Abstract
The objective of this work was to study the correlation between the variation of phenolic compounds and sensory characteristics in white wine during bottle storage and to explore the compounds that affected sensory evolution. Chardonnay (Vitis vinifera L. cv.) dry white wines were bottled under six types of stoppers and stored for 18 months. The composition of phenolic compounds was analyzed, and the sensory attributes of these wines were evaluated by professional panel. Multivariate statistical analysis demonstrated that bottle aging period exhibited a more important effect on phenolic compound evolution than stopper type. Most of the phenolic compounds disappeared after 18 months of bottle storage, whereas the wine sensory attributes were significantly improved after 15-month of bottle aging. No strong correlation existed between the phenolic variation and the dissolved oxygen content. Wine color characteristics developed towards better quality accompanying with the reduction of detectable hydroxycinnamic acid derivatives and flavan-3-ols, while the wine mouth-feel was related mainly to gallic acid and ferulic acid ester. This work provided some references for wine producers to select appropriate storage duration for bottled white wine.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2411-9) contains supplementary material, which is available to authorized users.
Keywords: Bottle aging, White wine, Phenolic compounds, Sensory analysis, Multivariate analysis
Introduction
Wines are a type of alcoholic beverages that have been confirmed to possess multiple nutritional and antioxidant features since they contain a number of functional components (Waterhouse 2002). Among these components, phenolic compounds have been considered one of the most important quality parameters of wines. They have been demonstrated to play important roles in wine sensory properties, such as color, astringency, and bitterness (Lee and Jaworski 1987), and also show multiple bioactive properties (Xanthopoulou et al. 2010).
Phenolic compounds patterns in wine are primarily determined by grape variety and maturity level (Paixão et al. 2007). Besides, terroir feature and cultivation management also significantly affect the pattern and distribution of phenolic compounds in wine (Li et al. 2011; Avizcuri-Inac et al. 2013). More importantly, wine fermentation, aging, and storage processes cause the evolution of phenolic compounds in wine (Zafrilla et al. 2003), and these alterations eventually determine the final sensory attributes and quality of wines (Sun et al. 2011). Therefore, phenolic compounds patterns have been considered an important marker for wine quality (Pozo-Bayón et al. 2003).
In white wines, the amounts of phenolic compounds are typically lower than red wines. Phenolic compounds in white wines are mainly hydroxycinnamic acids, hydroxybenzoic acids, flavonols and flavan-3-ols. Oxidation of these phenolic compounds is a major reaction that happens during both fermentation and storage processes (Waterhouse and Laurie 2006). Phenolic compounds are easily oxidized with the presence of oxygen due to their multiple hydroxyl groups in structure (Waterhouse and Laurie 2006). As a result, the evolution of phenolic compounds causes the changes of color and mouth-feel of wine. It has been widely accepted that bottle storage improved the quality of red wine, including color enhancement, astringency reduction and aroma improvement, since more phenolic compounds present in red wine are able to tolerate large amounts of oxygen and experience more complicated evolutions (Cartagena et al. 1994). However, bottle aging in white wine can induce non-enzymatic browning and over-maturation, which led to a quality defect of wines (Ribéreau-Gayon et al. 2000b). In white wines, hydroxycinnamic acids and flavonols were the main compounds involved in the reactions that cause oxidative browning (Simpson 1982; Cheynier et al. 1988). Since the wine’s shelf life is a primary concern and directly related to its resistance against oxidation (Fernandez-Zurbano et al. 1995), it is important to understand the phenolic compounds, the aging time, and the sensory changes during bottle aging. Much work has been made in evolution of phenolic compounds or sensory changes in red wine during bottle aging (Hopfer et al. 2013; Marquez et al. 2014; Saenz-Navajas et al. 2014). However, these studies have not been well investigated in white wine, especially for white wine in China. More importantly, correlation between evolution of phenolic compounds and sensory attribute alteration in white wine during bottle aging has not been established.
In this context, the aims of this work are (1) to investigate the evolution of phenolic compounds and the development of wine sensory properties in bottled white wines and (2) to assess which phenolic compounds played critical roles in the evolvement of sensory in Chardonnay wine during bottle-aging. Thus, Chardonnay wines after fermentation were bottled using six types of stoppers that provided different conditions for post-bottling maturation. The wines were stored in bottle for 18 months, and at each sampling point the wines were analyzed in terms of phenolic compounds patterns. In the meantime, the sensory characteristics of these wines were evaluated under a professional panel session. Furthermore, Hierarchical cluster analysis (HCA), principal component analysis (PCA), and correlation analysis were carried out to investigate the quality changes of wines with phenolic compound evolutions and correlation between the changes of phenolic compounds and sensory characteristics in these wines.
Materials and methods
Chemicals and standards
Gallic acid, (+)-catechin, caffeic acid, quercetin, and trans-resveratrol were purchased from Sigma–Aldrich (St. Louis, MO, USA). Methanol, formic acid, acetic acid, and acetonitrile (HPLC grade) were obtained from Fisher Co. (Fairlawn, NJ, USA). Deionized water (<18 MΩ resistivity) was obtained from a Milli-Q Elementwater purification system (Millipore, Bedford, MA, USA). Ethyl acetate (analytical grade) was purchased from Beijing Chemical Reagent Company (Beijing, China). All the other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless specifically noted.
Wine samples
White wine samples were produced in Beijing Dragon Seal Winery Company using the 2009 vintage of Chardonnay (Vitis vinifera L. cv.) grapes grown in Huailai County, Hebei Province, China. All wines were made following the traditional method of white winemaking. After destemming, juice was separated from the grapes by an atmosphere presser and pumped into the fermentation tank of 10 kilo-liter. Sulfur dioxide of about 100 mg/L was immediately added. Fermentation was started by commercial yeast application and carried out under at 16–18 °C. After alcohol fermentation, the wines were aged in a stainless steel tank and bottled into glass bottles with a capacity of 750 mL on January 21st, 2011 at the packaging line of this winery. The base parameters of the wines at the start of the experiment were as follows: alcohol content (12.0%, v/v), residual sugar (0.3%, w/v), pH 3.26, and free SO2 (30 mg/L). Six types of stoppers were used. A total of 100 bottles for each type of stopper were required. The bottles were held upright for at least 24 h before bottling. The bottled wines were delivered to the underground cellar in the Center for Viticulture and Enology for a period of 18 months bottle aging. During the aging, the wines were kept at a constant temperature of 16–18 °C with a 60–70% relative humidity. The pH values of the examined Chardonnay white wines varied from 3.17 to 3.29, independently of the stopper type. Ten bottles of the wines for each stopper type were sampled every 3 months. At each sampling point, 3 bottles were used for chemical analysis, whereas 7 bottles were used for sensory analysis.
Stoppers
Six types of stoppers were used in this study, including three types of polymer synthetic plugs (named as synthetic plug-1, synthetic plug-2, and synthetic plug-3, respectively), one agglomerated cork, one ‘1 + 1 technical’ cork, and one natural cork. They were represented by A, B, C, D, E, and F, respectively. All the stoppers were purchased from Yantai Yubao Cork Stopper Company (Yantai, Shandong, China). The physical characteristics of these stoppers were shown in Appendix Table 1 (Gao et al. 2015).
Determination of dissolved oxygen in wine
After opening of bottles which were sealed with the different stoppers, the dissolved oxygen was immediately measured under a nitrogen pressure of 1 bar. An inoLab Oxi730 dissolved oxygen analyzer equipped with an automatic mixing electrode (WTW Company, Germany) was used (Semerci and Çeçen 2007). The scale of sensor measurement ranges from 0 to 50 mg/L. Before each measurement, the electro-chemical equipment was calibrated. The dissolved oxygen concentration was obtained from the mean value of three readings after it was stabilized on a certain value.
Phenolic compound analysis
The wine sample (100 mL) was diluted with the same volume of distilled water, and then extracted three times with ethyl acetate (80 ml), followed by separating an organic layer through a separating funnel (Li et al. 2009). The combined ethyl acetate phase was evaporated on a rotary evaporation (SY-2000, Shanghai Yarong Biochemistry Factory, Shanghai, China) at 28 °C and then the resultant dryness was re-dissolved in 5 mL of methanol. Before HPLC–MS analysis, the resultant methanol solution was filtered by 0.22 μm organic membranes.
An Agilent 1200 series high-performance liquid chromatograph (HPLC) equipped with a G1322A degasser, a G1312B bin pump, a G1367C HiP-ALS, a G1316B TCC, and a G1314C VWD was used to analyze the phenolic compounds (Li et al. 2009). The mobile phase consisted of solvent A (1% acetic acid in water) and solvent B (acetonitrile). The gradient program was as follows: 5 min (0–5% B), 5 min (5–8% B), 5 min (8–12% B), 5 min (12–18% B), 2 min (18–22% B), 2 min (22–35% B), and 4 min (35–100% B). The flow rate was set at 1.0 mL/min and temperature on column was 25 °C. The injected volume was 2 μL and the detection wavelength on variable wavelength detector was 280 nm. Mass spectrometry conditions were as follows: electrospray ionisation (ESI), negative ion model; nebulizer pressure, 30 psi; dry gas flow, 10 mL/min; dry gas temperature, 325 °C; and scan range, m/z 100–1500. All the experiments were carried out in triplicate.
Some compounds were identified after comparison of MS information, elution order, and retention times to those of the commercially available standards, including quercetin, quercetin-3-O-glucoside, quercetin-3-O-galactoside, kaempferol-3-O-glucoside, gallic acid, caffeic acid, trans-resveratrol, (+)-catechin, (−)-epicatechin, (−)-epigallo, (−)-epicatechin and (−)-epicatechin-3-O-gallate. The remaining phenolic compounds were identified mainly by comparing molecular ions, product ions, and the elution orders of these compounds with those available in our peivious published literatures (Li et al. 2009; Zhao et al. 2013). The quantification of phenolic compounds was made using external standard calibration curves of area versus concentration. The relative content of flavan-3-ol, flavonol, hydroxybenzoic acid, and hydroxycinnamic acid was obtained as the equivalent of (+)-catechin, quercetin, gallic acid, and caffeic acid, respectively. The linear formulas, linear ranges and relative coefficients of the phenolic standards were listed in Appendix Table 2.
Sensory evaluation
A total of 20 students (ten males and ten females, 21–24 years old) majoring in Viticulture and Enology of our college were selected because of interest and their performance in the previous studies of our laboratory. They all had the knowledge of wine tasting and regular Chardonnay wine tasting experiences. A total of 40-h training was performed over four months in a session per week. The 100 positive points (OIV) (NN106/04 2004) was used for the evaluation of the sensory characteristics. During training, the judges discussed about the descriptive terms of Chardonnay wines. Finally, 15 sensory attributes were considered as best described the sensory characteristics of Chardonnay wines (Appendix Table 3). These attributes were composed of appearance (20 score points), mouth-feel (40 points), fragrance (30 points), and overall impressions (10 points) of wines. To explore correlation between these attributes and phenolic compounds, the appearance and mouth-feel values were taken into account in this study. Each panelist conducted the tasting test in each booth with a uniform source of lighting and noise-free environment. The wines sealed under six different types of stoppers were sensorially evaluated in triplicate in six sessions. Each participant was involved in the within-subject design evaluating all the wines in every task in both sessions. In each session the wines of the sample were presented in a completely randomized order. Before each session, concepts were harmonized for homogenizing the wine-tasting criteria. For further recovery, a 10-min break was taken between samples, during which still water and unsalted crackers were available for gustatory neutralization. To more objectively reflect the differences amongst the wine samples, the data were standardized as we previously reported (Gao et al. 2015). Wine sample, the average of the sensory evaluation scores, and standard derivation were named as wine-j, Mj, and σj, respectively. The raw sensory evaluation score of the wine-j sample was obtained and the confidence interval was in the range of Mj ± σj. The new set of data was generated as follows: (1) when the score that an individual taster gave was within this range, the raw score was applied; (2) when the score was outside this range, the standardization conversion was applied via the raw score plus or minus the σj value. These data were used for subsequent statistical analysis.
Statistical analysis
Unsupervised heat map and hierarchical clustering dendrogram generation were applied using the online software MetaboAnalyst (version 3.0) (http://www.metaboanalyst.ca). The ward’s method as the clustering method and the Euclidean distance as the distance measure were used to establish clusters. Two-way analysis of variance (ANOVA) with the storage time, stopper type, and their interaction as factors was performed for individual phenolic compound concentration, using SPSS version 14.0 (Chicago, IL, USA). PCA was carried out using MetaboAnalys (Xia et al. 2015) to evaluate the similarities and differences among the tested wine samples. Correlation analysis was performed to correlate phenolic components to the sensory attributes.
Results and discussion
Evolutions of dissolved oxygen during bottle aging
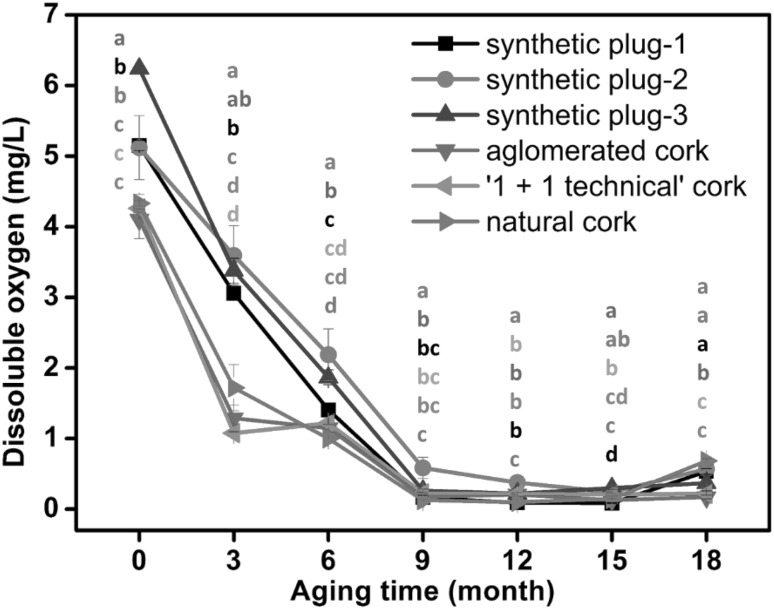
Figure 1 shows the changes of dissolved oxygen content in the Chardonnay dry white wines bottled with the six types of stoppers during aging. After bottled and held upright for 24 h, the initial dissolved oxygen amount was measured from the wines. The initial dissolved oxygen content in the wines in bottles with the synthetic plugs (synthetic plug-1, synthetic plug-2, and synthetic plug-3) exhibited significantly higher levels than those sealed with the other three types of stoppers. This might be due to the poor elasticity of polymer synthetic plugs, which allowed more oxygen to ingress into the bottle through the stopper body and the interface between the stopper and the bottle (Bhat 2002). Changes in dissolved oxygen reflected the net balance between the oxygen intake and consumption by wine (Guaita et al. 2013). In the first nine months of bottle storage, the concentration of dissolved oxygen in the wines dropped rapidly, indicating that the wines consumed oxygen very quickly during this aging period. After this, the dissolved oxygen concentration remained at a low level during 9 to 15 months of post-bottling period. This suggested that an equilibrium between oxygen intake through stopper and oxygen consumption by wine was achieved (Robertson 2009). However, wine in bottle becomes less at the later bottle-aging period, which increases the head space of bottle for oxygen ingress. As a result, the equilibrium between oxygen intake and consumption was broken, and oxygen amount in wine was expected to be increased. Indeed, a slight increase of dissolved oxygen was observed in the 18th month bottle-aged wines. During the whole period of aging, the dissolved oxygen content in the wines sealed with the synthetic plug-2 stoppers, the shortest stopper in length, showed relatively high levels. This further confirmed the important effect of stopper impermeability on the dissolved oxygen content. After 18 months of the post-bottling, wines sealed with the synthetic plug-1, synthetic plug-2, and natural cork contained significantly higher levels of dissolved oxygen compared with the other wines. These results further demonstrated that both synthetic and natural cork stoppers, possessed higher oxygen permeation capacity compared to screw-up and technical stoppers (Lopes et al. 2006). Since phenolic compounds are sensitive to oxygen and easily oxidized, different dissolved oxygen amount caused by different types of stoppers might alter the evolution pattern of phenolic compounds in white wine during bottle-aging.
Fig. 1.
Changes and differences in dissolved oxygen of Chardonnay dry white wines with the six types of stoppers during bottle aging (mg/L). Different letters indicate statistical differences (p < 0.05)
Evolution of phenolic compounds during bottle aging
In the analysis of individual phenolic contents, 15 phenolic compounds were identified and quantified (Table 1). The evolution of phenolic compounds during the wine bottle aging were assessed. Agglomerative hierarchical clustering algorithm (HCA) was applied to the sample set based on the detected phenolic compounds (Appendix Figure 1). A heat map (Appendix Figure 1a) was constructed to visualize the evolution of phenolic compounds during the wine bottle aging, whereas a dendrogram (Appendix Figure 1b) was generated to indicate the hierarchical structure of all the samples.
Table 1.
The classes and concentrations of phenolic compounds in Chardonnay dry wines sealed with different stoppers aging 18 months (mg/L)
| Aging time (months) | Phenolic compounds | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Cinnamic acid | Gallic acid acid | (+)-Catechin | Ferulic acid ester | Caffeic acid | Procyanidin B2 (P2) | Ethylgallate | p-Coumaric acid | |
| 0 | ||||||||
| 0.71 ± 0.01 | nd | 5.66 ± 0.22 | 0.64 ± 0.01 | 4.86 ± 0.04 | 1.23 ± 0.03 | 0.73 ± 0.02 | nd | |
| 3 | ||||||||
| A | 0.67 ± 0.01 | nd | 4.86 ± 0.29 | nd | 5.09 ± 0.15 | 0.83 ± 0.03 | tr | nd |
| B | 0.68 ± 0.01 | nd | 5.09 ± 0.20 | nd | 5.03 ± 0.08 | 0.89 ± 0.03 | 0.70 ± 0.02 | nd |
| C | 0.68 ± 0.02 | nd | 5.17 ± 0.07 | nd | 5.06 ± 0.05 | 0.87 ± 0.03 | 0.70 ± 0.01 | nd |
| D | 0.67 ± 0.02 | nd | 4.86 ± 0.29 | nd | 5.09 ± 0.15 | 0.83 ± 0.03 | tr | nd |
| E | 0.69 ± 0.01 | nd | 5.52 ± 0.15 | nd | 5.18 ± 0.07 | 1.00 ± 0.01 | tr | nd |
| F | 0.68 ± 0.01 | nd | 5.28 ± 0.23 | nd | 5.05 ± 0.04 | 0.89 ± 0.05 | 0.71 ± 0.02 | nd |
| 6 | ||||||||
| A | 0.66 ± 0.03 | nd | 4.60 ± 0.10 | 0.47 ± 0.01 | 4.34 ± 0.08 | 0.60 ± 0.05 | 0.59 ± 0.03 | nd |
| B | 0.65 ± 0.02 | nd | 5.29 ± 0.11 | 0.55 ± 0.02 | 4.31 ± 0.18 | 0.94 ± 0.04 | 0.62 ± 0.03 | nd |
| C | 0.67 ± 0.02 | nd | 5.30 ± 0.27 | 0.57 ± 0.02 | 4.37 ± 0.08 | 0.97 ± 0.05 | 0.64 ± 0.04 | nd |
| D | 0.66 ± 0.03 | nd | 4.60 ± 0.11 | 0.47 ± 0.01 | 4.34 ± 0.08 | 0.60 ± 0.05 | 0.59 ± 0.04 | nd |
| E | 0.69 ± 0.01 | nd | 5.37 ± 0.11 | 0.55 ± 0.01 | 4.32 ± 0.07 | 1.04 ± 0.07 | 0.64 ± 0.02 | nd |
| F | 0.74 ± 0.04 | nd | 5.83 ± 0.43 | 0.56 ± 0.03 | 4.59 ± 0.12 | 1.36 ± 0.22 | 0.72 ± 0.03 | nd |
| 9 | ||||||||
| A | 0.67 ± 0.01 | 1.42 ± 0.04 | 3.46 ± 0.27 | 4.07 ± 0.17 | 0.29 ± 0.13 | 1.54 ± 0.12 | 0.11 ± 0.03 | 0.16 ± 0.01 |
| B | 0.67 ± 0.01 | 1.40 ± 0.06 | 3.84 ± 0.10 | 4.40 ± 0.04 | 0.11 ± 0.01 | 1.72 ± 0.14 | 0.11 ± 0.04 | 0.17 ± 0.01 |
| C | 0.67 ± 0.02 | 1.41 ± 0.05 | 3.68 ± 0.40 | 4.06 ± 0.46 | 0.30 ± 0.27 | 1.71 ± 0.25 | 0.10 ± 0.02 | 0.16 ± 0.01 |
| D | 0.67 ± 0.01 | 1.42 ± 0.04 | 3.46 ± 0.27 | 4.07 ± 0.17 | 0.29 ± 0.13 | 1.54 ± 0.12 | 0.11 ± 0.03 | 0.16 ± 0.01 |
| E | 0.68 ± 0.01 | 1.48 ± 0.03 | 3.80 ± 0.32 | 4.42 ± 0.09 | 0.09 ± 0.01 | 1.80 ± 0.22 | 0.10 ± 0.02 | 0.16 ± 0.00 |
| F | 0.70 ± 0.04 | 1.55 ± 0.07 | 3.73 ± 0.46 | 4.43 ± 0.39 | 0.21 ± 0.03 | 1.89 ± 0.04 | 0.11 ± 0.02 | 0.17 ± 0.01 |
| 12 | ||||||||
| A | 0.67 ± 0.01 | 1.42 ± 0.04 | 4.42 ± 0.34 | 0.55 ± 0.02 | 4.04 ± 0.07 | 0.88 ± 0.04 | nd | 1.12 ± 0.01 |
| B | 0.67 ± 0.02 | 1.32 ± 0.01 | 3.97 ± 0.26 | 0.56 ± 0.02 | 4.07 ± 0.05 | 0.80 ± 0.00 | nd | 1.11 ± 0.02 |
| C | 0.67 ± 0.01 | 1.36 ± 0.10 | 4.21 ± 0.73 | 0.57 ± 0.04 | 4.11 ± 0.11 | 0.98 ± 0.00 | nd | 1.11 ± 0.06 |
| D | 0.68 ± 0.01 | 1.42 ± 0.04 | 4.42 ± 0.34 | 0.56 ± 0.02 | 4.04 ± 0.07 | 0.88 ± 0.04 | nd | 1.12 ± 0.02 |
| E | 0.69 ± 0.01 | 1.48 ± 0.09 | 5.02 ± 0.59 | 0.57 ± 0.03 | 4.08 ± 0.14 | 0.96 ± 0.14 | nd | 1.13 ± 0.04 |
| F | 0.69 ± 0.01 | 1.54 ± 0.04 | 4.67 ± 0.13 | 0.55 ± 0.01 | 4.15 ± 0.06 | 0.71 ± 0.11 | nd | 1.11 ± 0.02 |
| 15 | ||||||||
| A | 0.02 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.11 ± 0.00 | 0.01 ± 0.00 | tr | nd |
| B | 0.02 ± 0.00 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | tr | nd |
| C | 0.02 ± 0.00 | 0.06 ± 0.00 | 0.03 ± 0.02 | 0.02 ± 0.00 | 0.11 ± 0.01 | 0.01 ± 0.00 | tr | nd |
| D | 0.02 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.11 ± 0.00 | 0.01 ± 0.00 | tr | nd |
| E | 0.02 ± 0.00 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.00 | 0.13 ± 0.01 | 0.01 ± 0.00 | tr | nd |
| F | 0.02 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.01 | 0.12 ± 0.00 | 0.01 ± 0.00 | tr | nd |
| 18 | ||||||||
| A | tr | 0.34 ± 0.05 | 0.43 ± 0.19 | nd | nd | nd | 0.37 ± 0.08 | nd |
| B | tr | 0.27 ± 0.08 | 0.98 ± 0.23 | nd | 0.31 ± 0.07 | nd | 0.08 ± 0.05 | nd |
| C | tr | 0.29 ± 0.03 | 0.54 ± 0.06 | nd | 0.36 ± 0.03 | nd | 0.03 ± 0.01 | nd |
| D | tr | 0.34 ± 0.05 | 0.43 ± 0.19 | nd | nd | nd | 0.37 ± 0.08 | nd |
| E | tr | 0.32 ± 0.07 | 0.64 ± 0.13 | nd | 0.31 ± 0.02 | nd | 0.09 ± 0.01 | nd |
| F | tr | 0.31 ± 0.01 | 0.47 ± 0.01 | nd | 0.26 ± 0.03 | nd | 0.04 ± 0.00 | nd |
| Aging time (months) | Phenolic compounds | ||||||
|---|---|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| (−)-Epicatechin | Dihydroquercetin | Dihydroquercetin-3-O-rhamnoside | Ethyl caffeate | Ethyl p-coumarate | Caftaric acid ester | Glucose ester of trans-p-coumaric acid | |
| 0 | |||||||
| 7.73 ± 0.08 | nd | 1.20 ± 0.05 | 1.72 ± 0.03 | 0.35 ± 0.01 | 2.75 ± 0.28 | 0.02 ± 0.00 | |
| 3 | |||||||
| A | nd | nd | 0.59 ± 0.04 | 1.50 ± 0.04 | 0.32 ± 0.01 | nd | 4.86 ± 0.29 |
| B | nd | nd | 0.58 ± 0.02 | 1.45 ± 0.02 | 0.32 ± 0.01 | nd | 1.81 ± 0.53 |
| C | nd | nd | 0.56 ± 0.02 | 1.48 ± 0.02 | 0.32 ± 0.00 | nd | 4.79 ± 0.20 |
| D | nd | nd | 0.59 ± 0.04 | 1.50 ± 0.04 | 0.32 ± 0.01 | nd | 4.86 ± 0.29 |
| E | nd | nd | 0.57 ± 0.02 | 1.51 ± 0.02 | 0.32 ± 0.01 | nd | 4.72 ± 0.23 |
| F | nd | nd | 0.53 ± 0.01 | 1.41 ± 0.02 | 0.32 ± 0.01 | nd | 4.61 ± 0.06 |
| 6 | |||||||
| A | 7.22 ± 0.13 | nd | 1.11 ± 0.09 | 1.61 ± 0.02 | 0.31 ± 0.01 | 3.87 ± 0.01 | 0.05 ± 0.00 |
| B | 7.44 ± 0.18 | nd | 1.17 ± 0.13 | 1.63 ± 0.08 | 0.31 ± 0.01 | 2.64 ± 0.11 | 0.06 ± 0.01 |
| C | 7.49 ± 0.11 | nd | 1.18 ± 0.12 | 1.60 ± 0.02 | 0.32 ± 0.01 | 4.17 ± 0.16 | 0.05 ± 0.00 |
| D | 7.22 ± 0.13 | nd | 1.10 ± 0.09 | 1.61 ± 0.02 | 0.31 ± 0.01 | 3.87 ± 0.01 | 0.05 ± 0.00 |
| E | 7.52 ± 0.03 | nd | 1.18 ± 0.11 | 1.61 ± 0.01 | 0.31 ± 0.01 | 2.62 ± 0.11 | 0.03 ± 0.00 |
| F | 7.89 ± 0.27 | nd | 1.22 ± 0.14 | 1.69 ± 0.06 | 0.32 ± 0.01 | 1.03 ± 0.04 | 0.04 ± 0.00 |
| 9 | |||||||
| A | 9.61 ± 0.20 | 0.50 ± 0.01 | 1.20 ± 0.01 | 1.56 ± 0.06 | 0.36 ± 0.01 | 0.43 ± 0.01 | nd |
| B | 9.79 ± 0.25 | 0.49 ± 0.02 | 1.25 ± 0.02 | 1.52 ± 0.03 | 0.27 ± 0.16 | 0.51 ± 0.03 | 0.01 ± 0.00 |
| C | 9.80 ± 0.54 | 0.51 ± 0.02 | 1.21 ± 0.06 | 1.38 ± 0.24 | 0.34 ± 0.01 | 0.42 ± 0.02 | nd |
| D | 9.61 ± 0.20 | 0.50 ± 0.01 | 1.20 ± 0.01 | 1.56 ± 0.06 | 0.36 ± 0.01 | 0.43 ± 0.01 | nd |
| E | 9.99 ± 0.41 | 0.52 ± 0.03 | 1.25 ± 0.03 | 1.51 ± 0.04 | 0.36 ± 0.04 | 1.16 ± 0.02 | nd |
| F | 10.6 ± 0.61 | 0.54 ± 0.02 | 1.27 ± 0.08 | 1.60 ± 0.08 | 0.24 ± 0.19 | 0.49 ± 0.05 | nd |
| 12 | |||||||
| A | 3.00 ± 0.27 | nd | 1.40 ± 0.05 | 1.41 ± 0.02 | 0.35 ± 0.01 | 1.82 ± 0.04 | nd |
| B | 2.57 ± 0.19 | nd | 1.37 ± 0.02 | 1.39 ± 0.02 | 0.34 ± 0.03 | 1.83 ± 0.02 | nd |
| C | 2.77 ± 0.52 | nd | 1.37 ± 0.05 | 1.41 ± 0.05 | 0.36 ± 0.02 | 1.83 ± 0.06 | nd |
| D | 3.00 ± 0.28 | nd | 1.41 ± 0.05 | 1.42 ± 0.02 | 0.35 ± 0.01 | 1.82 ± 0.04 | nd |
| E | 3.40 ± 0.48 | nd | 1.40 ± 0.07 | 1.38 ± 0.05 | 0.32 ± 0.01 | 1.78 ± 0.06 | nd |
| F | 3.15 ± 0.06 | nd | 1.35 ± 0.03 | 1.44 ± 0.03 | 0.35 ± 0.02 | 1.88 ± 0.05 | nd |
| 15 | |||||||
| A | 0.20 ± 0.02 | nd | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.19 ± 0.01 | 0.01 ± 0.00 |
| B | 0.20 ± 0.00 | nd | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.19 ± 0.00 | 0.01 ± 0.00 |
| C | 0.19 ± 0.01 | nd | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.20 ± 0.00 | 0.01 ± 0.00 |
| D | 0.20 ± 0.02 | nd | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.20 ± 0.01 | 0.01 ± 0.00 |
| E | 0.22 ± 0.02 | nd | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.20 ± 0.00 | 0.01 ± 0.00 |
| F | 0.22 ± 0.01 | nd | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.01 ± 0.00 | 0.21 ± 0.01 | 0.01 ± 0.00 |
| 18 | |||||||
| A | 0.32 ± 0.03 | nd | nd | 0.08 ± 0.01 | 0.03 ± 0.01 | nd | nd |
| B | 0.29 ± 0.02 | nd | nd | 0.09 ± 0.01 | tr | nd | nd |
| C | 0.39 ± 0.08 | nd | nd | 0.10 ± 0.01 | tr | nd | nd |
| D | 0.32 ± 0.03 | nd | nd | 0.08 ± 0.01 | 0.03 ± 0.01 | nd | nd |
| E | 0.26 ± 0.04 | nd | nd | 0.11 ± 0.01 | tr | nd | nd |
| F | 0.26 ± 0.01 | nd | nd | 0.09 ± 0.01 | tr | nd | nd |
Although slight differences in phenolic compound concentration were observed among these wines using different types of stoppers, these wines showed the similar evolution patterns during the bottle aging period. The mean phenolic concentrations and standard deviations are included in Table 1, which shows that the phenolic profiles significantly changed in all the types of stoppers during aging. Two general evolution trends were observed: (1) phenolic compounds only showed a very high level in the wines that were bottle-aged for 9 and 12 months, and then turned to the similar or low levels in the other storage periods; (2) phenolic compounds in the wines appeared to be high at the early stage of bottle aging and then lowered at the later periods of aging, especially the 15–18th month bottle aging, although some fluctuation on phenolic compound level was observed. Phenolic compounds that followed the first evolution trend mainly included ferulic acid ester, dihydroquercetin, gallic acid, and p-coumaric acid. The other 11 phenolic compounds showed the second evolution pattern. Among these, ethyl caffeate, catechin, cinnamic acid, ethyl p-coumarate, and procyanidin B2 (P2) remained relatively high in the 0–12 months bottle-aged wines. However, (−)-epicatechin, dihydroquercetin-3-O-rhamnoside, caftaric acid ester, and ethyl gallate concentrations decreased significantly in the wines bottle-aged for 3 months. Glucose ester of trans-p-coumaric acid showed a significant increase in the concentration after 3 months of the storage. It should be noted that the wines showed a significant decrease in most of phenolic compounds after 18 months of bottle storage compared to their initial concentration and this significant decrease mainly occurred in the last 6 months of storage. The concentration of ferulic acid ester, procyanidin B2 (P2), p-coumaric acid, dihydroquercetin, dihydroquercetin-3-O-rhamnoside, caftaric acid ester and glucose ester of trans-p-coumaric acid even could not be detected after 18-month storage.
Phenolic compounds reduction in aging wines has been reported in previous literatures. Most of phenolic compounds showed a downward trend in the white wine during the later storage period (Recamales et al. 2006; Kallithraka et al. 2009), which was consistent with our investigation. Our previous study also reported that the low levels of phenolic compounds were observed in the red wines after 15–18 months bottle aging, except for some polymeric pigments (Gao et al. 2015). Particularly, phenolic compounds with low molecular weight, such as gallic, caffeic and ferulic acid, (+)-catechin and (−)-epicatechin generally exhibited a significant concentration reduction (Castellari et al. 2000). It was considered that during wine bottle storage phenolic compounds can undergo various chemical reactions, mainly including hydrolysis, oxidation and complexation reactions (Zafrilla et al. 2003). Some of the chemical reactions were oxygen-mediated reactions and others involving phenolic but not oxygen (Waterhouse and Laurie 2006). From our present data, it is inferred that the reduction of these substances during aging stage may have resulted from both phenolic oxidation and polymerization reactions. It was observed that dissolved oxygen contents rapidly dropped during first 9 months of bottle storage in all wines with different stopper types (Fig. 1), which may be directly related to a large variation of phenolic compounds from 9 to 12th month.
Effects of storage time and stopper types on phenolic compounds
Two-way ANOVA was carried out to investigate the effect of storage period and stopper type on the evolution of phenolic compounds during bottle aging. The results showed that both storage period (F1) and stopper type (F2) had significant effects on the concentration alteration of phenolic compounds (Table 2). For example, all the phenolic compounds in white wines showed significant differences in the concentration along with bottle storages, especially p-coumaric acid (F 1 = 19779.30), caffeic acid (F 1 = 13737.33), dihydroquercetin (F1 = 10896.73), and cinnamic acid (F 1 = 9497.45). However, only 60% of these phenolic compound evolutions relied on the stopper types and F2 values were much lower than F1 values. During the bottle storage, both oxygen content and storage time played important roles in triggering the hydrolysis, oxidation, and/or polymerization of phenolic compounds (Kallithraka et al. 2009; Wirth et al. 2010). Previous studies have demonstrated that stoppers possessed different air permeability capacity (Lopes et al. 2007; Poças et al. 2010), which influenced the content of oxygen dissolved in bottled wines. In the present study, although the stopper type significantly affected the phenolic compound evolutions, the storage time turned out to be a dominant factor for the evolution of phenolic compounds in these white wines.
Table 2.
Two-way ANOVA of various phenolic compounds in bottle-aged white wines sealed with different stoppers
| Number | Phenolic compound | F1 (Storage period) | F2 (Stopper type) | F1*F2 | p1 | p2 | p1*p2 |
|---|---|---|---|---|---|---|---|
| 1 | Cinnamic acid | 9497.45** | 7.09** | 1.92* | <0.001 | <0.001 | 0.011 |
| 2 | Gallic acid | 5827.73** | 6.23** | 2.43** | <0.001 | <0.001 | 0.001 |
| 3 | (−)-Catechin | 1380.18** | 7.29** | 2.44** | <0.001 | <0.001 | 0.001 |
| 4 | Ferulic acid ester | 3961.15** | 1.95 | 1.48 | <0.001 | 0.09 | 0.08 |
| 5 | Caffeic acid | 13737.33** | 2.78* | 3.08** | <0.001 | 0.023 | <0.001 |
| 6 | Procyanidin B2 (P2) | 1021.26** | 12.44** | 6.60** | <0.001 | <0.001 | <0.001 |
| 7 | Ethyl gallate | 2167.54** | 54.97** | 111.42** | <0.001 | <0.001 | <0.001 |
| 8 | p-Coumaric acid | 19779.30** | 0.05 | 0.25 | <0.001 | 1.00 | 1.00 |
| 9 | (−)-Epicatechin | 7078.90** | 5.17** | 2.25** | <0.001 | <0.001 | 0.002 |
| 10 | Dihydroquercetin | 10896.73** | 1.75 | 1.76* | <0.001 | 0.13 | 0.02 |
| 11 | Dihydroquercetin-3-O-rhamnoside | 2036.20** | 0.28 | 0.65 | <0.001 | 0.92 | 0.90 |
| 12 | Ethyl caffeate | 3684.78** | 1.27 | 1.44 | <0.001 | 0.28 | 0.10 |
| 13 | Ethyl p-coumarate | 271.40** | 1.08 | 0.92 | <0.001 | 0.38 | 0.59 |
| 14 | Caftaric acid ester | 2374.04** | 40.78** | 50.04** | <0.001 | <0.001 | <0.001 |
| 15 | Glucose ester of trans-p-coumaric acid | 3543.83** | 47.25** | 47.87** | <0.001 | <0.001 | <0.001 |
Data in bold are highlighted due to very high F value; ** represents significant level of p < 0.01; * represents significant level of p < 0.05
Differentiation of wines regarding phenolic compounds
Dendrogram was drawn to analyze the differences in wine characteristics with the storage periods using all the identified phenolic compounds as variables (Fig. 2a). The wines bottle-aged for 0–12 months (Cluster I) were primarily segregated from those stored for 15 and 18 months (Cluster II). The characteristics of the wine after 9 months of storage (Sub-cluster Ib) were significantly different from the rest of bottle-stored wines (Sub-cluster Ia). The wines stored in the bottle for 0, 3, and 6 months, compared to the 12-month bottle aged wine, showed a short distance on dendrogram, indicating that these wines possessed the similar characteristics. These results suggested that bottle storage time significantly affected the evolution of phenolic compounds in white wines, which significantly resulted in the characteristic alterations of these wines.
Fig. 2.
The dendrogram of hierarchical clustering for the white wine samples (a) and PCA of the phenolic contents differentiating the white wines at six different storage times (b, c). a, b Letters represent six bottle stoppers: synthetic plug-1 (A), synthetic plug-2 (B), synthetic plug-3 (C), agglomerated cork (D), ‘1 + 1 technical’ cork (E) and natural cork (F), respectively; Numbers after the letter represent aging time (months); The letter M represents month, and the number means the bottle storage time. c Numbers represent the phenolic compounds listed in Table 1
In order to confirm the cluster analysis results, principal component analysis was also carried out using the identified phenolic compounds as variables (Fig. 2b, c). The first two components accounted for 50.2 and 24% of the variation, respectively. The white wines with different storage periods were segregated and five distinct groups were assembled in terms of bottle aging periods, except for the 15- and 18-month stored wines, and 0- and 6-month bottle-aged wines. These results suggested that storage periods significantly exerted the impact on evolution of phenolic compounds, and thus led to the differences of these stored wines.
The first component (PC1) resulted in the segregation of the 15- and 18-month bottle-aged wines from the other wines (Fig. 2b), which was as similar as that in the hierarchical cluster analysis. Regarding the individual phenolic compounds, they were all located on the left of the vertical axis with negative loading values on PC1. Since both the 15- and 18-month stored wines had high positive values along PC1, these results indicated that the wines had low phenolic compound levels at the end of the bottle aging period, especially cinnamic acid (No. 1), (+)-catechin (No. 3), procyanidin B2 (P2) (No. 6), dihydroquercetin-3-O-rhamnoside (No. 11), ethyl caffeate (No. 12), and ethyl p-coumarate (No. 13).
The second component (PC2) differentiated the wines stored for 0, 3, and 6 months from those bottle-aged for 9, 12, 15 and 18 months. In the meantime, the 9th month bottle-aged wines showed the highest negative PC2 values, and were located at the bottom of the plot. This resulted in an obvious segregation from the other wine samples. This suggested that the 9th month bottle aging might be a significant turning point for the evolution of phenolic compounds, which was also observed in bottle-aged red wine in our previous study (Gao et al. 2015). The loading plot (Fig. 2c) revealed that the most important phenolic compound variables included ferulic acid ester (No. 4), dihydroquercetin (No. 10), and gallic acid (No. 2). For instance, as shown in Table 1, the concentration of ferulic acid ester (No. 4) was within 0 to 0.64 mg/L in the wine of first 6 months storage, and then increased up to 4.07–4.40 mg/L in the 9th month bottle-aged wines. However, its concentration finally decreased to 0–0.57 mg/L during the following period. It was considered that the esterification reaction could account for a certain proportion at the early bottle-aging storage period, and both hydrolytion should be main chemical reaction in the late stage of bottle aging.
Differentiation of wines regarding sensory characteristics
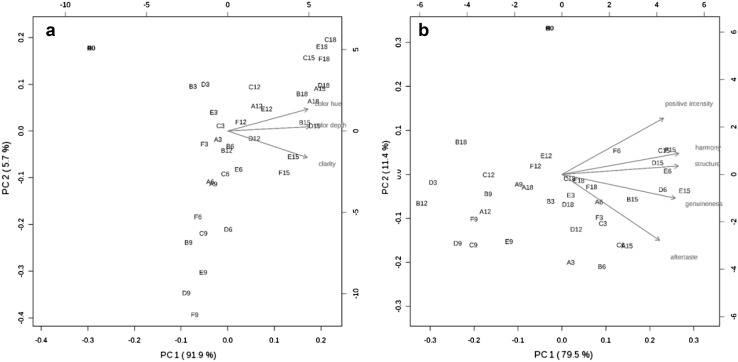
PCA was also carried out using sensory attributes of these stored wines as variables, and the biplots represented the positioning relationship between wines and sensory properties (Fig. 3). Figure 3a shows the PCA biplot of three appearance attributes, including clarity, color depth (saturation), and color hue (shade or tint). The x-axis and the y-axis represents the values for the first and the second principal component, respectively. The arrows indicate the proportion of the original variance explained by the first two principal components. The direction of the arrows indicates the relative loadings on the first and second principal components. In this figure, the relationships between those variables and the principal components can be viewed graphically. The appearance attributes were all located at the right side of the plot, corresponding to positive PC1 scores. The wines before the bottle storage were located at the left side of the plot, whereas the 3–12 months bottle stored wines were displayed in the middle. However, extending the bottle storage to 15–18 months obviously moved the wines towards the right side of the plot. These results suggested that high appearance scores were observed in the wines at the late periods of bottle aging. In another word, appropriately extending bottle storage period might benefit the appearance of white wines. Combined with the variation of dissolved oxygen during this period (Fig. 1), the evolution of the wine appearance quality did not seem to be directly related to the dissolved oxygen content. During the bottle storage of wines, access to additional oxygen was practically terminated and the subsequent reactions such as polymerization and condensation occurred under anaerobic conditions (Kallithraka et al. 2009). The data published showed that storage conditions might strongly affect the content of wine phenolic compounds and wine appearance quality (Recamales et al. 2006; Rocha-Parra et al. 2016). Since the present experiment took place at similar storage conditions and only the stoppers were different, the effect observed on the phenolic concentration and visual appearance of white wines could be attributed to storage time and stopper type. It was also noted that during the same bottle aging period, the wines were located close to each other in the biplot, indicating that storage time had more impact on wine appearance attributes compared with stopper type.
Fig. 3.
The PCA biplot on appearance characteristics (a) and mouth-feel characteristics (b) of bottle aged dry white wines sealed with different stoppers. Letters represent six bottle stoppers: synthetic plug-1 (A), synthetic plug-2 (B), synthetic plug-3 (C), agglomerated cork (D), ‘1 + 1 technical’ cork (E) and natural cork (F), respectively. Numbers after the letter represent aging time (months)
Figure 3b represents the mouth-feel characteristics of wine, including genuineness, positive intensity, structure, harmony, and aftertaste. These attributes were all located at the far right and correlated with each other. The highest values in this principal component were observed in the 15-month bottle-aged wines, whereas the 9-month stored wines were located at the left side of the plot. This indicated that the 9-month stored wines could be a turning point of development of the wine sensory attributes, which might be explained by the dramatic evolution of phenolic compounds at such a storage period (Marquez et al. 2014). It was also noted that the 12-month bottle-aged wines with the synthetic plug-3 (B12) and the 3-month stored wines using the agglomerated cork (D3) were located at the leftmost side of the plot, suggesting the poor mouth-feel appearances of these two wines. However, the wines bottle-aged for 6 months, especially those using the technical cork (D6) and agglomerated cork (E6) displayed a relatively favorable mouth-feel feature.
Additionally, the 15-month bottle-aged wines showed much higher mouth-feel scores than those stored for 18 months, which indicated that the mouth-feel attributes of white wines significantly weakened at 18 months of bottle storage. Considering the variation of dissolved oxygen during this period (Fig. 1), we speculated that the oxidation substrates were almost exhausted after 15 months of the post-bottling, at which time the mouth-feel properties of the wines reached the highest values. It has been known that wines basically experience a sensory improvement and then a quality loss during the shelf life (Ugliano 2013). Normally, wines are not consumed directly after production. Instead, most of high quality red wines normally spend a number of years for the best sensory features. However a short period of bottle storage is recommended for white wine before consumption (Ribéreau-Gayon et al. 2000a). The vast majority of wines are expected to be made for consumption within 12 months of production (Zraly 2008). Other research has also suggested that the white wines could be stored in bottles for nine months without any serious color alteration and their antioxidant even increased significantly activity. In this study, the white wines could be stored in bottles for 15 months with a relatively high sensory quality.
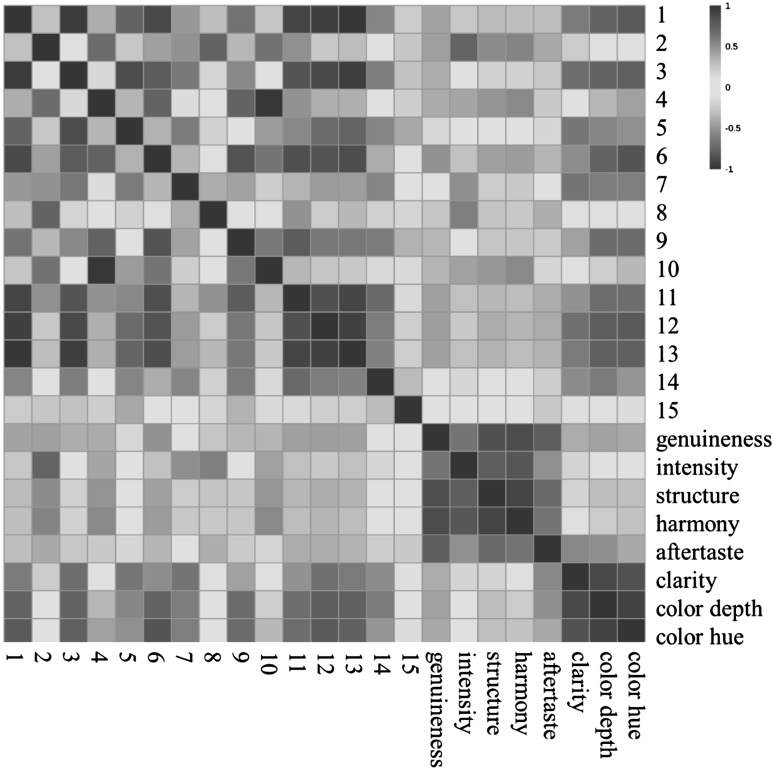
Correlation between phenolic compounds and sensory characteristics
The dramatic evolution of the phenolic compounds in these white wines was observed during the bottle aging period. In the meantime, the sensory attributes of these bottle-aged wines also significantly changed. Therefore, we hypothesized that the sensory alterations of white wines might be correlated with the evolution of phenolic compounds present in these wines. In this study, the correlations between 15 phenolic compounds and 9 sensory characteristics were assessed (Fig. 4). The development of three appearance indicators showed negative correlations with the variation of the most of phenolic compounds in the wines during bottle aging. The strongly negative correlation was observed from cinnamic acid (No. 1), (+)-catechin (No. 3), caffeic acid (No. 5), procyanidin B2 (P2) (No. 6), ethyl gallate (No. 7), dihydroquercetin-3-O-rhamnoside (No. 11), ethyl caffeate (No. 12) and ethyl p-coumarate (No. 13). In addition, clarity and color depth exhibited a highly negative correlation with caftaric acid ester (No. 14), whereas color depth and color hue were high negatively correlated with (−)-epicatechin (No. 9). These reflected that wines developed towards a good appearance with the reduction of these compounds in the concentration during bottle storage. These ten phenolic compounds mentioned above all followed the second evolution pattern in the heat map (Appendix Figure 2), which was higher levels at the early bottle aging period and then lower level at the late storage periods. It has long been known that, during wine aging, not all phenolic compounds are equally oxidisable, nor do their reaction products with the same brownness (Rossi and Singleton 1966). The former study has indicated that phenolic compounds with low molecular weight, such as (+)-catechin and (−)-epicatechin, caffeic and other hydroxycinnamic acids normally converted into bigger molecules along with wine aging, which leads to the yellow–brown pigments (Guyot et al. 1996). With particular regard to white wine, the concentrations of (+)-catechin and (−)-epicatechin had a good correlation with the susceptibility to wine browning and oxidation (Waterhouse and Laurie 2006). Cinnamic acid was also reported to be converted into browning pigments under oxidation (Wirth et al. 2010), which also enhanced the yellow color. In addition, some phenolic compounds undergo polymerization reactions in which oxygen plays an important role (Du Toit et al. 2006). These works also explained our present observations that the evolution of appearance attributes is mainly related with these five hydroxycinnamic acid derivatives and flavan-3-ols in the white wines.
Fig. 4.
Correlation analysis plot of phenolic compounds and sensory attributes. Red color indicates positive correlation, while blue color indicates negative correlation. Strong correlations are values of −1.0 to −0.5 (negative correlations) or 0.5–1; moderate correlations are values of −0.5 to −0.3 (negative correlations) or 0.3–0.5 (color figure online)
Similarly, negative correlation was also found between the sensory scores of mouth-feel and the level of most of phenolic compounds in these bottle-aged wines. The strongly negative correlation was established between the wine genuineness and procyanidin B2 (P2) (No. 6), whereas gallic acid (No. 2) and p-coumaric acid (No. 8) showed the highly negative correlations with the development of wine intensity. The significant negative correlation was launched between the wine structure and gallic acid (No. 2) and ferulic acid ester (No. 4). Meanwhile, gallic acid (No. 2), ferulic acid ester (No. 4) and dihydroquercetin (No. 10) were negatively correlated with the wine harmony. Regarding their evolution patterns (Table 1 and Appendix Figure 2), these phenolic compounds except for procyanidin B2 (P2) (No. 6) followed the first evolution trend, a dramatic evolution on phenolic compound content in the wines at the 9th month. This could explain why the 9th month bottle stored wines exhibited the poor mouth-feel quality. It should be noted that only ethyl gallate (No. 7) was positively correlated with the wine intensity, whereas no significant positive correlations were observed between the phenolic compounds and the wine mouth-feel characteristics.
Overall, the phenolic compounds that showed the second evolution pattern were associated not only with the development of wine appearance attributes, but also with the wine mouth-feel features in these white wines. It was also observed that these phenolic compounds had more significantly negative correlation with the wine appearance characteristics rather than the mouth-feel properties. However, not all the sensory attributes were explained by the phenolic compound evolution during bottle aging period. This was because volatile compound evolution, besides phenolic compounds, and interaction between volatile and phenolic compounds also played important roles in determining wine sensory attributes. Therefore, further study should be focused on correlation between wine sensory changes and volatile evolutions, and effect of interaction between volatile and phenolic compounds on wine sensory properties during bottle aging period.
Conclusion
This work provides a better knowledge of the evolution of phenolic compounds in Chardonnay wine during bottle aging period and sensory attribute alteration in these wines. Furthermore, the correlation between phenolic compound evolution and sensory changes was established. Bottle aging storage played a more important role in the development of white wine compared with stopper type. Phenolic compounds in white wines followed two evolution patterns, and most of them showed significant losses after 18 months of storage. The phenolic evolution during bottle aging appeared to have no strong correlation with the variation of wine dissolved oxygen content. The appearance attributes of these white wines moved towards a good quality at the last 6 months of bottle aging, whereas the highest mouth-feel scores were observed in the 15-month bottle stored wines. Negative correlations existed between most of phenolic compounds and sensory descriptors. The evolution of wine color characteristics was associated with most of detectable phenolic compounds, in particular hydroxycinnamic acid derivatives and flavan-3-ols. And the wine mouth-feel evolution during bottle aging was related mainly with the variation of two hydroxybenzonic acid derivatives (gallic acid and ferulic acid ester).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the financial support of the project of China Agriculture Research System (CARS-30).
References
- Avizcuri-Inac J-M, Gonzalo-Diago A, Sanz-Asensio J, Martínez-Soria M-T, López-Alonso M, Dizy-Soto M, Echávarri-Granado J-F, Vaquero-Fernández L, Fernández-Zurbano P. Effect of cluster thinning and prohexadione calcium applications on phenolic composition and sensory properties of red wines. J Agric Food Chem. 2013;61(5):1124–1137. doi: 10.1021/jf304257r. [DOI] [PubMed] [Google Scholar]
- Bhat SV (2002) Synthetic polymers. In: Biomaterials. Springer, pp 51–71
- Cartagena LG, Perezzuniga FJ, Abad FB. Interactions of some environmental and chemical-parameters affecting the color attributes of wine. Am J Enol Vitic. 1994;45:43–48. [Google Scholar]
- Castellari M, Matricardi L, Arfelli G, Galassi S, Amati A. Level of single bioactive phenolics in red wine as a function of the oxygen supplied during storage. Food Chem. 2000;69(1):61–67. doi: 10.1016/S0308-8146(99)00240-X. [DOI] [Google Scholar]
- Cheynier V, Owe C, Rigaud J. Oxidation of grape juice phenolic compounds in model solutions. J Food Sci. 1988;53(6):1729–1732. doi: 10.1111/j.1365-2621.1988.tb07828.x. [DOI] [Google Scholar]
- Du Toit W, Marais J, Pretorius I, Du Toit M. Oxygen in must and wine: a review. S Afr J Enol Vitic. 2006;27:76. [Google Scholar]
- Fernandez-Zurbano P, Ferreira V, Pena C, Escudero A, Serrano F, Cacho J. Prediction of oxidative browning in white wines as a function of their chemical composition. J Agric Food Chem. 1995;43(11):2813–2817. doi: 10.1021/jf00059a008. [DOI] [Google Scholar]
- Gao Y, Tian Y, Liu D, Li Z, Zhang X-X, Li J-M, Huang J-H, Wang J, Pan Q-H. Evolution of phenolic compounds and sensory in bottled red wines and their co-development. Food Chem. 2015;172:565–574. doi: 10.1016/j.foodchem.2014.09.115. [DOI] [PubMed] [Google Scholar]
- Guaita M, Petrozziello M, Motta S, Bonello F, Cravero MC, Marulli C, Bosso A. Effect of the closure type on the evolution of the physical-chemical and sensory characteristics of a Montepulciano d’Abruzzo Rose wine. J Food Sci. 2013;78:C160–C169. doi: 10.1111/1750-3841.12022. [DOI] [PubMed] [Google Scholar]
- Guyot S, Vercauteren J, Cheynier V. Structural determination of colourless and yellow dimers resulting from (+)-catechin coupling catalysed by grape polyphenoloxidase. Phytochemistry. 1996;42(5):1279–1288. doi: 10.1016/0031-9422(96)00127-6. [DOI] [Google Scholar]
- Hopfer H, Buffon PA, Ebeler SE, Heymann H. The combined effects of storage temperature and packaging on the sensory, chemical, and physical properties of a Cabernet Sauvignon wine. J Agric Food Chem. 2013;61:3320–3334. doi: 10.1021/jf3051736. [DOI] [PubMed] [Google Scholar]
- Kallithraka S, Salacha MI, Tzourou I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: accelerated browning test versus bottle storage. Food Chem. 2009;113:500–505. doi: 10.1016/j.foodchem.2008.07.083. [DOI] [Google Scholar]
- Lee CY, Jaworski A. Phenolic-compounds in white grapes grown in New-York. Am J Enol Vitic. 1987;38:277–281. [Google Scholar]
- Li Z, Pan Q-H, Jin Z-M, He J-J, Liang N-N, Duan C-Q. Evolution of 49 phenolic compounds in shortly-aged red wines made from Cabernet Gernischt (Vitis vinifera L. cv.) Food Sci Biotechnol. 2009;18:1001–1012. [Google Scholar]
- Li Z, Pan Q, Jin Z, Mu L, Duan C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011;125(1):77–83. doi: 10.1016/j.foodchem.2010.08.039. [DOI] [Google Scholar]
- Lopes P, Saucier C, Teissedre P-L, Glories Y. Impact of storage position on oxygen ingress through different closures into wine bottles. J Agric Food Chem. 2006;54:6741–6746. doi: 10.1021/jf0614239. [DOI] [PubMed] [Google Scholar]
- Lopes P, Saucier C, Teissedre P-L, Glories Y. Main routes of oxygen ingress through different closures into wine bottles. J Agric Food Chem. 2007;55:5167–5170. doi: 10.1021/jf0706023. [DOI] [PubMed] [Google Scholar]
- Marquez A, Serratosa MP, Merida J. Influence of bottle storage time on colour, phenolic composition and sensory properties of sweet red wines. Food Chem. 2014;146:507–514. doi: 10.1016/j.foodchem.2013.09.103. [DOI] [PubMed] [Google Scholar]
- Paixão N, Perestrelo R, Marques JC, Camara JS. Relationship between antioxidant capacity and total phenolic content of red, rose and white wines. Food Chem. 2007;105(1):204–214. doi: 10.1016/j.foodchem.2007.04.017. [DOI] [Google Scholar]
- Poças MF, Ferreira B, Pereira J, Hogg T. Measurement of oxygen transmission rate through foamed materials for bottle closures. Packag Technol Sci. 2010;23:27–33. [Google Scholar]
- Pozo-Bayón MÁ, Hernández MT, Martín-Álvarez PJ, Polo MC. Study of low molecular weight phenolic compounds during the aging of sparkling wines manufactured with red and white grape varieties. J Agric Food Chem. 2003;51:2089–2095. doi: 10.1021/jf021017z. [DOI] [PubMed] [Google Scholar]
- Recamales ÁF, Sayago A, González-Miret ML, Hernanz D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res Int. 2006;39:220–229. doi: 10.1016/j.foodres.2005.07.009. [DOI] [Google Scholar]
- Ribéreau-Gayon P, Dubourdieu D, Doneche B, Lonvaud A (2000a) Handbook of enology volume 1 the microbiology of wine and vinifications, 2nd edition
- Ribéreau-Gayon P, Glories Y, Maujean A, Dubourdieu D. Handbook of enology. The chemistry of wine and stabilisation and treatments. Chichester: Wiley; 2000. [Google Scholar]
- Robertson GL (2009) Food packaging and shelf life: a practical guide Food packaging and shelf life: a practical guide 416
- Rocha-Parra DF, Lanari MC, Zamora MC, Chirife J. Influence of storage conditions on phenolic compounds stability, antioxidant capacity and colour of freeze-dried encapsulated red wine. LWT-Food Sci Technol. 2016;70:162–170. doi: 10.1016/j.lwt.2016.02.038. [DOI] [Google Scholar]
- Rossi JA, Singleton Vernon L. Contributions of grape phenols to oxygen absorption and browning of wines. Am J Enol Vitic. 1966;17:231–239. [Google Scholar]
- Saenz-Navajas MP, Avizcuri JM, Ferreira V, Fernandez-Zurbano P. Sensory changes during bottle storage of Spanish red wines under different initial oxygen doses. Food Res Int. 2014;66:235–246. doi: 10.1016/j.foodres.2014.08.053. [DOI] [Google Scholar]
- Semerci N, Çeçen F. Importance of cadmium speciation in nitrification inhibition. J Hazard Mater. 2007;147:503–512. doi: 10.1016/j.jhazmat.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Simpson R. Factors affecting oxidative browning of white wine. Vitis. 1982;21(3):233–239. [Google Scholar]
- Sun B, Neves AC, Fernandes TA, Fernandes AL, Mateus N, De Freitas V, Leandro C, Spranger MI. Evolution of phenolic composition of red wine during vinification and storage and its contribution to wine sensory properties and antioxidant activity. J Agric Food Chem. 2011;59(12):6550–6557. doi: 10.1021/jf201383e. [DOI] [PubMed] [Google Scholar]
- Ugliano M. Oxygen contribution to wine aroma evolution during bottle aging. J Agric Food Chem. 2013;61:6125–6136. doi: 10.1021/jf400810v. [DOI] [PubMed] [Google Scholar]
- Waterhouse AL. Wine phenolics. Ann N Y Acad Sci. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Waterhouse AL, Laurie VF. Oxidation of wine phenolics: a critical evaluation and hypotheses. Am J Enol Vitic. 2006;57:306–313. [Google Scholar]
- Wirth J, et al. The impact of oxygen exposure before and after bottling on the polyphenolic composition of red wines. Food Chem. 2010;123:107–116. doi: 10.1016/j.foodchem.2010.04.008. [DOI] [Google Scholar]
- Xanthopoulou MN, Fragopoulou E, Kalathara K, Nomikos T, Karantonis HC, Antonopoulou S. Antioxidant and anti-inflammatory activity of red and white wine extracts. Food Chem. 2010;120:665–672. doi: 10.1016/j.foodchem.2009.10.058. [DOI] [Google Scholar]
- Xia J, Sinelnikov I, Han B, Wishart DS. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafrilla P, Morillas J, Mulero J, Cayuela JM, Martínez-Cachá A, Pardo F, López Nicolás JM. Changes during storage in conventional and ecological wine: phenolic content and antioxidant activity. J Agric Food Chem. 2003;51:4694–4700. doi: 10.1021/jf021251p. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Duan C-Q, Wang J. Components of non-anthocyanin phenolic compounds in wines of Vitis amurensis and its hybrids. Afr J Biotechnol. 2013;10:14767–14777. [Google Scholar]
- Zraly K. Windows on the world complete wine course. 2009. New York: Sterling; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.