Abstract
Comparative investigation of major phytoconstituents was performed from various parts of tea plant viz. apical bud, subtending 1st–5th leaf, stem, coarse leaves, flowers, fruits and roots. From the results of comparative RP-HPLC-DAD analysis it was found that underutilized tea parts especially coarse leaves, flowers and fruits contains abundant amount of phenolics (17.5%) and catechins (4–5%). From these underutilized tea plant parts the catechins were extracted and purified and then screened for their anticancer, immunomodulatory effect and antimicrobial activity against food borne pathogens. The results showed that tea fruit extract exhibited higher toxicity against oral cancer cells and also promotes proliferation of mice splenocytes. The results of antimicrobial studies revealed the inhibitory effect of these extracts against both gram positive and gram negative bacteria. These investigations clearly demonstrated that the underutilized tea plant parts could act as economical and sustainable bioresource of functionally active constituents which further lead to the development of new cost-effective nutraceuticals and other formulations.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2406-6) contains supplementary material, which is available to authorized users.
Keywords: Tea, Catechins, Phenolics, Antioxidants, Cytotoxicity
Introduction
Tea is a widely consumed beverage, which comes from processed and dried apical shoots of Camellia sinensis plant. It is cultivated mainly in tropical and subtropical areas, having an adequate moisture content, good water drainage and slightly acidic soil conditions (Anesini et al. 2008). Studies have shown that tea possesses a diverse range of phytochemicals (Manzocco et al. 1998) including phenolics, alkaloids and amino acids (Peng et al. 2008). The tender tea shoots comprising of two leaves and an apical bud are rich in phenolics with numerous therapeutic properties including antioxidants, anti-carcinogenic, anti-inflammatory, and anti-atherosclerotic activities (Dufresne and Farnworth 2001; Wang and Helliwell 2001; Atoui et al. 2005). Tea catechins such as epigallocatechin (EGC), epicatechin (EC), epigallocatechin gallate (EGCG), and epicatechin gallate (ECG) are the major phenolic compounds of tea (Peterson et al. 2005; Quan et al. 2007; Obuchowicz et al. 2011). These flavan-3-ols are renowned antioxidants which help in prevention and delay of several metabolic ailments (Cooper et al. 2005). The young tea shoots are the primary site for majority of these phenolics while a considerable decrease in phenolic content was noticed in woody axial tissues of the plant. Tea also contains an important amino acid called theanine (γ-glutamylethylamide). The synthesis of this unique amino acid occurs mainly in roots, from where it is translocated to aerial parts. It imparts pleasant umami taste to tea infusions and acts as a neural relaxant (Narukawa et al. 2008). Another important quality constituent of tea is caffeine (methyl xanthine alkaloid) which has brain stimulatory effect (Fernandez et al. 2000).
Various studies have been conducted in the recent past on health perspectives of major tea phytoconstituents against several diseases like obesity, diabetes, hypertension, artherosclerosis, prevention of tooth decay and oral cancer. Therefore, the demand for natural phenolic antioxidants in food, beverage, pharmaceutical and cosmetic industries is growing at a rapid pace. Hence, to meet the soaring demand of these high valuable phytoconstituents, there in emergent need to explore new economical and sustainable sources of these phytochemicals. Various studies have been performed for exploration of major tea constituents from underutilized leaves (Nor Qhairul Izzreen and Mohd Fadzelly 2013) and flowers of tea (Lin et al. 2003; Farhoosh et al. 2007). These studies revealed the use of underutilized plant parts as potent source of major bioactives. In current study, we focused on the simultaneous evaluation of each and every part of tea plant from apical bud to underground roots for assessment of phenolics, catechins, caffeine and theanine content. Thus, the prime aim of current study is to assess major bioactive constituents viz. catechins, caffeine and theanine in underutilized parts of the tea plant which are available in biomass. The study further aims sustainable extraction and purification of major phytochemicals and evaluation of their comparative functional properties.
Materials and methods
Chemicals
Tea catechins (GC, EGC, Catechin, EC, EGCG, GCG, ECG, CG), caffeine, theanine and Folin’s ciocalteau reagent were purchased from Sigma-Aldrich. All other solvents and chemicals were of analytical grade and obtained from Merck Specialites Pvt. Ltd. (Mumbai, India).
Sample collection
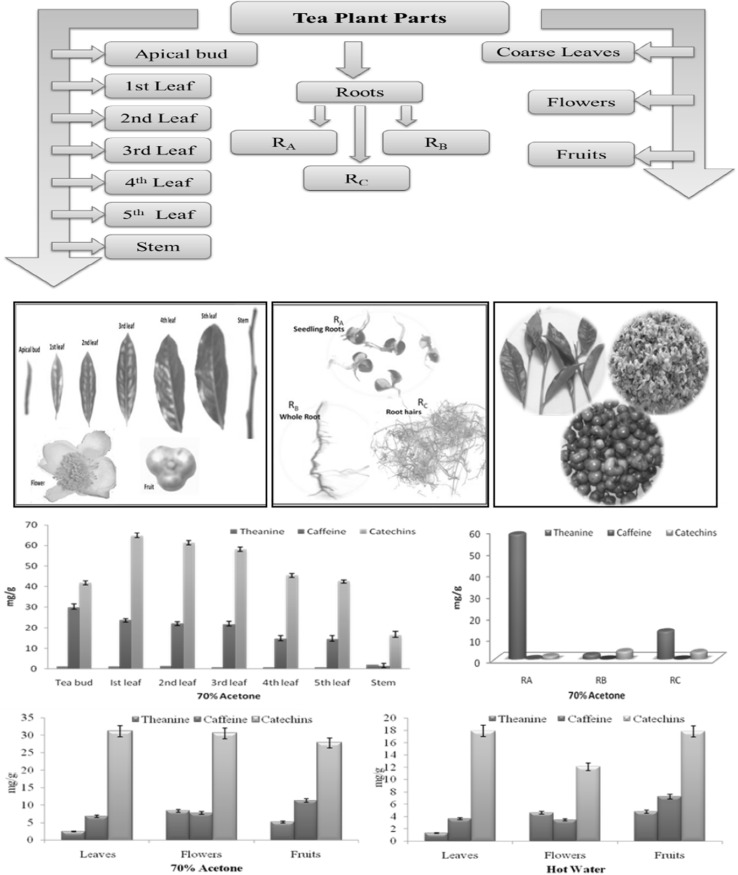
Different plant parts viz. apical bud, 1st–5th leaf, stem, coarse leaves, flowers, fruits and roots (Fig. 1) were collected individually during April to August. The plant material was collected from experimental tea farm of CSIR-IHBT, Palampur. Three types of tea root samples were collected viz. roots from germinating seeds (RA), whole root of tea plant (RB) and secondary roots (RC). All these samples were dried in a hot air oven (SHEL LAB) at 60 ± 2 °C, crushed to fine powder and stored at ambient temperature (25–27 °C) in a desiccator for further analysis.
Fig. 1.
Different parts of tea plant and content of major tea constituents in them
Extraction of different plant parts
Accurately weighed 500 mg dried sample of each plant part was extracted with 10 ml of aqueous acetone (70%) followed by centrifugation at 8000 rpm for 10 min at 4 °C (Sharma et al. 2005). The supernatant was collected and residue was re-extracted with 8 and 7 ml of 70% aqueous acetone to make the final volume to 25 ml. The obtained extracts were passed through 0.45 µm nylon filter (Millipore Merck India). The filtrates were stored at 4 °C in the freezer until further use. All the extractions were performed in triplicate.
Extraction of underutilized plant parts for purification of major bioactives
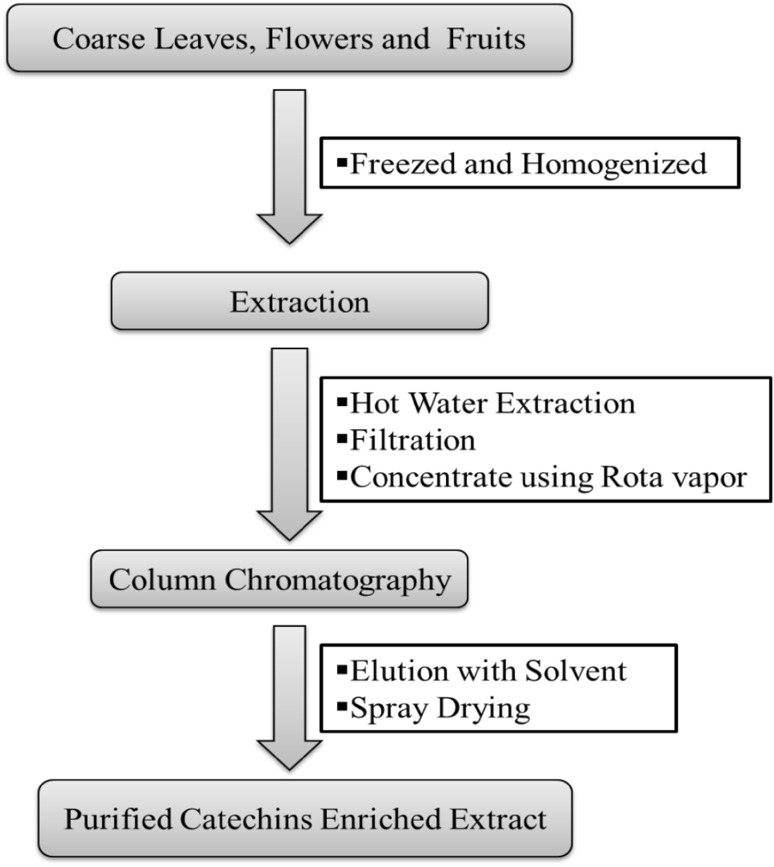
For quantitative determination and purification of major bioactive constituents from different underutilized tea plant parts, coarse tea leaves, flowers and fruits were collected. Extraction of major constituents from these underutilized parts was carried out using a green and sustainable patented process Singh and Rana (2014) as shown in (Fig. 2). The purified extracts were spray dried (Inlet temperature—140 °C, Outlet temperature—100 °C and 80% Aspiration) and obtained powder was analyzed for estimation of total phenolics, free radical scavenging antioxidants, catechins, caffeine and theanine content using spectroscopic and HPLC analysis.
Fig. 2.
Picture showing various parts of tea plant and content of major tea constituents in solvent extracts of these parts
HPLC analysis
Sample preparation
For HPLC analysis, samples dissolved in HPLC grade solvents were taken and filtered through 0.45 µm nylon membrane filters. The filtered extracts were injected into Waters HPLC system after diluting with equal volumes of distilled water.
Estimation of major tea constituents by RP-HPLC-PDA
For screening and quantification of major tea constituents our earlier published HPLC method Rana et al. (2015) was used. A Waters HPLC system equipped with 600 quaternary gradient pump, 2998-PDA and 717 auto sampler was employed. Separation of the compounds was achieved on a Phenomenex Synergi MAX-RP C12 column (4.6 × 250 mm) fitted with suitable guard column. The column oven temperature was maintained at 32 °C. The retention time and absorbance maximum of compounds were compared with authentic standards purchased from Sigma Aldrich. A gradient solvent system comprising A; acetonitrile and B; water with 0.01% TFA (trifluro acetic acid) was used. Starting from 10% A at 0 min to 15% at 3 min, and increased to 25% at 5 min, it further increased to 30% at 9 min. It was brought to 25% at 12 min and then 20% at 15 min, to 15% at 18 min, and finally reached to 10% A at 20 min.
Determination of total phenolics (TPC)
Total phenolic content of each sample extracts and purified fractions was estimated by Folin’s Ciocalteau method (Swain and Hillis 1959) with slight modifications (Rana et al. 2013). Aliquots 50 µl of various extracts from plant parts and purified catechins enriched extracts were taken in triplicate sets in 25 ml volumetric flasks. 1 N Folin’s Ciocalteau reagent (1 ml) was added followed by 1 ml of saturated Na2CO3 solution. The final volume was made up to 25 ml with distilled water. The reaction mixture were incubated for 30 min at room temperature. Absorbance was taken at 730 nm on Shimadzu UV–Vis spectrophotometer. Total phenolic content in different samples was expressed as gallic acid equivalent per gram.
Antioxidant activity
DPPH free radical scavenging assay
The free radical scavenging potential of antioxidant compounds present in sample extracts and purified fractions obtained from underutilized plant parts was assessed by using DPPH free radical assay (Turkmen et al. 2006). Reaction tubes were wrapped in aluminium foil and aliquots 200 µl of each extract was mixed with 1.8 ml of DPPH radical solution. Ethanol was used as control. The reaction mixture was vortex mixed and allowed to stand at 25 °C in dark for 30 min. Optical density measurements were performed at 517 nm using UV–Vis Shimadzu UV-2450 spectrophotometer. Antioxidant activity was estimated as percentage inhibition of DPPH radical and was determined by the following equation:
where AA—absorption of tested extract solution, AB—absorption of the blank sample.
Percentage of radical scavenging activity was plotted against the corresponding concentration of the extract to obtain IC50 values.
ABTS radical scavenging assay
ABTS radical cation decolorization test is a spectrophotometric method widely used for assessment of antioxidant activity of various compounds. This experiment was carried out using ABTS decolorization assay method (Re et al. 1999) with some modifications. ABTS (7 mM) was prepared in distilled water. ABTS radical cation was produced by reacting aqueous ABTS solution with potassium persulphate (2.45 mM). The mixture was allowed to stand in dark at room temperature for 12–16 h. The analysis was carried out by diluting the solution with ethanol to get absorbance of 0.700 ± 0.02 at 734 nm and equilibrated at 30 °C. After addition of 2.0 ml of diluted ABTS solution (A = 0.700 ± 0.020) to 50 µl of each sample extract, the absorbance was taken exactly after 4 min against appropriate solvent blank. All the determinations were carried out in triplicate. The percentage inhibition of absorbance at 734 nm was calculated and plotted as a function of the concentration of antioxidants. IC50 values for different extracts were calculated by plotting the percentage of radical scavenging activity against corresponding concentration of respective extracts.
Cytotoxicity studies against oral cancer cells
KB cell lines were procured from National Centre for Cell Sciences, grown in DMEM (Gibco, Invitrogen). The media was supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS; Gibco, Invitrogen) and antibiotic antimycotic (1%) (Gibco, Invitrogen). Cells were maintained at 37 °C with 5% CO2 and 95% humidity in an incubator (Thermoscientific). Once 70–80% confluence was achieved in the culture flask, KB cells were harvested and taken up for toxicity study. Different purified extracts were assayed for anti-proliferative activity against KB cells using Sulphorhodamine-B assay (Skehan et al. 1990). The cell monolayer was trypsinized and final suspension of 2 × 105/ml was prepared using DMEM. 0.1 ml (around 20,000 cells) of this suspension was added to required number of wells in 96-well microtiter plates and incubated at 37 °C with 5% CO2 in 95% humidified atmosphere. Insult at required concentrations was given for 24, 48 and 72 h in triplicate following which cell monolayer was fixed with 50% trichloroacetic acid (w/v) and subjected to colorimetric study where cells were stained with 0.1 ml of anionic/aminoxanthine 0.4% SRB dye. Allowed 30 min staining to ensure its optimum electrostatic binding where it forms a complex with basic amino acids on the live cell membrane. After that, unbound dye was washed with 1% acetic acid and protein-bound dye was dissolved in 10 mM Tris base [tris (hydroxymethyl) aminomethane] solution. OD540 was determined using microplate reader [BioTek Synergy H1 Hybrid Reader] and percentage inhibition was calculated using following formula and Standard Deviation (SD) was calculated using Microsoft excel.
where C = OD540 of Control and S = OD540 of Sample
Lymphocyte proliferation assay
Splenocytes were isolated from mice spleen and were maintained in RPMI (Gibco, Invitrogen). The isolation protocol of splenocytes from mice was approved by Institutional Animal Ethical Committee (IAEC) at CSIR-IHBT in compliance relevant with State and Federal Regulations (IHBT-P10 dated February 21, 2013). Splenocytes were cultured and processed to undertake cell proliferation study of each extract on 1 × 105 cells/well. Accordingly, SRB assay was performed and OD was measured using microplate reader. The % proliferation was calculated using following formula while SD was calculated using Microsoft excel.
where C = OD540 of control and S = OD540 of sample
Antimicrobial activity
Food borne pathogens; Bacillus subtilis, Staphylococcus aureus (gram positive bacteria) and Escherichia coli and Klebsiella pneumoniae (gram negative bacteria) were collected from Institute of Microbial Technology MTCC Chandigarh (India). The antimicrobial activity was evaluated by cup method by using nutrient agar as growth media. The microorganism was activated by inoculating a loopful of the species in the nutrient broth and incubated for 24 h at 37 °C. The sub culture in broth was used as the inoculums for seeding the agar or Muller Hinton agar plate. The growth media were prepared and sterilized as directed by the manufacturer of the media and poured into presterilized petri dishes on a flat horizontal surface to a depth of 4 mm and allowed to solidify. The plates were inoculated with 100 µl inoculums using micropipettes. With the help of sterile spreader, the inoculums were spread uniformly to obtain a nearly confluent lawn of growth covering the whole surface of plate. The wells were bored into the inoculated petriplates with the help of sterile borer depending upon the number of concentrations of extracts. 100 µl aliquot from various dilutions of the extracts i.e. 5, 10 and 20 mg/ml were added into the wells.
Results and discussion
The escalating demand for green tea and tea-based products has prompted several investigations related to compositional analysis, and assessment of biological properties associated with major tea constituents. It is well established that major tea constituents play a significant role in prevention and delay of various metabolic disorders like diabetes, obesity, hypertension, cancer and cardiovascular disorders (Stangl et al. 2007). Thus, exploration of new economical and reliable sources other than tender tea shoots (two leaves and apical bud) is highly required. With this viewpoint, we performed a comparative investigation of different tea plant parts (apical bud, 1st–5th leaf, stem, coarse leaves, flowers, fruits and roots) (Fig. 1) for evaluation of total phenolics, free radical scavenging antioxidants along with major bioactive tea constituents viz. catechins, caffeine and theanine. The prime aim of this comparative investigation was the underutilized tea plant parts, especially coarse tea leaves, flowers and immature green fruits. Although, few studies have been conducted in the recent past for evaluation of phenolic antioxidants from underutilized tea leaves, tea flowers (Vuong et al. 2012; Yang et al. 2007) and tea fruit peel (Xu et al. 2012), but there is hardly any report on simultaneous evaluation of total phenolic antioxidants accompanied by quantitative evaluation of major tea constituents (catechins, caffeine and theanine) in these vary parts. There is scarcity of data related to the chemical composition of tea roots and tea fruits. Hence, different parts of tea plant were quantitatively evaluated for major chemical constituents for exploring horizons from underutilized tea plant parts by harvesting major phenolic antioxidants from them.
Thus, for the qualitative assessment of various parts of the tea plant, the dried and powdered parts were extracted with 70% acetone for the determination of total phenolics, theanine, caffeine and catechins content. The use of aqueous organic solvents such as methanol, ethanol and acetone has been preferred for extraction of major tea constituents in earlier studies (Wang and Helliwell 2001; Vuong et al. 2010). We have conducted the extraction in 70% acetone and spectral determinations of total phenolics in acquired extracts were performed by standard Folin’s Ciocalteau method, and results were reported as gallic acid equivalents (GAE). These results revealed variations in total phenolics content ranging from 36.63 to 102.24 mg/g in different plant parts (Table 1, Fig. S1). The results showed good correlation with earlier investigations of Rusaczonek et al. (2007) who have reported the occurrence of high yield of phenolics in tea leaves followed by stems and leaf stalk. Also, Nor Qhairul Izzreen and Mohd Fadzelly (2013) reported higher phenolic content in young tea shoots (80.27 mg/g) in comparison to mature leaves (56.63 mg/g). This decrease in phenolics with maturity of plant part might be due to the morphological changes that occur in leaf with age (Farhoosh et al. 2007). The solvent extracts of different tea plant parts [apical bud, 1st leaf, 2nd leaf, 3rd leaf, 4th leaf, 5th leaf, stem, mature leaves, flowers, fruits, roots (RA, RB, RC)] were evaluated for individual catechins, caffeine and theanine content using published RP-HPLC-DAD (Rana et al. 2015). The HPLC results showed highest catechins content in 1st leaf and lowest in stem. The order of total catechins content was recorded as; 1st leaf > 2nd leaf > apical bud > 3rd leaf > 4th leaf > 5th leaf > mature leaves > flowers > fruits > stem. Higher caffeine content was recorded in young tea shoots (apical bud and 1–3rd leaf) compared to older leaves (4–5th leaf) and stem portion. Lin et al. (2003) reported the lower content of caffeine in old leaves compared to young tea leaves. Though, tender tea shoots which are generally used for making tea contain higher amount of catechins and caffeine, but coarse tea leaves along with other underutilized plant parts (fruits and flowers) also contain appreciable content of catechins and caffeine.
Table 1.
Major tea constituents in different parts of tea plant
| Compound | Apical bud | 1st Leaf | 2nd Leaf | 3rd Leaf | 4th Leaf | 5th Leaf | Stem |
|---|---|---|---|---|---|---|---|
| Theanine | 1.32 ± 0.001 | 1.13 ± 0.020 | 1.42 ± 0.009 | 0.92 ± 0.007 | 0.82 ± 0.001 | 0.82 ± 0.000 | 1.98 ± 0.000 |
| GC | 0.13 ± 0.008 | 1.18 ± 0.001 | 1.49 ± 0.010 | 1.33 ± 0.020 | 0.62 ± 0.050 | 0.72 ± 0.020 | 0.53 ± 0.020 |
| EGC | 2.10 ± 0.060 | 15.90 ± 0.311 | 16.02 ± 0.030 | 15.90 ± 0.070 | 10.80 ± 0.110 | 10.44 ± 0.040 | 5.95 ± 0.070 |
| Catechin | ND | ND | ND | ND | ND | ND | ND |
| Caffeine | 29.91 ± 0.200 | 23.80 ± 0.040 | 22.09 ± 0.90 | 21.80 ± 0.140 | 14.70 ± 0.120 | 14.50 ± 0.020 | 1.54 ± 0.030 |
| EC | 1.53 ± 0.070 | 7.05 ± 0.080 | 6.71 ± 0.050 | 6.45 ± 0.070 | 4.33 ± 0.006 | 4.52 ± 0.0800 | 3.95 ± 0.010 |
| EGCG | 25.10 ± 0.110 | 28.90 ± 0.071 | 26.15 ± 0.150 | 24.90 ± 0.140 | 22.50 ± 0.080 | 20.63 ± 0.021 | 4.75 ± 0.110 |
| GCG | ND | ND | ND | ND | ND | ND | ND |
| ECG | 12.89 ± 0.040 | 11.60 ± 0.071 | 10.98 ± 0.090 | 9.35 ± 0.120 | 7.11 ± 0.010 | 6.41 ± 0.120 | 1.14 ± 0.001 |
| CG | ND | ND | ND | ND | ND | ND | ND |
| Phenolicsa | 74.93 ± 1.16 | 102.24 ± 0.55 | 91.02 ± 1.09 | 84.44 ± 0.75 | 64.20 ± 0.85 | 61.76 ± 0.62 | 36.63 ± 1.25 |
Results are expressed as mg/g and mean of three replicates ±SD
GC gallocatechin, EGC epigallocatechin, EC epicatechin, EGCG epigallocatechin gallate, GCG gallocatechin gallate, ECG epicatechin gallate, CG catechin gallate, ND not detected
aGallic acid equivalent
The roots are also considered as an important part of the plant, and in case of tea (C. sinensis), the roots are primary site of theanine biosynthesis which is a novel non-proteogenic amino acid (Vuong et al. 2011). From roots theanine gets translocated to aerial parts. Theanine is well known for its brain relaxing properties and is also responsible for unique Umami taste in tea infusion (Henriquez-Aedo et al. 2013). Three different root samples: RA (seedling roots), RB (whole roots) and RC (secondary roots) were taken for assessment of catechins, caffeine and theanine content. Higher theanine content in roots as compared to other parts (2.15–58.17 mg/g) (Table S1) clearly showed that roots are prime source of theanine. Few earlier reports have also showed highest theanine content in roots as compared to other plant parts (leaves, stems and cotyledons). Baptista et al. (2012) have found high theanine content in young leaves compared to mature leaves. The highest catechins were recorded in RB (3.9 mg/g) followed by RC (3.57 mg/g). The caffeine was found to be highest in RA but not detected in RC. Our study on different samples of tea roots has provided a very distinct and clear profile of major phytoconstituents of tea roots. From these results, it is evident that germinating seedling roots could act as a good source of theanine.
Consequently, the results of simultaneous investigations of various tea plant parts for phenolic content and HPLC analysis of major phytoconstituents have unveiled that underutilized plant parts such as mature leaves, flowers and fruits contain adequate content of phenolic antioxidants, catechins, caffeine and theanine. Therefore, huge potential lies in valorization of this agro biomass which otherwise go waste. The results of phenolic content of underutilized tea plant parts (mature leaves, flowers and fruits) have noticeably recorded highest phenolic content in tea fruits (175.14 mg/g) as compared to tea flowers and mature leaves. Comparative HPLC investigations for catechins, caffeine and theanine content had been performed in 70% acetone and hot water extracts of these underutilized parts (Table S2). These results clearly revealed that 70% acetone extracts showed slightly higher extractability of catechins, caffeine and theanine as compared to hot water extracts. But keeping in view the safety and toxicity issues of solvent, water was preferred over acetone as green and economical solvent. Thus, we collected underutilized tea plant parts (coarse leaves, flowers and fruits) for extraction, purification and characterization of major phytoconstituents. A patented process (Singh and Rana 2014) was opted for extraction and purification of major tea constituents from underutilized tea plant parts using hot water (80–90 °C) (Fig. 2). Hot water was chosen as a green and economical solvent. The obtained aqueous extracts were concentrated using rotavapor to 1/4th of its original volume and then passed through resin column packed in water. The adsorbed compounds were eluted with aqueous ethanol, and elutions were spray dried to obtain powdered catechins enriched extracts. Moreover usage of hot water as preferred extraction solvent also avoids the chances for unwanted residues, which could have to occur in fractions obtained by using organic solvents (Vuong et al. 2010).
Quantitative analyses of purified extracts obtained from different underutilized parts have revealed the presence of sufficient amount of phenolics (838–941 mg/g) as gallic acid equivalent in these purified extracts. Higher content of gallic acid equivalent phenolics was reported in fruit extracts (941 ± 0.78 mg/g) followed by mature leaves extracts (897 ± 0.73 mg/g) and least was accounted in flower extracts (838 ± 0.57 mg/g) (Table S3).
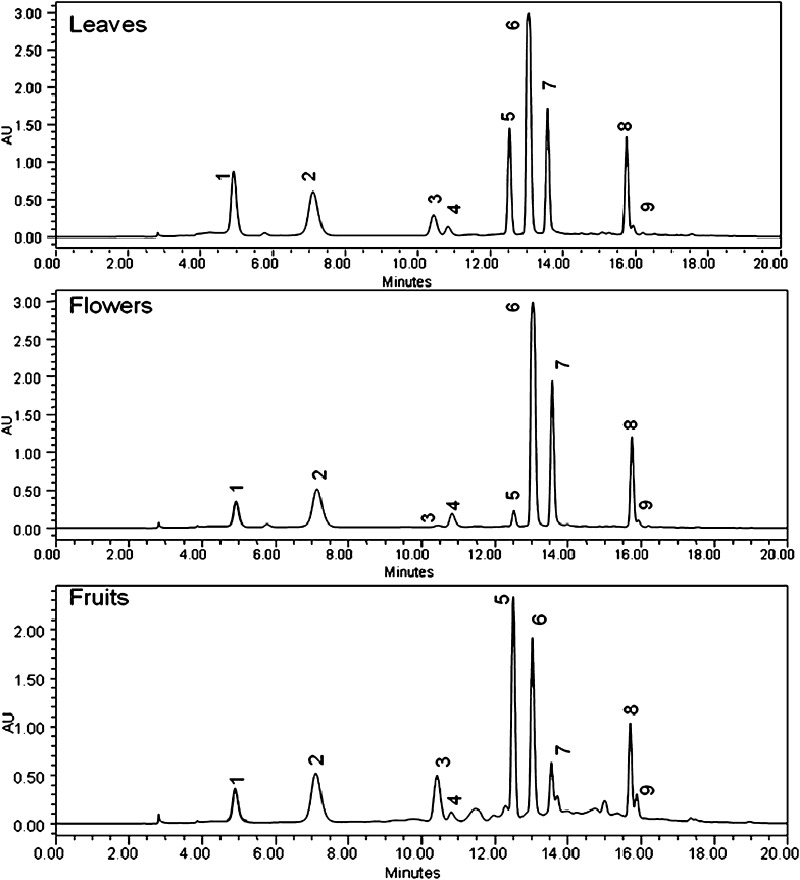
The purified catechins enriched extracts obtained from underutilized tea plant parts were also evaluated for estimation of catechins and other constituents by RP-HPLC-DAD analysis (Fig. 3). The results of HPLC analysis (Table 2) of purified extracts obtained after processing of underutilized plant parts showed higher catechins content in coarse leaves (746.06 mg/g) followed by flowers (680.46 mg/g). HPLC results clearly demonstrated that adequate content of major tea constituents is available in the fractions obtained from various underutilized tea plant parts. Higher EGCG content was found in the extracts obtained from coarse leaves, while tea fruit extracts had higher content of EC. The lower caffeine content was also found in fruits extracts as compared to leaves and flowers extract.
Fig. 3.
RP-HPLC chromatograms of purified catechin enriched extracts of underutilized tea plant parts. Peaks are labelled as: 1 GC, 2 EGC, 3 Catechin, 4 Caffeine, 5 EC, 6 EGCG, 7 GCG, 8 ECG and 9 CG
Table 2.
Data showing major constituents (mg/g) in purified extracts obtained from underutilized tea plant parts
| Compound | Coarse leaves | Flowers | Fruits |
|---|---|---|---|
| GC | 60.51 ± 0.100 | 41.81 ± 0.120 | 42.75 ± 0.630 |
| EGC | 70.20 ± 0.120 | 88.56 ± 0.500 | 93.58 ± 0.700 |
| Catechin | 14.19 ± 0.150 | 1.09 ± 0.007 | 48.87 ± 0.080 |
| Caffeine | 33.74 ± 0.110 | 21.63 ± 0.080 | 6.66 ± 0.120 |
| EC | 36.16 ± 0.090 | 8.27 ± 0.100 | 108.55 ± 0.050 |
| EGCG | 303.20 ± 0.070 | 298.96 ± 0.400 | 140.12 ± 0.500 |
| GCG | 178.92 ± 0.150 | 176.82 ± 0.110 | 39.83 ± 0.300 |
| ECG | 79.47 ± 0.250 | 63.20 ± 0.080 | 48.72 ± 0.120 |
| CG | 3.41 ± 0.080 | 1.75 ± 0.001 | 8.97 ± 0.090 |
Results are expressed as mg/g and mean of three replicates ±SD
GC gallocatechin, EGC epigallocatechin, EC epicatechin, EGCG epigallocatechin gallate, GCG gallocatechin gallate, ECG epicatechin gallate, CG catechin gallate
The free radical scavenging antioxidants in extracts of underutilized plant parts was assessed by DPPH and ABTS free radical scavenging assays. DPPH and ABTS assays widely used standard methods employed for screening of the antioxidant potential of plant extracts. Both free radical scavenging assays rely on the ability to accept donated hydrogen by antioxidant compounds present in the plant extracts. DPPH and ABTS radical scavenging activity of purified fractions are tabulated in (Table S3) with their IC50 values. In the case of DPPH radical scavenging activity of extracts of coarse leaves, fruits and flowers, highest and least scavenging activity were recorded in tea fruits (IC50 = 18.54 µg/ml) and tea flowers (IC50 = 36.62 µg/ml) respectively. ABTS radical scavenging activity of mature leaves, flowers and fruits also varied from 15.01–31.4 µg/ml whereas in purified catechins enriched extracts, IC50 values varied from 4.48–5.57 µg/ml. It has been observed that purified extracts of tea fruits impart higher antioxidant potential with IC50 values of 4.92 and 4.48 µg/ml against DPPH and ABTS free radicals, respectively. The results of both DPPH and ABTS assay showed good correlation (y = 0.674x + 0.156, R2 = 0.980) with each other as well as with total phenolics (phenolics vs DPPH; y = −0.3067x + 34.149, R2 = 0.9855 and phenolics vs ABTS; y = −0.1211x + 15.706, R2 = 0.9878) (Fig. S2).
There are reports that tea polyphenols selectively induce apoptosis in oral carcinoma cells (Hsu et al. 2002) and thus can act as potential preventive agents for the same. Hence, purified catechins enriched extracts obtained from underutilized plant parts were studied at different concentrations on human oral cancer cells. From the results, it was found that within 24 h of treatment with tea fruit extract, KB cells became apoptotic. Toxicity ranged from 71.6 to 74.9% at the concentration of 50–200 μg/ml (IC50 = 36.08 μg/ml) which was found comparable to standard vinblastine at 24 h (74.2% at 2 μg/ml concentration; Fig. S3, Table S4). The toxicity declined for 48 and 72 h and reached to 8.3%. Interestingly, extract obtained from tea fruits positively induce proliferation (63.4%) in mice splenocytes within 24 h at 200 μg/ml which too declined gradually (Fig. S3, Table S5). The extracts from flower and leaf of tea neither showed significant toxicity nor proliferation of splenocytes. The catechin enriched extract obtained from tea fruits was able to induce toxicity on oral cells and at the same time, it proliferates the mixed cell population obtained from mice spleen. The suppressive effect of EGCG alone on mice whole spleen population was earlier reported (Shazaan et al. 2009) and our results have revealed that tea fruits extract exhibited more promising results which may be accounted due to synergistic effect among other phytochemicals that are present in the fruit extracts along with catechins. The increase in splenocyte population by fruit extract also indicated an immuno-modulatory effect of this extract which is required to be further validated by exploring the other responsible phytochemicals and exact mechanism of action behind underlined synergy with catechins.
The antimicrobial potential of different extracts was evaluated against selected gram postive and gram negative bacterial stains. The results of the study (Table 3) showed that catechins enriched extracts obtained from underutilized tea parts showed promising antibacterial activity. Among tested extracts, tea flower extract showed maximum zone of inhibition at 20 mg/ml. The results of antimicrobial study clearly highlights the importance of these extracts as possible candidate for preparation of biopreservatives and for development of antimicrobial formulations against food borne pathogens.
Table 3.
Antimicrobial activity of extracts obtained from purified extracts obtained from underutilized tea parts
| Extracts | Conc. (mg/mL) | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| Gram positive | Gram negative | ||||
| B. sublitis | S. aureus | K. pneumoniae | E.coli | ||
| Tea leaf | 5 | ND | ND | ND | ND |
| 10 | 6.25 ± 0.3 | 7.5 ± 0.7 | 6.0 ± 0.0 | 6.0 ± 0.1 | |
| 20 | 12.0 ± 0.0 | 10.5 ± 0.7 | 10.5 ± 0.7 | 10.0 ± 0.0 | |
| Tea flower | 5 | ND | ND | ND | ND |
| 10 | 8.0 ± 0.0 | 8.0 ± 0.0 | 9.0 ± 1.4 | 9.0 ± 0.0 | |
| 20 | 11.0 ± 0.0 | 12.5 ± 0.7 | 12.5 ± 0.7 | 12.0 ± 0.0 | |
| Tea fruit | 5 | ND | ND | ND | ND |
| 10 | 8.0 ± 1.4 | 6.0 ± 0.0 | 9.0 ± 0.0 | 6.0 ± 0.0 | |
| 20 | 10.5 ± 0.7 | 10.5 ± 0.7 | 10.5 ± 0.7 | 9.0 ± 0.0 | |
| Streptomycin | 5 | 20.0 ± 2.0 | 21.0 ± 1.5 | 20.0 ± 2.0 | 22.0 ± 3.0 |
Means are expressed as ±SD, ND not detected
Conclusion
For tea manufacture only tender tea shoots are harvested, leaving other plant biomass unutilized in amount of thousands of tons every year. The current study unveils the comparative evaluation of major bioactive constituents from each and every part of C. sinensis plant, right from apical bud to underground roots. The results of spectrophotometric and RP-HPLC-PDA investigations of different solvent extracts of tea plant parts showed variable content of major bioactives. The results of quantitative analysis significantly highlights the occurrence of sufficient amount of major bioactive tea constituents in underutilized plant parts. Hence, these valuable phytoconstituents were extracted and purified followed by screening of their bioactivities. The purified extracts showed promising free radical scavenging potential with anticancer activity against KB cell line. Apart these extracts exhibit immunomodulatory effect on mice splenocytes and antimicrobial activity against some food borne pathogens. Hence, it has established that underutilized tea plant parts could be used as economical and reliable bioresource for harvesting major tea constituents for further development of nutraceuticals and formulations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the financial support from Council of Scientific and Industrial Research (CSIR) New Delhi, India through BSC-0107 and MLP-0070. Kiran Rawat thanks D.S.T. for the award of S.R.F.
Contributor Information
Ajay Rana, Email: ajayrana@ihbt.res.in.
Ashu Gulati, Email: ashugulati@ihbt.res.in.
References
- Anesini C, Graciela FE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem. 2008;56:9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- Atoui AK, Mansouri A, Boskou G, Kefalas P. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem. 2005;89:27–36. doi: 10.1016/j.foodchem.2004.01.075. [DOI] [Google Scholar]
- Baptista J, Lima E, Paiva L, Andrade AL, Alves MG. Comparison of Azorean tea theanine to teas from other origins by HPLC/DAD/FD. Effect of fermentation, drying temperature, drying time and shoot maturity. Food Chem. 2012;132:2181–2187. doi: 10.1016/j.foodchem.2011.12.050. [DOI] [Google Scholar]
- Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea: part II. Review of anticancer properties. J Altern Complement Med. 2005;11:639–652. doi: 10.1089/acm.2005.11.639. [DOI] [PubMed] [Google Scholar]
- Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12:404–421. doi: 10.1016/S0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Farhoosh R, Golmovahhed GA, Khodaparast MHH. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.) Food Chem. 2007;100:231–236. doi: 10.1016/j.foodchem.2005.09.046. [DOI] [Google Scholar]
- Fernandez PL, Martín MJ, Gonzalez AG, Pablos F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. R Soc Chem Anal. 2000;125:421–442. doi: 10.1039/a909219f. [DOI] [PubMed] [Google Scholar]
- Henriquez-Aedo K, Vega M, Aranda A. Evaluation of tea functionality: determination of l-Theanine content in green and black teas by liquid chromatography. J Chil Chem Soc. 2013;58:1651–1654. doi: 10.4067/S0717-97072013000400057. [DOI] [Google Scholar]
- Hsu SD, Singh BB, Lewis JB, Borke JL, Dickinson DP, Drake L, Caughman GB, Schuster GS. Chemoprevention of oral cancer by green tea. Gen Dent. 2002;50:140–146. [PubMed] [Google Scholar]
- Lin YS, Tsai YJ, Tsay JS, Lin JK. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem. 2003;51:1864–1873. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- Manzocco L, Anese M, Nicoli MC. Antioxidant properties of tea extracts as affected by processing. LWT Food Sci Technol. 1998;31:694–698. doi: 10.1006/fstl.1998.0491. [DOI] [Google Scholar]
- Narukawa M, Morita K, Hayshi Y. L-Theanine elicits an umami taste with inosine 5-monophosphate. J Sci Technol Dev. 2008;10:6–10. doi: 10.1271/bbb.80328. [DOI] [PubMed] [Google Scholar]
- Nor Qhairul Izzreen MN, Mohd Fadzelly AB. Phytochemicals and antioxidant properties of different parts of (Camellia sinensis) leaves from Sabah Tea Plantation in Sabah Malaysia. Int Food Res J. 2013;20:307–312. [Google Scholar]
- Obuchowicz J, Engelhardt UH, Donnelly K. Flavanol database for green and black teas utilising ISO 14502-1 and ISO 14502-2 as analytical tools. J Food Comp Anal. 2011;24:411–417. doi: 10.1016/j.jfca.2010.07.006. [DOI] [Google Scholar]
- Peng L, Song S, Shi S, Li J, Ye C. An improved HPLC method for simultaneous determination of phenolic compounds, purine alkaloids and theanine in Camellia species. J Food Comp Anal. 2008;21:559–563. doi: 10.1016/j.jfca.2008.05.002. [DOI] [Google Scholar]
- Peterson J, Dwyer J, Bhagwat S, Haytowitz D, Holden J, Eldridge AL, Beecher G, Aladesanmi J. Major flavonoids in dry tea. J Food Comp Anal. 2005;18:487–501. doi: 10.1016/j.jfca.2004.05.006. [DOI] [Google Scholar]
- Quan PT, Hang TV, Ha NH, Gang BL. Total polyphenols, total catechins content and DPPH free radical scavenger activity of several types of Vietnam commercial green tea. J Sci Technol Dev. 2007;10:6–10. [Google Scholar]
- Rana A, Bhangalia S, Singh HP. A new phenylethanoid glucoside from (Jacaranda mimosifolia) Nat Prod Res. 2013;27:1167–1173. doi: 10.1080/14786419.2012.717290. [DOI] [PubMed] [Google Scholar]
- Rana A, Singh HP, Gulati A. Concurrent analysis of theanine, caffeine, and catechins using hydrophobic selective C12 stationary phase. J Liq Chromatogr Relat Technol. 2015;38:709–715. doi: 10.1080/10826076.2014.962144. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente RC. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rusaczonek A, Zebrowska M, Waszkiewicz-Robak B, Edyta S. Evaluation of phenolic compounds content and antioxidant capacity of herbs. Polish J Food Nutr Sci. 2007;57(4):483–488. [Google Scholar]
- Sharma V, Gulati A, Ravindranath SD, Kumar V. A simple and convenient method for analysis of tea biochemicals by reverse phase HPLC. J Food Comp Anal. 2005;18(6):583–594. doi: 10.1016/j.jfca.2004.02.015. [DOI] [Google Scholar]
- Shazaan H, Lalithapriya J, Amy BH, Devang B, Dipti LB, Dana RC. Select phytochemicals suppress human T-lymphocytes and mouse splenocytes suggesting their use in autoimmunity and transplantation. Nutr Res. 2009;29:568–578. doi: 10.1016/j.nutres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh HP, Rana A (2014) An economical process for purification of free bio amino acids. CN104105685 A
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Stangl V, Dreger H, Stangl K, Lorenz M. Molecular targets of tea polyphenols in the cardiovascular system. Int J Cardiovasc Res. 2007;73:348–358. doi: 10.1016/j.cardiores.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of (Prunus domestica) the quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Turkmen N, Velioglu YS, Sari F. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteau methods. Food Chem. 2006;99:835–841. doi: 10.1016/j.foodchem.2005.08.034. [DOI] [Google Scholar]
- Vuong QV, Golding JB, Nguyen MH, Roach PD. Extraction and isolation of catechins from tea. J Sep Sci. 2010;33:3415–3428. doi: 10.1002/jssc.201000438. [DOI] [PubMed] [Google Scholar]
- Vuong QV, Bowyer MC, Roach PD. L-Theanine: properties, synthesis and isolation from tea. J Sci Food Agric. 2011;91:1931–1939. doi: 10.1002/jsfa.4373. [DOI] [PubMed] [Google Scholar]
- Vuong QV, Golding JB, Nguyen MH, Roach PD. Production of caffeinated and decaffeinated green tea catechin powders from underutilised old tea leaves. J Food Eng. 2012;110:1–8. doi: 10.1016/j.jfoodeng.2011.12.026. [DOI] [Google Scholar]
- Wang H, Helliwell K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res Int. 2001;34:223–227. doi: 10.1016/S0963-9969(00)00156-3. [DOI] [Google Scholar]
- Xu P, Bao Z, Gao J, Zhou T, Wang Y. Optimization of extraction of phenolic antioxidants from tea (Camellia sinensis L.) fruit peel biomass using response surface methodology. Bioresources. 2012;7:2431–2443. [Google Scholar]
- Yang Z, Xu Y, Jie G, He P, Tu Y. Study on the antioxidant activity of tea flowers (Camellia sinensis) Asia Pacific J Clin Nutr. 2007;16:148–152. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.