Abstract
Conventional gnocchi are small Italian dumplings made from potatoes, flour, and eggs. In this study, a range of gnocchi-type products containing navy bean and beef meat (10–40% w/w) were developed. The nutritional, physicochemical and sensory properties of the formulated gnocchi were determined, and a Modified in vitro Stomach Stir Tank (MISST) system was used to determine in vitro digestibility. Adding meat significantly increased the fat and protein content of cooked gnocchi type products compared to the control sample. Addition of navy bean and meat also significantly increased hardness, springiness, and chewiness, of most gnocchi type products compared to control sample. In vitro studies showed that pH increased faster in samples high in meat and navy bean content during the initial 30 min to control. The addition of high levels of meat emulsion and navy bean increased, springiness, beany, and meaty flavour. Gnocchi with 20% meat emulsion was similar to control upto some extent being characterized to have flocculent, soft, chewy, and wheaty in flavour. The addition of meat and navy bean did not affect the digestibility of starch in the gastrointestinal tract. Fortified gnocchi with meat and bean was showed a promising vehicle to deliver nutritive values without any changes in starch digestibility.
Keywords: Gnocchi, In vitro digestibility, Physicochemical analysis, Meat, Navy bean
Introduction
Conventional gnocchi are small Italian dumplings made from potatoes, wheat flour, and sometimes eggs. These dumplings are rich in carbohydrates but poor in protein content. Incorporation of legume flour (Petitot et al. 2010; Shogren et al. 2006; Zhao et al. 2005) and algae (Fradique et al. 2010; Zouari et al. 2011) into pasta can increase protein content. In addition, incorporation of 50% soy flour into spaghetti increased the content of proteins and essential amino acids: lysine, threonine, methionine, isoleucine and leucine (Shogren et al. 2006). In another study, Petitot et al. (2010) showed that the incorporation of 35% faba bean flour increased protein content of durum pasta. The textural properties of raw and cooked pasta were however altered with fortification. Fortification of durum wheat pasta with split pea or faba bean flour increased raw pasta hardness (Petitot et al. 2010). Similarly, Zhao et al. (2005) found that incorporation of green and yellow pea, or chickpea flour increased the firmness of cooked spaghetti.
Meat proteins provide all essential amino acids. Navy (haricot) beans (Phaseolus vulgaris) on the other hand are low-cost and a rich source of protein as well as fibre, oligosaccharides and other carbohydrates. Ingestion of a considerable amount of resistant starch present in navy beans has been found to reduce postprandial glycaemic responses (Gallegos-Infante et al. 2010). Furthermore, Osorio-Diaz et al. (2005) showed that these starches were high in slowly digestible carbohydrates with a relatively low hydrolysis index (HI = 36%).
Our study is the first investigation on a novel gnocchi-type food made from a combination of potatoes, navy bean flour, and constituents of beef. The present work was undertaken to investigate the effects of navy bean and beef emulsion addition on the physicochemical, textural, sensorial and digestibility characteristics of gnocchi.
Materials and methods
Materials
Potatoes (Solanum tuberosum) of the Agria cultivar were purchased from a local supermarket and stored under dark conditions between 7 and 8 °C. Semolina flour (Sun Valley Foods, New Zealand) and dried navy (haricot) beans (Phaseolus vulgaris) were purchased from a local supermarket and stored under dry and dark conditions prior to the experiment. Minced beef muscle from New Zealand dairy bulls (18–24 months-old) was provided by AgResearch Ltd. Delmaine classic potato gnocchi (Delmaine Fine Foods, New Zealand) was chosen as a commercial sample.
Gnocchi preparation
Potatoes were boiled for 30 min until soft. The skin was removed and potatoes were mashed using a potato-ricer (HB710, Kenwood, New Zealand). Minced beef (200 g), salt (2 g), and water (150 mL) were homogenized at 7000 rpm for 5 min using a high speed homogenizer (L5 M-A Laboratory Mixer, Silverson®, USA) to make a 57% meat emulsion. Navy beans were soaked overnight in water and boiled for 2 h, then drained, cooled, and mashed into a paste using a food processor (FP734, Kenwood, New Zealand) for 20 min at high speed.
Mashed potato, meat emulsion, and navy bean paste were then combined with semolina flour. Seventeen formulations of gnocchi dough were prepared using a dough maker (KM210, Kenwood, New Zealand). A lemon-size piece of dough (4 g) was rolled into a rope of approximately 2 cm in diameter, and then cut into short pieces (2 cm × 2 cm). Gnocchi was cooked within 2–3 h using a steamer for 20 min, or stored frozen at −15 °C until further use. Two batches of each gnocchi formulation were prepared.
Proximate composition
Fat, protein, ash, moisture and total carbohydrate contents of gnocchi were determined following standard methods (AOAC 2000).
Texture analysis
Texture Profile Analysis (TPA) of cooked gnocchi samples were carried out as described by Alessandrini et al. (2010) with slight modification using a texture analyser (TA.XTplus, Stable Micro Sysytems, UK). The samples were placed on a Heavy Duty Platform (HDP/90) of the TA-XT2 texture analyser with a cylindrical aluminum probe (5 cm in diameter) using a 50 kg load cell. The crosshead speed was 0.5 mm/s, with a rest period of 5 s between cycles and the deformation was observed upto 50% of the original length. The compression test was used for determination of hardness, springiness, and chewiness. Textural data were normalized by dividing the respective numerical values by the weight of each different sample (Alessandrini et al. 2010).
Colour analysis
Colour of each sample was evaluated using a Hunter Lab Color meter (Flex EZ 45°/0° benchtop spectrophotometer, HunterLab, USA). Readings were taken three times for each gnocchi sample and recorded as L* (lightness), a* (green to redness), and b* (blue to yellowness). Values of a* and b* were transformed to chroma C* (C* = [a2 + b2]½). L*, a*, and b* colour space was modeled after a color-opponent theory (Trouillas et al. 2016) stating that two colors cannot be red and green at the same time or yellow and blue at the same time. L* indicates lightness, a* is the red/green coordinate, and b* is the yellow/blue coordinate. A positive L* is a measure of lightness, ranging from 0 (black) to +100, while a* value ranges from −100 (greenness) to +100 (redness), and b* value ranges from −100 (blueness) to +100 (yellowness). A calculated C* which represents chroma would indicate sample colour contrast. A positive chroma would indicate a brighter colour, while a negative chroma indicated a duller colour.
Sensory evaluation of cooked gnocchi
Six gnocchi formulations and control samples were chosen for sensory projective mapping. Gnocchi was served with diluted Leggo’s classic tomato sauce in a ratio of 7:3 sauce to water. Gnocchi sample (10 g each) was mixed with 5 mL pasta sauce and placed into 25 mL heat resistant plastic containers coded with three-digit random numbers that were maintained at 40 °C in an oven. The order of serving was randomized to avoid sample order and carry-over effects (Wakeling and MacFie 1995). Filtered water was served as a palate cleanser and testing was conducted under red light to mask colour differences between samples. Ethics approval for this study was obtained from the Auckland University of Technology Ethics Committee (AUTEC 13/21).
Twelve university staff and students aged between 20 and 65 years-old who consumed gnocchi at least once a month participated in this study. Product codes were positioned on the computer screen and the panellists keyed in sensory attributes associated with each product. Panellists selected and grouped sample codes, and then positioned it on a rectangular map (200 × 300 mm). They were also asked to describe the sensory attributes that corresponded to their product groupings. The data obtained was recorded using the FIZZ sensory data acquisition software (FIZZ Network v2.46b, Biosystèmes, France). A total of 11 panellists completed the sensory projective mapping of seven gnocchi products over three replicate sessions.
In-vitro digestibility
Digestive juice
Simplified artificial saliva was prepared according to Yoo and Chen (2007) by dissolving 2 g of fungal (Aspergillus oryzae) α-amylase (Grindamyl™ A5000, 5000U/g, Danisco 071314, Danisco A/S, Denmark) in 200 mL deionized water. The prepared artificial saliva was then kept at 37 °C until use. Simplified gastric juice was prepared using 2.2 g L−1 KCl (Sigma-Aldrich, USA), 1 g L−1 pepsin from porcine gastric mucosa (Sigma-Aldrich P-7000, E.C.3.4.23.1., Sigma-Aldrich, USA), 5 g L−1NaCl (Sigma-Aldrich, USA), 0.22 g L−1 CaCl2 (Sigma-Aldrich, USA), 1.5 g L−1 NaHCO3 (Sigma-Aldrich, USA) and 0.15 g L−1 HCl (RCI Labscan Ltd., Thailand).
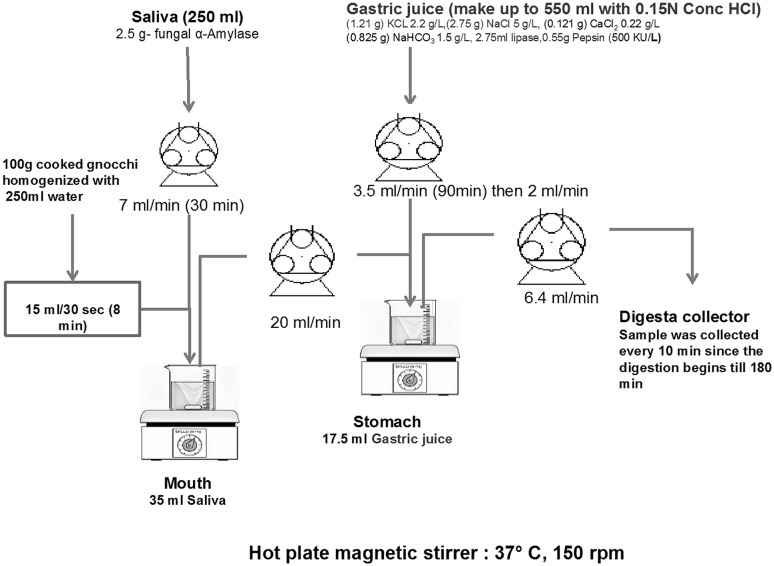
The modified in vitro stomach stir tank (MISST) model used in this study was modified from the Simulated Human Intestinal Microbial System (SHIME) model developed by Molly et al. (1993). A schematic of the MISST design used in this study is shown in Fig. 1.
Fig. 1.
Schematic diagram of the Modified in vitro Stomach Stir Tank (MISST) reactor modified from the Simulated Human Intestinal Microbial System (SHIME). The mouth compartment is filled with artificial saliva, while the stomach compartment is filled with gastric juice. Samples are pumped through the mouth compartment where it is hydrolyzed, and further digested in the stomach compartment. The digesta was then collected after in vitro digestion
Digestion steps
The mouth reactor contained 35 mL of artificial saliva before ingestion to simulate the cephalic phase of digestion. The saliva was pumped into a mouth reactor at 7.0 mL min−1. Gnocchi sample was homogenized with drinking water (1:2.5) to make a paste-like food bolus, and was then introduced into the mouth reactor at a speed of 30 mL min−1. The digested bolus was removed from the mouth reactor to the stomach reactor at a rate of 20 mL min−1. Gastric juice was pumped into the stomach at 3.5 mL min−1 from the start until 90 min, and then pumped at 2 mL min−1 from 90 to 120 min. Prior to digestion, the stomach reactor contained 17.5 mL of gastric juice to simulate the cephalic phase of digestion. The mouth and stomach reactors were maintained at 37 ± 1 °C on a hot plate magnetic stirrer with a stirring speed of 150 rpm (Molly et al. 1993). The chyme was pumped out of the stomach at a rate of 6.7 mL min−1. Two replicates were carried out for each gnocchi formulation.
Online pH monitoring and digestion analysis
An auto-logging pH meter (HI 4212, Hanna Instrument, Italy) was used to record the pH and temperature in the stomach reactor every 30 s for 120 min. The chyme mixture (10 mL aliquots) was collected from the stomach reactor every 10 min until the 120 min mark. The collected sample was immediately placed into a boiling water bath to stop any further enzymatic digestion. Samples were centrifuged (400e, Labofuge) at 700×g for 5 min and stored at 4 °C for further analysis.
Digested starch was analyzed using a total starch kit (K-TSTA, Megazyme, Ireland) according to the method (996.11) of AOAC (2000), which was based on amyloglucosidase (McCleary et al. 1997). Total water-soluble carbohydrate was analyzed using the phenol–sulphuric acid assay (Dubois et al. 1956).
The hydrolysis of starch in the collected chyme was expressed as the percentage of digested starch (including glucose, maltose, maltotriose and maltodextrin) in total carbohydrates of the ingested gnocchi sample. The hydrolysis values were calculated by dividing the mass of digested starch by the mass of total carbohydrates of the gnocchi sample as shown in Table 1 (%w/w carbohydrates × 100 g).
Table 1.
Nutritional composition and physicochemical properties of gnocchi samples containing meat and navy bean (Mean ± SE)
| Samples* | Nutritional composition (g/100 g wet basis) | Physicochemical properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Fat | Protein | Ash | Carbohydrates | Hardness (N) | Springiness (dimensionless) | Chewiness (N) | L* | a* | b* | |
| 10 M | 38.52 ± 0.13a | 0.81 ± 0.21ab | 9.94 ± 0.58bcd | 1.15 ± 0.04abcd | 49.59 | 668 ± 35i | 0.06 ± 0.01efg | 84 ± 18hi | 61 ± 0d | 4.7 ± 0.2 g | 24.5 ± 0.5bc |
| 10M10B | 40.49 ± 0.25bc | 1.00 ± 0.07bc | 9.15 ± 0.63abc | 1.03 ± 0.04abc | 48.32 | 1233 ± 209fg | 0.05 ± 0.00 fg | 125 ± 27fghi | 64 ± 0c | 3.2 ± 0.1j | 23.6 ± 0.4bcd |
| 10 M20B | 42.45 ± 0.21efg | 0.72 ± 0.03ab | 10.30 ± 0.97bcd | 1.07 ± 0.01abc | 45.46 | 1285 ± 187efg | 0.08 ± 0.01bc | 231 ± 26cdefgh | 61 ± 0d | 4.2 ± 0.1i | 23.7 ± 0.2bcd |
| 10M30B | 44.18 ± 0.16hi | 0.72 ± 0.01ab | 10.44 ± 0.13bcde | 0.82 ± 0.02a | 43.83 | 1052 ± 199ghi | 0.07 ± 0.01cdef | 139 ± 43fghi | 58 ± 0f | 4.4 ± 0.1hi | 22.4 ± 0.4cdef |
| 20M | 39.74 ± 0.07ab | 1.06 ± 0.02bcd | 11.11 ± 0.38cdef | 1.23 ± 0.15abcd | 46.86 | 995 ± 186ghi | 0.07 ± 0.01bcde | 171 ± 33efghi | 61 ± 0e | 4.2 ± 0.1hi | 22.1 ± 0.1defg |
| 20M10B | 41.85 ± 0.28def | 1.04 ± 0.12bcd | 11.59 ± 0.52cdefg | 1.16 ± 0.05abcd | 44.37 | 1148 ± 26gh | 0.07 ± 0.00bcde | 171 ± 4efghi | 60 ± 0e | 4.4 ± 0.1h | 21.9 ± 0.1defgh |
| 20M20B | 42.97 ± 0.68fgh | 1.17 ± 0.01bcde | 12.25 ± 0.16defghi | 0.995 ± 0.04ab | 42.62 | 1601 ± 90def | 0.08 ± 0.00bcd | 273 ± 60cde | 58 ± 0g | 5.6 ± 0.1ef | 22.3 ± 0.1cdef |
| 20M30B | 44.21 ± 0.08hi | 1.16 ± 0.02bcde | 11.73 ± 0.22cdefgh | 0.99 ± 0.00ab | 41.91 | 1962 ± 52bcd | 0.04 ± 0.00 fg | 182 ± 5efghi | 58 ± 0g | 4.9 ± 0.1 g | 20.3 ± 0.1fghij |
| 30 M | 39.23 ± 0.02a | 1.28 ± 0.11cde | 14.51 ± 0.47hij | 1.2 ± 0.00abcd | 43.79 | 1053 ± 216ghi | 0.08 ± 0.01bcde | 195 ± 54defgh | 58 ± 0f | 5.5 ± 0.1ef | 21.1 ± 0.2efghi |
| 30M10B | 40.91 ± 0.41bcd | 1.44 ± 0.35cdef | 11.62 ± 1.94 cdefg | 1.01 ± 0.03abc | 45.02 | 1676 ± 89de | 0.06 ± 0.02cdefg | 243 ± 43cdef | 56 ± 0i | 5.6 ± 0.2ef | 25.2 ± 5.6b |
| 30M20B | 43.14 ± 0.17gh | 1.43 ± 0.01cdef | 13.19 ± 0.91efghij | 0.95 ± 0.11ab | 41.29 | 1745 ± 174cd | 0.09 ± 0.02b | 303 ± 30cd | 54 ± 0j | 6.1 ± 0.1c | 20.6 ± 0.2fghi |
| 30M30B | 45.28 ± 0.30i | 1.59 ± 0.03ef | 13.79 ± 0.94fghij | 0.92 ± 0.11ab | 38.43 | 2512 ± 251a | 0.07 ± 0.00bcde | 455 ± 63a | 57 ± 0h | 5.7 ± 0.0def | 19.2 ± 0.2ij |
| 40 M | 38.86 ± 0.15a | 1.48 ± 0.05def | 14.77 ± 0.24ij | 1.45 ± 0.25cd | 43.44 | 824 ± 198hi | 0.09 ± 0.01bcd | 178 ± 77efghi | 57 ± 0h | 5.5 ± 0.1f | 20.2 ± 0.1fghij |
| 40M10B | 43.05 ± 0.80fgh | 1.73 ± 0.03f | 14.57 ± 0.22hij | 1.54 ± 0.04d | 39.12 | 1736 ± 305cd | 0.12 ± 0.03a | 335 ± 44bc | 52 ± 0k | 6.4 ± 0.1b | 20.1 ± 0.3ghij |
| 40M20B | 42.54 ± 0.04 fg | 1.84 ± 0.09f | 15.22 ± 0.12j | 1.15 ± 0.30abcd | 39.26 | 2216 ± 105ab | 0.10 ± 0.00b | 538 ± 74a | 53 ± 0k | 5.8 ± 0.1d | 18.3 ± 0.1j |
| 40M30B | 45.38 ± 0.17i | 1.83 ± 0.10f | 14.09 ± 1.01ghij | 1.28 ± 0.10bcd | 37.41 | 2112 ± 444abc | 0.08 ± 0.01bcd | 440 ± 161ab | 53 ± 0k | 7.0 ± 0.1a | 19.8 ± 0.1hij |
| Control | 41.22 ± 0.08cde | 0.40 ± 0.04a | 7.80 ± 0.05ab | 0.88 ± 0.01ab | 49.7 | 800 ± 0hi | 0.04 ± 0.01g | 66 ± 0i | 70 ± 0a | 0.7 ± 0.1l | 27.4 ± 0.1a |
| Commercial | 42.13 ± 0.20defg | 1.00 ± 0.01bc | 6.92 ± 0.09a | 1.01 ± 0.00abc | 48.94 | 699 ± 127i | 0.06 ± 0.01defg | 120 ± 17ghi | 69 ± 0b | 2.0 ± 0.1k | 23.3 ± 0.5bcde |
| p value | |||||||||||
| Meat | 0.001 | 0.001 | 0.001 | 0.001 | NA | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.003 |
| Navy bean | 0.001 | 0.068 | 0.045 | 0.002 | NA | 0.001 | 0.014 | 0.001 | 0.001 | 0.001 | 0.058 |
| Meat × Navy bean | 0.01 | 0.158 | 0.105 | 0.417 | NA | 0.009 | 0.021 | 0.025 | 0.001 | 0.001 | 0.139 |
a,b,c,d,e,f,g,h,i,j,k,l mean values of nutritional composition and physicochemical properties with different superscripts within the same column differ significantly (p < 0.05)
Statistical analysis
A full factorial design was generated from four levels of meat emulsion (10, 20, 30 and 40% w/w), and four levels of navy bean paste (0, 10, 20 and 30% w/w) using Minitab (v16, Minitab Inc, USA). Meat, bean, semolina flour (30% w/w) and complementary amounts of mashed potato were used in each gnocchi formulation. A formulation containing 70% mashed potato and 30% semolina was used as control (Saveur 2008). Gnocchi samples were coded according to their percentage of meat emulsion (M) and navy bean (B) content, as shown in Table 1.
One-way analysis of variance (Giner et al. 2001) and post hoc comparison of means was carried out using the Fisher’s least significant difference (LSDs) method at a significance level of 0.05 (XLSTAT version 2013.5.01, Addinsoft, USA).
Full factorial design analysis and general factorial regression analysis (Minitab v16, Minitab Inc, USA) were further carried out to study the effects of meat, navy bean and their interactions on the physicochemical properties of reformulated gnocchi samples. The contour plots were plotted to indicate significant interactions between meat and navy bean (p < 0.05).
Analysis of projective mapping results was performed according to Balbas et al. (2015) using Addinsoft XLSTAT-MX version 2011.5.01. Multiple Factor Analysis (MFA) was used to obtain Random Variable (RV) coefficient to check panellists’ agreement and consistencies in terms of product characteristics. Generalized Procrustes Analysis (GPA) was used to obtain overall product coordinates, and Principal Component Analysis (PCA) was carried out to visualize the product on the extracted coordinates. Sensory attributes that occurred for a minimum of five times across panelists per product were included as supplementary variables in the PCA biplots.
Results and discussion
Physicochemical properties
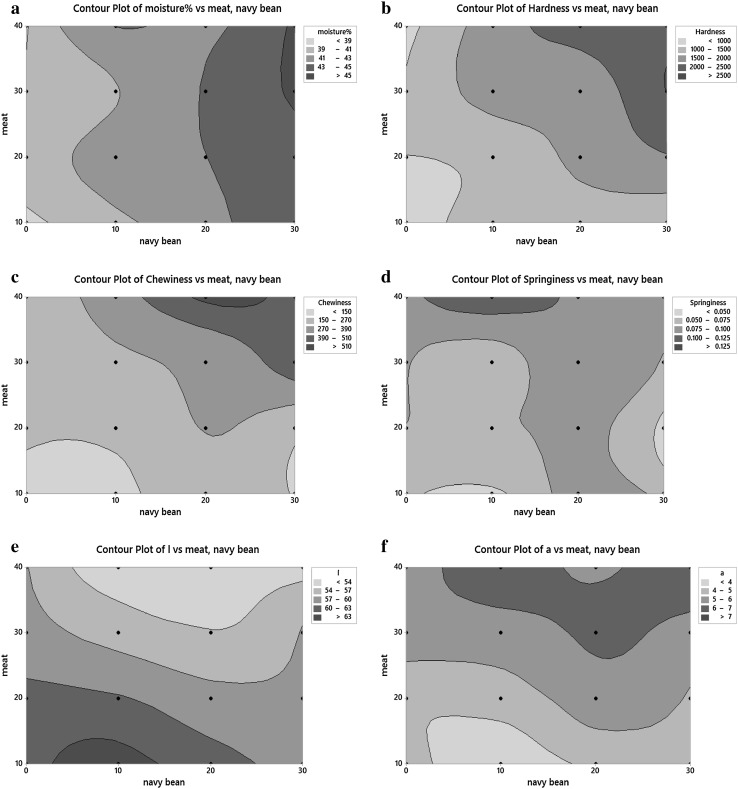
There were significant effects of meat addition on the physicochemical properties of gnocchi (p < 0.05). Addition of navy bean had significant effects on almost all physicochemical properties measured except for fat and b* values. There were significant interactions effects between meat emulsion and navy bean on moisture, hardness, chewiness, springiness, and L* and a* value (Table 1). Contour plots were plotted to further illustrate the relationship between meat emulsion and navy bean addition in the formulated gnocchi. All contour curves with significant interaction displayed considerable curvature implying that the interaction term was important (Fig. 2).
Fig. 2.
Contour plots showing changes in moisture, hardness chewiness, springiness, L* value and a* value with varying meat and navy bean content (meat × navy bean, p < 0.05)
The proximate composition of gnocchi is summarized in Table 1. Carbohydrate content of the gnocchi samples ranged from 37.4 to 49.7%. This value was much lower than the total carbohydrates of cooked pasta, which was reported to be between 60–77% (Gallegos-Infante et al. 2010; Petitot et al. 2010). This is because mashed potato in gnocchi made up 12.8% (wet basis) of the carbohydrate content. This was much lower than semolina flour, which contained about 22% carbohydrates (FSANZ 2013). The ash content of all gnocchi was around 1% and was not significantly different between samples.
The gnocchi with 30 and 40% meat emulsion (30M, 30M10B 30M20B, 30M30B, 40M, 40M10B, 40M20B, 40M30B) had significantly higher fat and protein content (p < 0.05) than that containing 10% meat emulsion (Table 1). The contour plot (Fig. 2a) showed that increasing the amount of navy bean increased moisture content. Navy bean has a high fiber content that was reported to be between 10 and 20% of dry mass (Kereliuk and Kozub 1995), which might had contributed to a higher water holding capacity (Chen et al. 1984).
Texture of cooked gnocchi
Most navy bean and meat emulsion combinations added to the formulation significantly increased the hardness, springiness and chewiness of cooked gnocchi compared to control, commercial gnocchi samples (Table 1). The contour plots (Fig. 2b) showed that hardness increased with increasing amount of incorporation of both meat emulsion and navy bean. Fortification of pasta with legume flour (navy bean, pinto bean, lentil, green pea, faba bean, split pea) or protein concentrates was reported to increase pasta firmness and hardness (Alireza Sadeghi and Bhagya 2008; Zhao et al. 2005). Malcolmson (1991) also reported that spaghetti firmness improved with increasing semolina protein content. The improvement in firmness has been attributed to higher number of polypeptide chains associated with higher protein levels that increased their chance for proteins interaction and form an insoluble network. This insoluble protein network can entrap swollen and gelatinized starch granule, which may have increased firmness (Malcolmson 1991).
The presence of meat increased chewiness in gnocchi with high navy bean samples (20 and 30% navy bean) (Fig. 2c) and was pronounced at higher meat concentration (30 and 40% meat). High springiness value was observed for the 40M10B gnocchi sample (Fig. 2d). The increased chewiness and springiness may be attributed to unfolding and aggregation of myofibrillar proteins upon heating that resulted into formation of three-dimensional cross-linked protein network, which traps fat and macroparticulates within the gel matrix (Sun and Holley 2011).
Colour of raw and cooked gnocchi
Colour, as one aspect of appearance, is an indicator of quality, freshness, flavour expectation and commercial value (Fradique et al. 2010). A slight yellow colour in fresh pasta was generally considered an important positive quality attribute (Alessandrini et al. 2010).
The gnocchi with 70% mashed potato (control) exhibited the highest b* value (27.4 ± 0.1) among all the samples. Agria potatoes, which has deep yellow flesh was used in this study to make gnocchi. This yellow color may be due to the presence of high amount of lutein and zeaxanthin (two kinds of carotenoid in yellow-flesh potatoes) (Brown et al. 2005). Commercial gnocchi sample on the other hand had a lower b* value (23.3 ± 0.5), which might be due to the use of potato puree made from white potato starch, which may be low in carotenoids (Álvarez et al. 2012).
The increased incorporation of meat emulsion increased a* value and decreased L* value (Fig. 2e, f; Table 1). Higher navy bean concentration resulted in lower L* value in gnocchi samples, which contained 20% or less meat concentration. When meat content was kept constant at 20 or 30%, a* value was only higher at 20% navy bean concentration. The higher redness and corresponding decrease in lightness could be associated with the color of myoglobin in meat emulsion that quickly changed to the bright red oxymyoglobin when exposed to air (Mancini and Hunt 2005).
Sensory characteristics
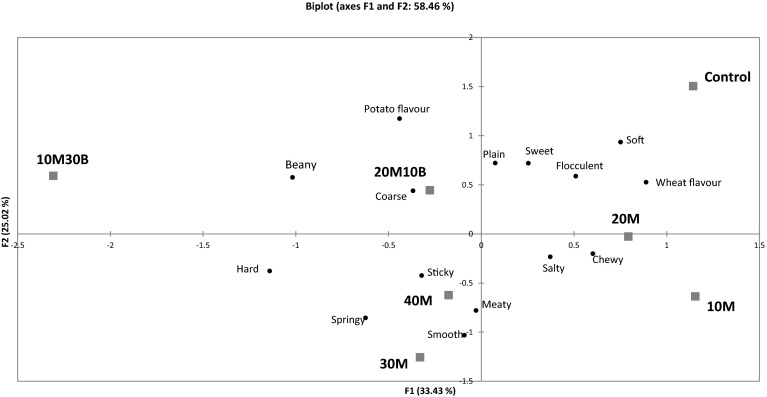
Panellists showed high consistency in terms of product groupings in replicated sessions and between panels (as assessed by MFA) (RV > 0.500) (results not shown). Product and attribute maps are shown in Fig. 3. The control sample was clearly separated from the experimental samples due to its soft texture, which was consistent with texture analysis results (Table 1). The panellists were able to separate other gnocchi samples in terms of texture. 40M, 30M and 20M10B samples had hard texture, while 20M and 10M samples were chewy, and neither hard nor soft in texture. 40M and 30M samples were associated with smooth mouth feel and meaty flavour. The smooth mouth feel of samples with high meat content may resulted from the interaction of proteins and fat to form a gel network (Malcolmson 1991; Mastromatteo et al. 2012). Gnocchi samples containing navy bean (20M10B and10M30B) were different due to their beany flavour and coarse mouth feel. This might be due to the fact that the navy bean was not finely ground in this study. Consumer testing for this reformulated gnocchi was also carried out. However, there were no significant differences in the reformulated gnocchi with the commercial products, in terms of overall liking, flavour, taste, texture, and odour (results not shown).
Fig. 3.
Biplot of first two dimensions of principal component analysis of seven gnocchi samples over the combined three sensory projective mapping trials. The sensory description used to describe the samples was supplemented as loadings. 10 M30B: 10% meat emulsion and 30% bean gnocchi; 20M10B: 20% meat emulsion and 10% bean gnocchi; 20M: 20% meat emulsion gnocchi; 30M: 30% meat emulsion gnocchi; 40M: 40% meat emulsion gnocchi
Digestibility studies using MISST
pH profile over the course of digestion
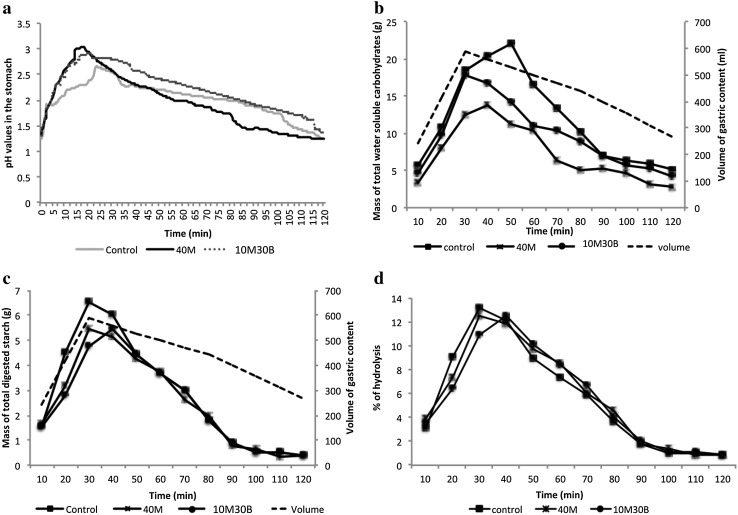
High meat (40M), high bean (10M30B), and control gnocchi samples were selected for further digestibility studies as they had similar textural properties to that of commercial gnocchi sample. The in vitro digestibility of starch and pH profiles of these samples in the stomach reactor of the MISST system are shown in Fig. 4a.
Fig. 4.
In vitro digestibility of gnocchi samples
The pH in the stomach reactor ranged from 1.3 to 3.5 over the 120 min digestion period (Fig. 4a), and closely simulated the conditions in human stomach. Kalantzi et al. (2006) reported that the median pH of the upper gastrointestinal (GI) lumen of human subjects increased from 1.7 to 2.4 after administration of 500 mL Ensure plus® (a complete nutrient drink). This initial rapid increase in gastric pH was indicative of the quick utilization of acid in the digestion process (Yoo and Chen 2007), and dilution of gastric fluids with saliva.
For all digested gnocchi samples, the highest pH after 20 min (Fig. 4a) were: 3.04, 2.95, and 2.66 respectively for 40 M, 10 M30B, and control gnocchi samples. Buffering capacity indicates the ability of food to resist changes in pH (Xu et al. 2012). The highest pH in the 40M gnocchi sample may be attributed to inorganic phosphate, protein-bound l-histidine residues, free l-histidine or histidine-related dipeptides in meat that played a key role in buffering (Culbertson et al. 2010). Gnocchi with 10% meat emulsion and 30% bean content showed a higher buffering capacity than control sample, even though there was no significant difference (p > 0.05) between samples in terms of protein content. The high buffering capacity in 10M30B might be due to the fiber in navy bean that has been reported to be positively correlated with buffering capacity (Al-Dabbas et al. 2010).
The pH of all samples decreased after 20 min due to stomach evacuation and constant gastric juice delivery into the stomach reactor. Towards the end of the 120 min digestion period, the pH of all samples decreased to 1.30 ± 0.05. This pH value was similar to that of gastric juice due to most of the chyme being pumped out of the stomach reactor, and the gastric acid content approaching 100% in the stomach reactor. In the human digestive tract, the acidic chyme from stomach stimulated the release of secretin, inducing the pancreatic bicarbonate and pancreatic fluid secretion, which were essential to neutralize gastric chime (Kvietys 2014). Protein was known to stimulate pancreatic enzyme and bicarbonate secretions more than carbohydrate. Hence with higher pH, the digestibility of protein containing gnocchi was expected to be slower than control.
Total water-soluble carbohydrates
Mass of total water-soluble carbohydrates indicates the total amount of available carbohydrates in the stomach reactor at given times. Because the gnocchi samples were rich in carbohydrates (37–50%, Table 1), total water-soluble carbohydrates were measured to study the transit pattern of bolus and chyme in the stomach reactor. Initially, to simulate the cephalic phase of gastric digestion, 3.5 mL min−1 of gastric juice was delivered to the stomach reactor for 5 min. Due to gastric secretion, delivery of bolus and gastric emptying, the volume of the gastric content increased to about 560 mL after 40 min, and then decreased to about 180 mL after 120 min. Measured concentrations of total water-soluble carbohydrates were converted to mass for a more accurate indication of carbohydrate digestion (Fig. 4b). The initial mass of water-soluble carbohydrates was 5.6 g for control, 3.3 g from 40M sample, and 4.5 g for 10M30B sample. This was followed by an increase in mass of water-soluble carbohydrates to a maximum of 22.11 g for control, 13.81 g for 40M, and 17.73 g for 10M30B sample. Therefore, more carbohydrates were available for digestion in the control sample.
Hydrolysis of starch in the MISST
Starch, the dominating water soluble carbohydrate in the gnocchi samples can be hydrolysed by alpha amylase in the reactor to produce low molecular weight molecules such as maltodextrins, maltotriose, and small portions of maltose and glucose that then enters the stomach (Southgate 1995). The control sample had the highest mass of total digested starch in the stomach reactor from 10 until 50 min (Fig. 4c). The higher the concentration of digested starch, the more the interactions between starch and alpha amylase occurred. Protein or lipid outside the starch granule has been reported to hinder the starch-amylase reaction (Butterworth et al. 2011).
Figure 4d shows the percentage hydrolysis of starch that contributes to total water-soluble carbohydrates in the collected chyme. The values were calculated by dividing the mass of digested starch by the mass of total carbohydrates in gnocchi. The highest % starch hydrolysis reached 13% (at 30 min), 12% (at 30 min), and 12.5% (after 40 min) for control, 40M and 10M30B samples respectively. The hydrolysis of starch decreased with no significant differences between the three samples thereafter due to the inactivation of alpha amylase by the acidic condition in the stomach reactor (Yoo and Chen 2007). Our results were similar findings by Gallegos-Infante et al. (2010), where the in vitro starch hydrolysis rate of spaghetti containing 45% common bean flour was 15%, which was significantly lower than spaghetti made with pure semolina (25%). Gastrin, the main stimulant of acid secretion during a meal, was released in response to dietary proteins, amino acids, and amines. Gastrin appeared to have a role in inducing satiety (Wilson 2003). Therefore, the protein-rich gnocchi formulated in this study may potentially maintain longer satiety.
Conclusion
Consumers are constantly demanding food products with high nutritional value. In this study gnocchi was used as a vehicle to incorporate meat and bean to deliver high nutrition. The enrichment of gnocchi has been shown to be a successful way to enhance its protein composition. In this study meat and navy bean has been incorporated into gnocchi without any adverse effects on flavour, taste, texture, and odour. The production of reformulated gnocchi will not only increase the nutritional quality of gnocchi but may also have a positive implication for human health. Further research needs to be further carried out on the shelf life and storage conditions of spaghetti fortified with meat and navy bean.
References
- Al-Dabbas MM, Al-Ismail K, Taleb RA, Ibrahim S. Acid-base buffering properties of five legumes and selected food in vitro. Am J Agric Biol Sci. 2010;5:154. doi: 10.3844/ajabssp.2010.154.160. [DOI] [Google Scholar]
- Alessandrini L, Balestra F, Romani S, Rocculi P, Rosa MD. Physicochemical and sensory properties of fresh potato-based pasta (Gnocchi) J Food Sci. 2010;75:S542–S547. doi: 10.1111/j.1750-3841.2010.01842.x. [DOI] [PubMed] [Google Scholar]
- Alireza Sadeghi M, Bhagya S. Quality characterization of pasta enriched with mustard protein isolate. J Food Sci. 2008;73:S229–S237. doi: 10.1111/j.1750-3841.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- Álvarez MD, Jiménez MJ, Olivares MD, Barrios L, Canet W. Texture perception determined by soy protein isolate and inulin addition in potato puree: links with mechanical and microstructural features. J Texture Stud. 2012;43:361–374. doi: 10.1111/j.1745-4603.2012.00347.x. [DOI] [Google Scholar]
- AOAC (2000) Official methods of analysis of the association of official analytical chemists. The Association
- Balbas J et al (2015) Comparison of physicochemical characteristics, sensory properties and volatile composition between commercial and New Zealand made wakame from Undaria pinnatifida Food Chemistry [DOI] [PubMed]
- Brown C, Culley D, Yang C-P, Durst R, Wrolstad R. Variation of anthocyanin and carotenoid contents and associated antioxidant values in potato breeding lines. J Am Soc Hortic Sci. 2005;130:174–180. [Google Scholar]
- Butterworth PJ, Warren FJ, Ellis PR. Human α-amylase and starch digestion: an interesting marriage. Starch-Stärke. 2011;63:395–405. doi: 10.1002/star.201000150. [DOI] [Google Scholar]
- Chen J, Piva M, Labuza T. Evaluation of water binding capacity (WBC) of food fiber sources. J Food Sci. 1984;49:59–63. doi: 10.1111/j.1365-2621.1984.tb13668.x. [DOI] [Google Scholar]
- Culbertson JY, Kreider RB, Greenwood M, Cooke M. Effects of beta-alanine on muscle carnosine and exercise performance: a review of the current literature. Nutrients. 2010;2:75–98. doi: 10.3390/nu2010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Fradique M, Batista AP, Nunes MC, Gouveia L, Bandarra NM, Raymundo A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: preparation and evaluation. J Sci Food Agric. 2010;90:1656–1664. doi: 10.1002/jsfa.3999. [DOI] [PubMed] [Google Scholar]
- FSANZ (2013) Food standards code, vol 2
- Gallegos-Infante JA, Bello-Perez LA, Rocha-Guzman NE, Gonzalez-Laredo RF, Avila-Ontiveros M. Effect of the addition of common bean (Phaseolus vulgaris L.) flour on the in vitro digestibility of starch and undigestible carbohydrates in spaghetti. J Food Sci. 2010;75:H151–H156. doi: 10.1111/j.1750-3841.2010.01748.x. [DOI] [PubMed] [Google Scholar]
- Giner J, Gimeno V, Barbosa-Cánovas GV, Martín O. Effects of pulsed electric field processing on apple and pear polyphenoloxidases. Food Sci Technol Int. 2001;7:339–345. [Google Scholar]
- Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23:165–176. doi: 10.1007/s11095-005-8476-1. [DOI] [PubMed] [Google Scholar]
- Kereliuk G, Kozub G. Chemical composition of small white (navy) beans LWT-Food. Sci Technol. 1995;28:272–278. [Google Scholar]
- Kvietys PR (2014) Physiology of the gastrointestinal microcirculation
- Malcolmson LJ (1991) Spaghetti optimization using response surface methodology: effects of drying temperature, durum protein level and farina blending
- Mancini R, Hunt M. Current research in meat color. Meat Sci. 2005;71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Mastromatteo M, Chillo S, Civica V, Iannetti M, Suriano N, Del Nobile M. A multistep optimization approach for the production of healthful pasta based on nonconventional flours. J Food Process Eng. 2012;35:601–621. doi: 10.1111/j.1745-4530.2010.00610.x. [DOI] [Google Scholar]
- McCleary BV, Gibson TS, Mugford DC. Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: collaborative study. J AOAC Int. 1997;80:571–579. [Google Scholar]
- Molly K, Woestyne MV, Verstraete W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl Microbiol Biotechnol. 1993;39:254–258. doi: 10.1007/BF00228615. [DOI] [PubMed] [Google Scholar]
- Osorio-Diaz P, Tovar J, Paredes-Lopez O, Acosta-Gallegos JA, Bello-Perez LA. Chemical composition and in vitro starch bioavailability of Phaseolus vulgaris (L) cv Mayocoba. J Sci Food Agric. 2005;85:499–504. doi: 10.1002/jsfa.2012. [DOI] [Google Scholar]
- Petitot M, Boyer L, Minier C, Micard V. Fortification of pasta with split pea and faba bean flours: pasta processing and quality evaluation. Food Res Int. 2010;43:634–641. doi: 10.1016/j.foodres.2009.07.020. [DOI] [Google Scholar]
- Saveur M (2008) Saveur cooks authentic italian. Chronicle books
- Shogren R, Hareland G, Wu Y. Sensory evaluation and composition of spaghetti fortified with soy flour. J Food Sci. 2006;71:S428–S432. doi: 10.1111/j.1750-3841.2006.00061.x. [DOI] [Google Scholar]
- Southgate D. Digestion and metabolism of sugars. Am J Clin Nutr. 1995;62:203S–210S. doi: 10.1093/ajcn/62.1.203S. [DOI] [PubMed] [Google Scholar]
- Sun XD, Holley RA. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr Rev Food Sci Food Saf. 2011;10:33–51. doi: 10.1111/j.1541-4337.2010.00137.x. [DOI] [Google Scholar]
- Trouillas P, Sancho-García JC, De Freitas V, Gierschner J, Otyepka M, Dangles O (2016) Stabilizing and modulating color by copigmentation: insights from theory and experiment chemical reviews [DOI] [PubMed]
- Wakeling IN, MacFie HJ. Designing consumer trials balanced for first and higher orders of carry-over effect when only a subset of k samples from t may be tested. Food Qual Prefer. 1995;6:299–308. doi: 10.1016/0950-3293(95)00032-1. [DOI] [Google Scholar]
- Wilson JA (2003) Gastroenterologic disorders. In: Geriatric medicine. Springer, pp 835–851
- Xu R-K, Zhao A-Z, Yuan J-H, Jiang J. pH Buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sediments. 2012;12:494–502. doi: 10.1007/s11368-012-0483-3. [DOI] [Google Scholar]
- Yoo M, Chen X (2007) Physico-chemical modeling of the human stomach. In: Proceedings of the 35th Australasian Chemical Engineering Conference, CHEMECA, pp 223–230
- Zhao YH, Manthey FA, Chang SK, Hou HJ, Yuan SH. Quality characteristics of spaghetti as affected by green and yellow pea, lentil, and chickpea flours. J Food Sci. 2005;70:s371–s376. doi: 10.1111/j.1365-2621.2005.tb11458.x. [DOI] [Google Scholar]
- Zouari N, Abid M, Fakhfakh N, Ayadi M, Zorgui L, Ayadi M, Attia H. Blue-green algae (Arthrospira platensis) as an ingredient in pasta: free radical scavenging activity, sensory and cooking characteristics evaluation. Int J Food Sci Nutr. 2011;62:811–813. doi: 10.3109/09637486.2011.582461. [DOI] [PubMed] [Google Scholar]