional food formulations. Accordingly, the aim of the study was to investigate the phytoconstituents, minerals composition and stability of pigment from the ripened fruits of B. rubra.
Materials and methods
Materials
HPLC grade methanol, ethanol, ascorbic acid, and standard mineral solutions were obtained from Sisco Research Laboratory (Mumbai, India). Tocopherol standard, butylated hydroxyanisole (BHA), hydrochloric acid, DNS, phenol, sodium sulphite, sulphuric acid, Anthrone, Folin–Ciocalteu’s reagent and sodium carbonate were procured from Himedia Chemicals (Mumbai, India). All other chemicals used were of analytical grade. For HPLC analysis, degassed and 0.22 mm membrane filtered Milli-Q water was used.
Source of fruits
The ripened red-violet fruits of Basella rubra (150–200 g) were collected from 3-month-old twine of greenhouse maintained potted plants at CFTRI, Mysore from March 2013 to April 2013. The Herbarium sheet was deposited at the Herbarium Collection Centre, University of Mysore (Reference No. 02/08/05/13) upon confirmation of its taxonomical features. For experiment purpose, 150–200 g fresh fruits were collected from the single plant and from this lot, only the required quantity of fruits was used for biochemical analysis. Similarly, fruits from the same plant were used for pigment extraction and stability studies. The harvested fruits were immediately used for pigment extraction and frozen at −20 °C until nutritional profile was performed.
Pigment extraction
Manually deseeded fruits flesh (10 g) was extracted using distilled water (100 ml) with a mortar and pestle until the macerate becomes colourless. Then, the macerate was centrifuged at 12,000 rpm for 10 min. All clear supernatants were pooled and stored at −20 °C until use.
Qualitative chemical test
Identification of betacyanin pigments was performed as described (Harborne 1998). The clear fruit extract was separately treated with 2 M HCl at 100 °C and with a few drops of 2 M NaOH. The disappearance of pink colour and the change of pink to yellow colour respectively confirmed the presence of betacyanin pigments. Quantification of total betalain content (0.34 g/100 g FW) was performed using a UV–visible spectrophotometer (UV 1800, Shimadzu, Kyoto, Japan) and identification and characterization during ontogeny of B. rubra fruit extracts by HPLC and MS were shown using an HPLC pump equipped with a C18 column (Sunfire, Waters Corporation, Milford, MA, USA) of 250 × 4.6 mm i.d. The UV detector was recorded at 477 and 535 nm (Kumar et al. 2015b, d).
Determination of nutritional components of ripened B. rubra fruits
The total carbohydrates (anthrone reagent), reducing sugars (DNS reagent) and protein (Lowry’s method) were analysed following standard methods based on the absorbance of coloured complexes developed when reacted with corresponding reagents (Sadasivam and Manickam 2008). Fruits were dried at 50 °C overnight in a hot air oven and the reduction in weight was calculated as moisture content following method Da 2a-48 (AOCS, 2003). Total soluble solids (TSS) in the juice were recorded using a digital refractometer RX-500 (Atago Co. Ltd., Tokyo, Japan).
Dietary fibre content was analysed in dry defatted fruit pulp by using an enzymatic method (Asp et al. 1983). Briefly, triplicate 1.0 g defatted sample was suspended in a 0.1 M phosphate buffer (pH 6.0). The sample was digested with 0.1 ml of heat stable α-amylase (Termamyl-Sigma, St. Louis, USA) and boiled for 15 min in a boiling water bath with occasional shaking. The contents were cooled to room temperature, and then 20 ml of distilled water was added followed by adjustment of pH of the slurry to 1.5 with 4 M hydrochloric acid. To this 100 mg pepsin was added and incubated at 40 °C for 1 h with agitation. Subsequently, 20 ml of distilled water was added and, pH was adjusted to 6.8 with 4 M NaOH. Then 100 mg pancreatin was added and incubated at 40 °C for 1 h with agitation. The pH of the digested sample was again adjusted to 4.5 with 4 N HCl. The volume was made up to 100 ml and the contents were used to determine total, soluble and insoluble dietary fibre. The digested sample was filtered through celited pre-weighed crucible, washed successively with 20 ml of 75% ethanol, 40 ml of 95% ethanol, dried for 5 h at 105 °C and weighed. The residues were incinerated at 550 °C, cooled and weighed for insoluble dietary fibre. To the filtrate from insoluble fibre determination, 4 volumes of warm 95% ethanol were added and allowed to stand overnight. The precipitated fibre was filtered and washed as mentioned earlier for insoluble fibre. The residue was dried, weighed and incinerated and weighed again. The sum of insoluble and soluble dietary fibre content gives the total dietary fibre.
Energetic value (kcal/100 g deseeded fruits) was calculated as,
Lipids were extracted using hexane at 40 °C for 8 h from dried (moisture-free) fruit pulp following Soxhlet extraction method Ba 5b-68 (AOCS, 2003). Fatty acid methyl esters (FAME) were prepared by treating the extracted oil, from B. rubra fruits, with methanolic KOH (2 mol equivalent/l) and analysed as reported. FAME was identified by using reference standard methyl esters. Iodine value was determined from the fatty acid composition by using official method Cd 1c-85 (AOCS, 2003).
Atherogenicity and thrombogenicity indices were calculated following the method described (Ulbricht and Southgate 1991).
Determination of niacin, ascorbic acid, and total tocopherol content
Niacin was determined in sulphuric acid extract of ripened fruit pulp following the standard procedure (Sadasivam and Manickam 2008) wherein, the extract reacts with cyanogen bromide in the presence of aniline to form coloured complex that was measured at 420 nm.
Ascorbic acid was extracted (under subdued light) from the fresh fruit pulp of B. rubra, according to the modified method (Vanderslice et al. 1990). Briefly, one gram fresh deseeded fruit pulp was homogenized with 2 ml methanol and 10 ml of cold extraction solution, containing 3% metaphosphoric acid (MPA, w/v), 0.05% ethylenediamine tetraacetic acid (EDTA, w/v) and 0.8% glacial acetic acid (v/v). The slurry was centrifuged for 15 min at 5240 g in a cooling centrifuge (4 °C), and the supernatant was collected. Samples in duplicates were filtered through 0.22 µm membranes into amber HPLC vials. The samples (20 µl) were then directly injected into the HPLC system. Chromatographic analysis of ascorbic acid was carried out in LC 20 AD (M/s Shimadzu Corporation, Kyoto, Japan) under the following conditions. Chromatopak column C18 (150 × 4.6 mm i.d., 5 µ particle size); the mobile phase was 50 mM K2HPO4, pH 7 (Solvent A) and 100% methanol (Solvent B). The gradient elution was 1% B for 5 min, linear gradient of 1–30% B for 15 min, followed by 30% B in the next 10 min at a flow rate of 1 ml/min. Ascorbic acid was monitored at 254 nm.
Total tocopherol content in the ripened fruits was done according to the method (Wong et al. 1988).
Mineral analysis
Minerals were estimated by atomic absorption flame emission spectroscopy (Model AA-6701F; Shimadzu Corporation) with a graphite furnace attachment. Dried and deseeded fruit pulp was incinerated in a muffle furnace for 3 × 90 min at 550 °C to ensure complete carbon burning. The ash obtained was taken in aqua regia. Zinc, iron, copper, magnesium, sodium, potassium, and calcium were analysed after diluting with the respective acid solution. Sodium and potassium were analysed using strontium chloride (2%) as matrix modifier, whereas, for calcium, lanthanum chloride (2%) was employed. The minerals were quantified using reference standards.
Carbon, hydrogen, nitrogen and sulphur analysis
Dried B. rubra fruit pulp was ground and approximately 5.0–10.0 mg of the sample was used to fill up the tin capsule and weighted using electronic balance (Sartorius Balance). The sample was analysed using carbon, hydrogen, nitrogen and sulphur Elemental Analyser (Vario EL III). Sulphanilic acid was used as a standard. The percentage carbon, hydrogen, nitrogen and sulphur content in B. rubra fruit pulp were determined.
Stability studies of crude betalain pigment extract of fruits
Effect of pH
The deseeded fruit pulp (10 g) was pulverised using mortar and pestle in the presence of neutralised sand particles with distilled water until the macerate was colourless and then centrifuged at 12,000 rpm for 10 min. The clear supernatant was collected in aluminium foil wrapped screw capped tubes and studied at pH 4.0, 5.0 and 6.0 under room temperature for 2 months and then analysed for its pigment stability (Ozela et al. 2007).
Effect of temperature
The fresh fruit pulp (10 g) was weighed and homogenised in distilled water (10/100 w/v) using mortar and pestle and centrifuged at 12,000 rpm 10 min. The clear supernatant was collected and transferred to aluminium foil wrapped screw-cap tubes. The tubes were maintained at 4 °C (refrigerator), 20 °C (growth chamber), 60 °C (heating module), 90 °C (water bath) and 120 °C (heating module) (Lin et al. 2010). Up to 3 h, incubation was carried out and samples at 1 h interval were collected from respective temperatures and immediately cooled in an ice-bath following the absorbance determination at 535 and 477 nm in triplicate.
Effect of light
The fruit pulp was weighed and homogenised in distilled water (10/100 w/v) using mortar and pestle and centrifuged at 12,000 rpm 10 min. The clear supernatant was collected and transferred to screw-cap tubes (Kunnika and Pranee 2011). The tubes were continuously exposed to light (Constant fluorescence irradiation) and maintained at room temperature for 7 days. The pigment retention was determined in triplicate on a UV 1800 double beam spectrophotometer and the results were compared with control (day 0).
Influence of acids, salt and sugars
To study the influence of acids, salts and sugars on crude B. rubra pigments, solutions of ascorbic acid (0.1–1.0%) and citric acid (0.1–1.0%), sodium chloride (1–5%) and sugars (1–10%) such as glucose, fructose and sucrose were prepared. Then, 10 g of deseeded fruit pulp was separately extracted with 100 ml of the above prepared acids, salts and sugar solutions and kept at room temperature in the dark for 20 days. These extracts were compared with control without acids, salts and sugars maintained under same conditions (Kunnika and Pranee 2011).
Statistical analysis
All values presented are mean ± SEM of three analyses. Data were subjected to one-way ANOVA followed by post hoc Duncan’s Multiple Range Test (DMRT) using SPSS 17 (SPSS Inc., Chicago, IL, USA) for determining significant differences. A difference was considered significant when p < 0.05.
Results and discussion
Qualitative test
Water extract of B. rubra fruit was subjected to qualitative chemical tests to confirm the presence of the red-violet betacyanin pigments. The addition of few drops of 2 M NaOH converted the pink colour pigment to light yellow colour (Supplementary Data). Similarly, the sample was treated with hot aqueous HCl, which led to the destruction of the pink colour. Thus, this test leads to the conformation of betalains in B. rubra fruits rather than anthocyanins.
Nutritional composition of fruits
The proximate composition of B. rubra fruits revealed the presence of different components such as total carbohydrates (1.64 ± 0.01), reducing sugars (0.49 ± 0.01), protein (0.051 ± 0.001), total lipid (1.38 ± 0.04), Iodine value (62.15), ash content (2.48 ± 0.10) in g/100 g and moisture of 81.76% ± 1.52 (FW basis) along with total soluble solids (5.69°Brix), and dietary fibre content 32.52 ± 1.41 and 12.34 ± 0.5 g of total and soluble dietary fibre content (100 g DW). Total dietary fibre content was compared to the crude fibre content of Rivina humilis berries and Tinospora cordifolia fruits (Khan et al. 2011, 2012). As the carbohydrate and protein content was low, the overall energetic value of the fruit was found to be less (19.18 kcal/100 g) (Table 1).
Table 1.
Nutrient composition of ripened deseeded fruits of B. rubra
| Nutrient | Content (g/100 g FW) |
|---|---|
| Carbohydrate | 1.64 ± 0.01 |
| Reducing sugar | 0.49 ± 0.01 |
| Non-reducing sugar | 1.15 ± 0.03 |
| Cellulose | 0.025 ± 0.001 |
| Total soluble solidsb | 5.69 ± 0.023 |
| Proteins | 0.051 ± 0.001 |
| Total lipids | 1.38 ± 0.04 |
| Total dietary fibrea | 32.52 ± 1.41 |
| Soluble dietary fibrea | 12.34 ± 0.5 |
| Insoluble dietary fibrea | 20.16 ± 0.91 |
| Ash content | 2.48 ± 0.10 |
| Caloric value (kcal) | 19.18 |
| Iodine value | 62.15 |
| Moisturec | 81.76 ± 1.52 |
Values are mean ± SEM of triplicates
Non reducing sugar content was obtained by subtracting reducing sugar content from total carbohydrate content
aExpressed in dry weight basis
bExpressed in ˚Brix
cValues are expressed in percent
Fatty acid composition of lipids extracted from the dry pulp of B. rubra is presented in Table 2. Palmitic acid was the major saturated fatty acid (24.63 g) whereas oleic acid (15.65 g) and linolenic acid (9.10 g) major MUFA and PUFA. It was observed that the content of SFAs, MUFA and PUFA were 57.52, 20.62 and 22.28 g in 100 g lipids respectively. The ratio of SFA: MUFA: PUFA was 2.79:1:1.08. For balanced fat intake, the proposed ratio is approximately 1:1:1 in India. The fatty acid composition was comparable with the fatty acid composition of R. humilis berries reported (Khan et al. 2012).
Table 2.
Fatty acid composition of dried deseeded fruits of B. rubra
| Fatty acid | Relative% |
|---|---|
| Caprylic acid (C8:0) | 1.17 ± 0.17 |
| Lauric (C12:0) | 1.98 ± 0.23 |
| Myristic (C14:0) | 3.06 ± 0.05 |
| Palmitic (C16:0) | 24.63 ± 1.02 |
| Palmitoleic (C16:1) | 1.34 ± 0.10 |
| Stearic (C18:0) | 6.21 ± 0.21 |
| Oleic (C18:1) | 15.65 ± 1.04 |
| Linoleic (C18:2) | 9.10 ± 0.58 |
| Linolenic (C18:3) | 12.10 ± 0.58 |
| Arachidic (C20:0) | 2.95 ± 0.02 |
| Behenic acid (C22:0) | 2.34 ± 0.28 |
| Erucic acid (C22:1) | 2.0 ± 0.21 |
| Lignosenic acid (C24:0) | 15.87 ± 0.47 |
| Total saturated | 57.52 ± 0.34 |
| Total unsaturated | 42.48 ± 0.34 |
| MUFA | 20.62 ± 0.41 |
| PUFA | 22.28 ± 0.08 |
| Atherogenicity index | 0.89 ± 0.01 |
| Throbogenisity index | 1.54 ± 0.02 |
Values are mean ± SEM of three triplicates analysis
Atherogenicity (AI) and thrombogenicity indices (TI) indicated the propensity of a diet or fat to influence the incidence of heart disease. AI (0.89) and TI (1.54) of lipids extracted from dry B. rubra fruit pulp are presented in Table 2. The values of AI and TI were in agreement with reported AI (0.97) and TI (2.0) of lipids extracted from dry R. humilis berries (Khan et al. 2012). Oils with low (≤1) AI and TI values have been considered good for health (Ulbricht and Southgate 1991). The British diet has an AI of 0.93 and TI of 1.2, whereas the oil rich in saturated fatty acids such as coconut oil and palm oil have been reported to have AI of 12.6 and 0.88, and TI of 6.18 and 1.7 respectively (Ulbricht and Southgate 1991).
Vitamins like niacin (0.5 ± 0.011 mg), ascorbic acid (89.33 ± 5.82 mg) and total tocopherols (1.27 ± 0.09 mg) were found in substantial quantity in fruit pulp per 100 g FW. The niacin content in cereals and peanuts are about 5–7 and 10 mg/100 g as listed in USDA Food Composition Database (available at http://ndb.nal.usda.gov/ndb/foods/show/6421?fg=&sort=&offset=&format=Full& new=&measureby=, as on April 15, 2014). Niacin content is very low in fruits. Niacin has been involved directly or indirectly in various biological activities such as DNA repair, cancer prevention, fighting reactive oxygen species at a range of 16 and 14 mg/day for males and females respectively (Hageman and Stierum 2001). Ascorbic acid appeared to be high (89.33 mg) in B. rubra fruits which is a potential antioxidant compound present in fruits. The recommended U.S daily intake of Ascorbic Acid (AA) is 90 mg/day/Adult. The total tocopherol content of the fruit was apparently high (1.27 mg/100 g FW) compared to berries of T. cordifolia and R. humilis (Khan et al. 2012, 2011). Tocopherols protect bio-membranes from lipid peroxidation that causes cell lysis resulting in many diseases (Kris-Etherton et al. 2004).
Mineral composition of fresh deseeded B. rubra fruits showed (mg/100 g FW) microelements such as iron (0.66), copper (0.083) and zinc (0.32) were ≤1 mg (Table 3). The macro element, potassium content (96.33 mg) was better to that of raspberries, grapes and other fruits (Heghedus-Mindru et al. 2014). The sodium (6.77), magnesium (9.66) and calcium (21.00) content were in the usual range and were comparable to other betalains rich fruits (Khan et al. 2012).
Table 3.
Mineral composition (mg/100 g FW) of ripened B.rubra deseeded fruits
| Sample | Iron | Zinc | Copper | Sodium | Potassium | Magnesium | Calcium |
|---|---|---|---|---|---|---|---|
| B.rubra | 0.66 ± 0.056 | 0.32 ± 0.02 | 0.083 ± 0.007 | 6.77 ± 0.18 | 96.33 ± 4.62 | 9.66 ± 0.63 | 21.00 ± 0.48 |
Values are mean ± SEM of three triplicates analysis
Carbon, hydrogen, nitrogen and sulphur composition (%) of dry deseeded B. rubra fruits indicates the content of carbon (38.8 ± 1.4) as very high followed by hydrogen (6.03 ± 0.29) and nitrogen (3.36 ± 0.22), but sulphur (0.034 ± 0.012) content was less.
Stability studies of crude B. rubra fruit betalain pigment extract
Effect of pH
The effect of pH on pigment extract subjected to a pH range of 4–6 on pigment degradation in pulp of B. rubra fruits up to 8 weeks of storage at room temperature. At pH 5 the lowest pigment degradation was observed, followed by pH 4 and 6. The pigment was least stable at pH 6 (Fig. 1a). The presence of ‘1,7-diaza’ substructure driven some researchers to claim betalains as chromoalkaloids but their stability in slightly acidic pH disproved the claim (Khan 2016). These results were comparable with betacyanin colour stability values of flesh and peel of red dragon fruits (Kunnika and Pranee 2011). Stintzing and Carle (2007) had also reported similar pH range of betacyanin colour stability in red dragon fruit from Israel (pH 4.5–5.5) but with a wider range compared to red dragon fruit from Taiwan (pH 5) and in beetroot (pH 5.5).
Fig. 1.
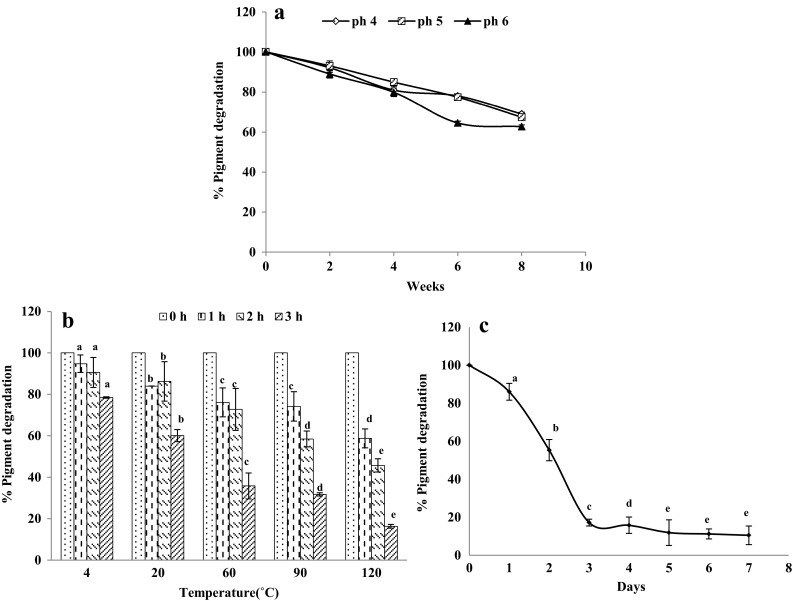
Stability studies of crude betalain pigment extract of B. rubraripened fruits, a effect of pH, b effect of temperature,c effect of light. Values are mean ± S.E of three replicates and significance was tested by Duncan Multiple Range Test at p < 0.05, and values with different superscript were found not significant difference from each other
Effect of temperature
The effect of temperature on crude pigment extract incubated at various temperatures for 3 h has been analysed using a spectrophotometer. At 60 °C, the degradation of the pigment was quite high. At higher temperatures, 90 and 120 °C, very higher rates of pigment degradation was observed (Fig. 1b). At temperatures of 4, 20 and 60 °C, a slight decrease in pigment degradation was observed up to 2 h of incubation. However, after 3 h incubation, the pigment degradation was high at 60, 90 and 120 °C compared to 4 and 20 °C. These values were earlier reports (Lin et al.2010). In view of this, B. rubra fruit pigments were observed to be a novel source of natural colourant for minimally processed foods, such as desserts, drinks, yoghurts, ice creams, etc. (Kumar et al. 2015d).
Effect of light
The crude pigment extract showed least stability to light when the extracts were exposed to light for 3–7 days at room temperature (26 ± 2 °C). The rate of degradation of pigments in fruit extracts incubated in dark condition was slower compared in the presence of light (Fig. 1c). It can be explained that light affected the electrons of double bonds in betacyanins molecules to be in an excited state, resulting the higher degradation of betacyanin in the presence of light compared to pigment stored in dark condition (Cai et al. 2005). A similar result was reported for betacyanin pigments of red dragon fruits that were kept under observation for not more than 2 days (Kunnika and Pranee 2011).
Influence of acids, salt and sugars
The influence of acids such as ascorbic acid (Fig. 2a) and citric acid (Fig. 2b) on B. rubra crude pigment extract showed that at lower (0.1%) concentrations of these acids, and citric acid betalains were more stable compared to at higher concentrations. As the concentration of ascorbic acid increased from 0.25 to 1%, almost 50–60% reduction in pigment content was observed at 1% ascorbic acid concentration. However, at all concentrations of ascorbic acid, the pigment stability was good compared to control even after 20 days of incubation. Overall, pigment retention was found to be more in the presence of ascorbic acid than citric acid. Khan and Giridhar (2014) reported that the ascorbic acid (0.25%) and selenium (40 g) concentration gave good pigment content in R. humilis berries.
Fig. 2.
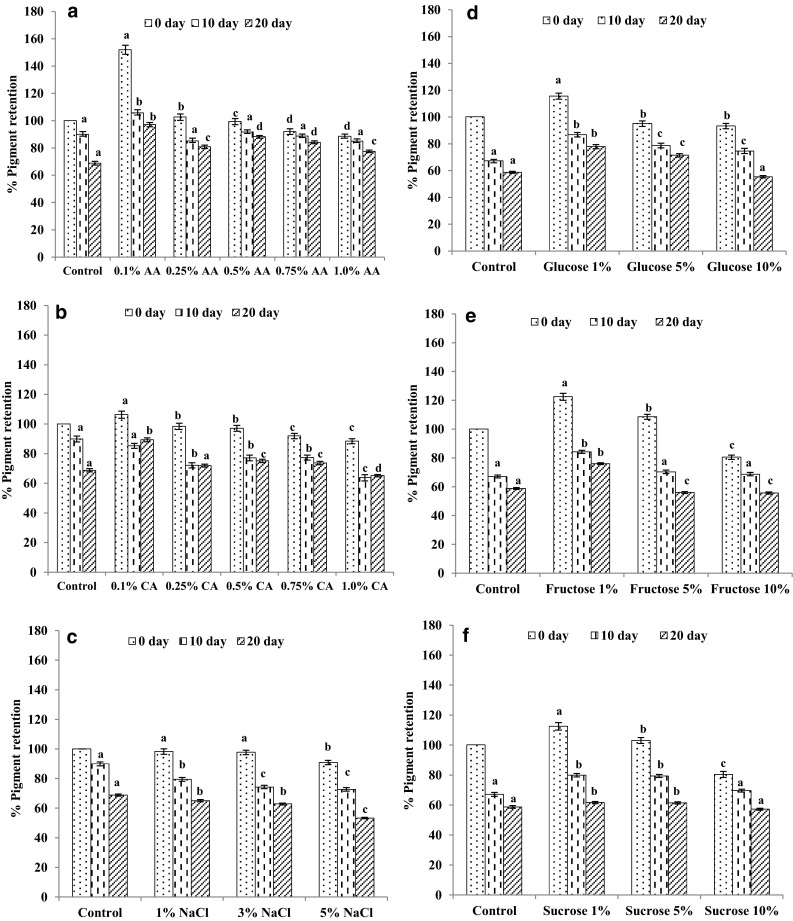
Influence of acid, a ascorbic acid (AA), b citric acid (CA), c salt (NaCl), and sugars d glucose, e fructose, f sucrose on crude betalain pigments extracts of B. rubra ripened fruits. Values are mean ± SEM of three replicates and significance was tested by Duncan Multiple Range Test at p < 0.05, and values with different superscript were found not significant difference from each other
The influence of salts on crude pigment extract showed that at higher concentrations of salt, the degradation of pigments were higher when compared to control (without salt). At 5%, NaCl concentration based extraction 10% decline in pigment content was noticed compared to control samples (Fig. 2c). However, the reports on the effect of NaCl on colour stability of anthocyanins in elderberry and black current showed 32, 42 and 55% degradation at 1, 3 and 5% NaCl concentration respectively (Hubbermann et al. 2006). During subsequent incubation at room temperature for 20 days, there was a gradual decline in pigment content (40–50%) in control and NaCl based extraction.
The influence of sugars such as glucose (Fig. 2d), fructose (Fig. 2e) and sucrose (Fig. 2f) on B. rubra crude pigment extract showed that at 1% concentration, positive influence on pigment content has been observed. Among the above-analysed sugar solutions on betalain stability, glucose and fructose brought the best stability. It can be explained that adding sugars can decrease water activity. Betalains are highly stable at low water activities. This phenomenon has been collaborated by a water-glucose system where degradation was reduced by decreasing water activity (Delgado-Varges and Lopez 2000). However, the sugar treatment did not help significantly, to retain the pigment content after 20 days incubation at room temperature. The addition of glucose into red dragon fruit samples can increase colour values has been reported earlier (Kunnika and Pranee 2011).
Conclusion
Fruits of B. rubra were investigated for its nutritional composition mainly focusing on biochemical constituents such as nutrient composition, vitamins (niacin, ascorbic acid, tocopherols) and minerals. In view of its easy growing in the backyards, pigments (betalains) rich fruits of B. rubra are worthy as a functional food. Though the levels of total betalains content of leaves and fruits of B. rubra were less as compared to tubers of beet, nutritionally rich phytoconstituents and mineral content was an asset to this fruit for consumption as an affordable and sustainable alternative source. Moreover, B. rubra has good prospects as a prolific leafy vegetable with tremendous nutritional properties, notable resistance to diseases and pests, appropriate for backyard cultivation as well as for small-scale market gardens. Hence, it is of merit to identify nutritional and stability studies of betalains for value addition to B. rubra fruit as a possible source of natural colourant and nutrients. These findings provide value addition to B. rubra fruit as a potential source of natural nutrients as a functional food to the food industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to Department of Biotechnology, Government of India, New Delhi for financial assistance (BT/PR1238/FNS/20/524/2011). We greatly acknowledge the Director, CSIR-CFTRI for his kind support.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
References
- AOCS . Methods-soxhlet extraction of oil, Ba 5b-68, moisture content, Da 2a-48, iodine value, Cd 1c-85. In: Firestone D, editor. Official methods and recommended practices of the American Oil Chemical Society. 5. Champaign: AOCS Press; 2003. [Google Scholar]
- Asp NG, Johansson CG, Hallmer H, Siljestrom M. Rapid enzymatic assay for soluble and insoluble dietary fibre. J Agric Food Chem. 1983;31:476–482. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- Cai YZ, Sun M, Corke H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci Technol. 2005;16:370–376. doi: 10.1016/j.tifs.2005.03.020. [DOI] [Google Scholar]
- Cyunel E. Basella alba L.: in vitro culture and the production of betalains. Biotechnol Agric For. 1989;7:47–68. [Google Scholar]
- Delgado-Varges F, Lopez OP. Natural pigments, carotenoids, anthocyanins, and betalains. Characteristics, biosynthesis processing and stability. Crit Rev Food Sci Nutr. 2000;40:173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- Delgado-Varges F, Lopez OP. Betacyanins and phenolic compounds from Beta vulgaris L. roots. Food Chem. 2002;58:255–258. doi: 10.1016/S0308-8146(96)00163-X. [DOI] [Google Scholar]
- Hageman GJ, Stierum RH. Niacin, poly (ADP-ribose) polymerase-1 and genomic stability. Mutat Res. 2001;475:45–56. doi: 10.1016/S0027-5107(01)00078-1. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. London: Chapman A & Hall; 1998. [Google Scholar]
- Harsha PSC, Khan MI, Prabhakar P, Giridhar P. Cyanidin-3-glucoside, nutritionally important constituents and in vitro antioxidant activities of Santalum album L. berries. Food Res Int. 2013;50:275–281. doi: 10.1016/j.foodres.2012.10.024. [DOI] [Google Scholar]
- Heghedus-Mindru RC, Heghedus-Mindru G, Negrea P, Sumalan R, Negrea A, Stef D. The monitoring of mineral elements content in fruit purchased in supermarkets and food markets in Timisoara, Romania. Ann Agric Environ Med. 2014;21(1):98–105. [PubMed] [Google Scholar]
- Hubbermann EM, Heins A, Stockmann H, Schwarz K. Influence of acids, salts, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur Food Res Technol. 2006;223:83–90. doi: 10.1007/s00217-005-0139-2. [DOI] [Google Scholar]
- Khan MI. Plant Betalains: safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr Rev Food Sci Food Saf. 2015;15(2):316–330. doi: 10.1111/1541-4337.12185. [DOI] [PubMed] [Google Scholar]
- Khan MI. Stabilization of betalains: a review. Food Chem. 2016;197:1280–1285. doi: 10.1016/j.foodchem.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Khan MI, Giridhar P. Enhanced chemical stability, chromatic properties and regeneration of betalains in Rivina humilis L. berry juice. LWT-Food Sci Technol. 2014;58:649–657. doi: 10.1016/j.lwt.2014.03.027. [DOI] [Google Scholar]
- Khan MI, Harsha PSC, Giridhar P, Ravishankar GA. Pigment identification, antioxidant activity and nutritional composition of Tinospora cordifolia (willd.) Mixers ex Hook.f & Thoms fruits. Int J Food Sci Nutr. 2011;62:239–249. doi: 10.3109/09637486.2010.529069. [DOI] [PubMed] [Google Scholar]
- Khan MI, Harsha PSC, Giridhar P, Ravishankar GA. Pigment identification, nutritional composition, bioactivity and in vitro cell cytotoxicity of Rivina humilis L. berries, potential source of betalains. LWT-Food Sci Technol. 2012;47:315–323. doi: 10.1016/j.lwt.2012.01.025. [DOI] [Google Scholar]
- Khan MI, Harsha PSC, Chauhan AS, Vijayendra SVN, Asha MR, Giridhar P. Betalains rich Rivina humilis L. berry extract as natural colorant in product (fruit spread and RTS beverage) development. J Food Sci Technol. 2015;52:1808–1813. doi: 10.1007/s13197-013-1175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–538. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Manoj P, Giridhar P. Nutrition facts and functional attributes of foliage of Basella spp. LWT-Food Sci Technol. 2015;64:468–474. doi: 10.1016/j.lwt.2015.05.017. [DOI] [Google Scholar]
- Kumar SS, Manoj P, Giridhar P, Shrivastava R, Bharadwaj M. Fruit extracts of Basella rubra that are rich in bioactives and betalains exhibit antioxidant activity and cytotoxicity against human cervical carcinoma cells. J Funct Foods. 2015;15:509–515. doi: 10.1016/j.jff.2015.03.052. [DOI] [Google Scholar]
- Kumar SS, Manoj P, Giridhar P. A method for red-violet pigments extraction from fruits of Malabar spinach (Basella rubra) with enhanced antioxidant potential under fermentation. J Food Sci Technol. 2015;52:3037–3043. doi: 10.1007/s13197-014-1335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Manoj P, Shetty NP, Prakash M, Giridhar P. Characterization of major betalain pigments-Gomphrenin, Betanin and Isobetanin from Basella rubra L. fruit and evaluation of efficacy as a natural colourant in product (ice cream) development. J Food Sci Technol. 2015;52:4994–5002. doi: 10.1007/s13197-014-1527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnika S, Pranee A. Influence of enzyme treatment on bioactive compounds and colour stability of betacyanin in flesh and peel of red dragon fruit Hylocereus polyrhizus (Weber) Britton and Rose. Int Food Res J. 2011;18:1437–1448. [Google Scholar]
- Lin SM, Lin BH, Hsieh NM, Ko HJ, Lin C, Chen LG, et al. Structural identification of Bioactivities of red-violet pigments present in Basella alba fruits. J Agric Food Chem. 2010;58:10364–10372. doi: 10.1021/jf1017719. [DOI] [PubMed] [Google Scholar]
- Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Nöthlings U, Schulze MB, Weikert C, Boeing H, van der Schouw YT, Bamia C, Peeters PH. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr. 2008;138(4):775–781. doi: 10.1093/jn/138.4.775. [DOI] [PubMed] [Google Scholar]
- Ozela E, Shingheta PC, Chauea MC. Stability of Anthocyanins in spinach vine (Basella rubra) fruits. Ciencia e Investigacion Agraria. 2007;34:85–90. [Google Scholar]
- Palada MC, Crossman SMA. Evaluation of tropical leaf vegetables in the Virgin Islands. In: Janick J, editor. Perspectives on new crops and new uses. Alexandria: ASHS Press; 1999. pp. 388–393. [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods. 8. New Delhi: New Age International Publishers; 2008. [Google Scholar]
- Stintzing FC, Carle R. Betalain-emerging prospects for food scientists. Trends Food Sci Technol. 2007;18:514–525. doi: 10.1016/j.tifs.2007.04.012. [DOI] [Google Scholar]
- Toshiyuki M, Kazuhiro H, Masayuki Y. Structures of new oleanane-Type triterpene oligoglycosides, basella saponins A, B, C, and D, from the fresh aerial parts of Basella rubra L. Chem Pharm Bull. 2001;49:776–779. doi: 10.1248/cpb.49.776. [DOI] [PubMed] [Google Scholar]
- Ulbricht TLV, Southgate DAT. Coronary heart disease: seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-M. [DOI] [PubMed] [Google Scholar]
- Vanderslice JT, Higgs DJ, Hayes JM, Block G. Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J Food Compost Anal. 1990;3:105–118. doi: 10.1016/0889-1575(90)90018-H. [DOI] [Google Scholar]
- Wealth of India . A dictionary of Indian raw materials and industrial products (CSIR) New Delhi: NISCAIR; 2000. p. 159. [Google Scholar]
- Wong ML, Timms RE, Goh EM. Colorometric determination of total tocopherols in palm oil, olein and stearin. J Am Oil Chem Soc. 1988;65:258–261. doi: 10.1007/BF02636412. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


