Abstract
The present work was undertaken to evaluate the chemical composition (proximate, minerals and dietary fibre), colour parameters, antioxidant activity and polyphenol profiles of different fruits (pomegranate, kinnow, mango, banana, jambolan, grapes and sapodilla) and vegetables (beetroot, brinjal, orange carrot, bitter gourd, mentha and spinach). The amount of insoluble dietary fibre was higher than soluble dietary fibre for all fruits and vegetables. Vegetables showed superior mineral composition (higher amounts of K, Ca and Fe) as compared to fruits. Total phenolic content (TPC) and antioxidant activity (ABTS and DPPH) ranged from 354.9 to 1639.7 mg GAE/100 g, 2.6 to 5.5 and 3.0 to 6.3 mM TE/g, respectively for different fruits, while it ranged from 179.3 to 1028.6 mg GAE/100 g, 2.1 to 4.7 and 2.0 to 5.0 mM TE/g, respectively for different vegetables. Gallic acid, protocatechuic acid, catechin, caffeic acid, ferulic acid, sinapic acid, quercetin, resveratrol and kaempferol were detected and quantified in different fruits and vegetables. The results highlighted that fruit peels could be used as valuable sources of minerals and polyphenols having high antioxidant activity.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2412-8) contains supplementary material, which is available to authorized users.
Keywords: Fruits, Vegetables, Antioxidant activity, Dietary fibre, Polyphenols
Introduction
Fruits and vegetables are generally good sources of vitamins as well as minerals. In addition, these are rich sources of several bioactive compounds (mainly polyphenols) which have many health promoting effects (Singh et al. 2016a). These compounds have the capacity to scavenge free radicals and show synergistic effects contributing to anti-inflammatory, antimicrobial, anti-mutagenic, anti-tumour and neuroprotective properties (Kang et al. 2011; Fujita et al. 2013; Zielinski et al. 2014; Singh et al. 2016d). A wide range of polyphenols have been reported in fruit and vegetables. In addition, these also provide sufficient quantities of dietary fibre which is important for the maintenance of intestinal health as well as prevention of cardiovascular diseases, cancer, obesity and diabetes (Elleuch et al. 2011). Therefore, a regular consumption of fruits and vegetables is recommended in the human det.
India is the largest producer of fresh fruits and second largest producer of vegetables in the world (FAOSTAT 2012). A wide variety of fruits and vegetables are commonly grown and consumed in India. Mango (Mangifera indica), banana (Musa sp.), jambolan (Syzygium cumini, commonly known as jamun), sapodilla (Manilkara zapota), pomegranate (Punica granatum), kinnow (Kinnow mandarin) and grapes (Vitis vinifera) are the some of the important fruits, while beetroot (Beta vulgaris), brinjal (Solanum melongena), carrot (Daucus carota), bitter gourd (Momordica charantia), mentha (Mentha arvensis) and spinach (Spinacia oleracea) are some important vegetables commonly available in India. These fruits and vegetables have been evaluated thoroughly for their nutritional value. However, only limited information is available on the presence and profiling of various bioactive constituents (such as polyphenols and dietary fibre) in them.
There is an increased interest in the utilisation of bioactive constituents from fruits and vegetables (either in raw or extracted form) in the food and pharmaceutical industry. Fruits are commonly processed into pulp, leaving behind a substantial amount of residues in the form of peels. These residues form a bulk of organic waste, which is often disposed in open spaces or in municipal bins leading to environmental pollution. However, these contain significant amounts of essential minerals as well as bioactive constituents and their utilisation in the production of nutraceuticals will not only act as value addition but also help in reducing environmental pollution. The antioxidants from fruit wastes have been suggested to be useful in the food, cosmetic and pharmaceutical industries (Makris et al. 2007; Babbar et al. 2011) where these can be used as substitutes of synthetic antioxidants that provide protection against oxidative degradation from free radicals.
Phenolic contents and antioxidant properties of different fruits and vegetables such as banana, mango, carrot, kinnow, jambolan, grapes and pomegranate (Faller and Fialho 2010; Babbar et al. 2011; Rai et al. 2011; Zielinski et al. 2014; Malviya et al. 2014) have been evaluated recently. Faller and Fialho (2010) evaluated the polyphenol content of peels and pulps of different fruits and vegetables grown in Brazil such as banana, mango and carrot. They reported that banana pulp had the highest polyphenol content among them. Babbar et al. (2011) evaluated the phenolic content and antioxidant activities of kinnow peels and banana peels. These authors reported that kinnow peels had highest phenolic and antioxidant activity. Rai et al. (2011) studied the effect of long term storage of jambolan under modified atmosphere packaging and reported an increase in phenolic content and antioxidant activity after storage. Zielinski et al. (2014) determined the phenolic content and antioxidant activity of 19 Brazilian frozen pulps and reported the highest values for grapes. Malviya et al. (2014) studied the antioxidant potential of pomegranate peel extracts. They reported that these peels had good antioxidant activities and could be added in various food items. However, none of the earlier studies compared minerals, dietary fibre and phenolic composition of diverse fruits and vegetables under similar conditions of estimation. Therefore, the present work was undertaken to evaluate the variability in chemical composition, antioxidant activities and polyphenol profiles of common Indian fruits and vegetables.
Materials and methods
Materials
Fresh fruits (pomegranate, kinnow, mango, banana, jambolan, grapes and sapodilla) and vegetables (beetroot, brinjal, orange carrot, bitter gourd, mentha and spinach) were procured from a supermarket (Hyper City Retail India Ltd.) in Amritsar, India. Fresh black carrots were procured from Nijjer Agro Foods Ltd. (Jandiala, Amritsar, India). The fruits and vegetables were washed with water and then separated into inedible (peels) and edible (pulp) portions. The seeds were removed manually and discarded. Afterwards, these portions were homogenised in a blender, dried immediately using a freeze-dryer (Christ Beta 2–8 LD plus, Germany), powdered and then kept at −20 °C till further analysis. These powdered samples were used for all analysis except colour determination which was carried out on fresh homogenised material. Polyphenol standards (quercetin, gallic acid, kaempferol, protocatechuic acid, sinapic acid, catechin, caffeic acid, ferulic acid and resveratrol) with purity >97.5%, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), Folin-Ciocalteu reagent, aluminium chloride, sodium nitrite, ultrapure HPLC grade water, 2,2′-azinobis3-etheylbenzothiazoline-6-sulfonic acid (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma–Aldrich Company Ltd (St Louis, USA). HPLC grade methanol and acetic acid glacial were purchased from Finar Ltd (Ahmedabad, India). Total dietary fibre assay kit (K-TDFR) was purchased from Megazyme International (Wicklow, Ireland). All other chemicals used were of at least analytical grade.
Colour properties
Colour parameters (L*, a* and b*) of different fruit and vegetables were recorded using an Ultra Scan VIS Hunter Lab (Hunter Associates Laboratory Inc., Reston, VA, USA) as described elsewhere (Singh et al. 2016b).
Chemical composition
Protein, fat, ash and dietary fibre (soluble, insoluble and total) content of fruits and vegetables were estimated following standard AOAC (2000) methods. Mineral composition was determined using an atomic absorption spectrometer (Agilent Technologies, Santa Clara, CA, USA). Powdered samples (0.5 g) were placed in a porcelain crucible and heated to 550 °C in a muffle furnace for 6 h. The resulting ash was dissolved in 50 ml of 1% nitric acid and then filtered. The filtrate was analyzed for macroelements (K, Mg, Na and Ca) and microelements (Cu, Fe, Mn and Zn). The instrument was calibrated with standard stock solutions of various minerals and the content in different fruits portions and vegetables was reported in mg/100 g.
Total phenolics, flavonoids and antioxidant activity
Fruits and vegetables (0.25 g) were mixed with 80% methanol (10 ml) and shaken for 2 h using an orbital shaker at 200 rpm (Remi, India), followed by centrifugation at 4000g for 10 min at 25 °C. The extraction was repeated twice; supernatants were pooled; then filtered for phenolic, flavonoid and antioxidant assays.
Total phenolic content (TPC)
TPC was determined following the method of Singleton et al. (1999) with slight modifications (Singh et al. 2015). Sample extract (100 µl) was diluted to 4.8 ml by distilled water and 300 µl of Folin–Ciocalteau reagent was added. 20% sodium carbonate (900 µl) was added with mixing after 8 min, and the solution was left at 40 °C for 30 min before taking its absorbance at 765 nm on a spectrophotometer. Gallic acid was used as a standard and the results were reported as mg GAE (gallic acid equivalents)/100 g.
Total flavonoid content (TFC)
TFC of the fruits and vegetables was determined by the method of Heimler et al. (2005). Quercetin was used as a standard and results were expressed as mg QE (quercetin equivalents)/100 g.
Antioxidant activity assays (DPPH and ABTS)
The total antioxidant activity of fruits and vegetables was determined using DPPH and ABTS assays. DPPH assay was done according to the method of Brand-Williams et al. (1995) as modified by Singh et al. (2015). ABTS assay was performed according to the method of Ozgen et al. (2006) as modified by Singh et al. (2015). A calibration curve was constructed by plotting percentage inhibition against concentration of trolox in both the assays. The antioxidant capacity of the fruits and vegetables was expressed as mM TE (trolox equivalents)/g.
HPLC analysis
Samples for HPLC analysis were extracted using the method of Sakakibara et al. (2003) with modifications. Briefly, powdered samples (0.5 g) were dissolved in 80% methanol (15 ml) containing 0.5% acetic acid. This mixture was placed in an ultrasonic bath for 15 min at 25 °C, followed by centrifugation at 4000g for 10 min (at 25 °C). The extraction was repeated twice and the supernatants were pooled, evaporated and reconstituted in 2 ml of 80% methanol containing 0.5% acetic acid. The final extracts were filtered through a 0.2 mm filter (Pall Corporation, New York, USA) before analysis. HPLC system (Infinity 1260, Agilent Technologies, Santa Clara, CA, USA) having a quaternary gradient pump (G1311C quaternary pump VL, Agilent Technologies) and a diode array detector (G1315D 1260 DAD VL, Agilent Technologies) was used for HPLC analysis. The chromatographic analysis was done using a C18 column (250 mm × 4.6 mm, 5 µm particle size; Enable G, Spinco Biotech Pvt. Ltd., Chennai, India). The injection volume was 10 µl and the running temperature was set at 35 °C. The polyphenols were detected at 280 nm at a 0.6 ml/min constant flow rate. A mobile phase was a mixture of ultrapure water having 0.1% acetic acid, whereas B mobile phase was a mixture of methanol and 0.1% acetic acid. The gradient used was: 5% B, 0–5 min; 10% B, 5–10 min, 20% B, 10–25 min; 23% B, 25–35 min; 28% B, 35–45 min; 35% B, 45–60 min; 50% B, 60–75 min; 55% B, 75–80 min; 75% B, 80–85 min; 35% B, 85–90 min; 5% B, 90–95 min. Peaks were identified in the chromatograms by comparing the retention times with those of the standards and were quantified using a calibration curve between peak area and concentration (Singh et al. 2016d).
Statistical analysis
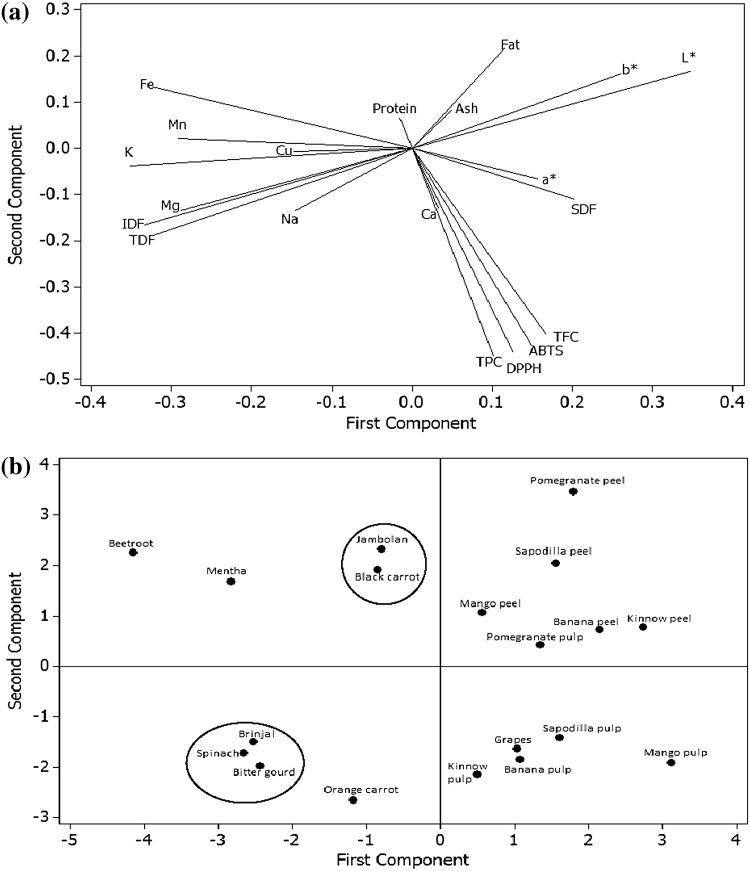
The data is the average of triplicate observations. This was subjected to analysis of variance (ANOVA) by Duncan’s test (P < 0.05) using the Minitab Statistical Software (State College, PA). The results were reported on dry weight basis. Principal component analysis (PCA) was also carried out using Minitab Statistical Software. PCA loading plot (Fig. 1a) showed an overview of relations amongst different properties of common Indian fruits and vegetables, while score plot (Fig. 1b) showed the similarities and differences amongst them on the basis of the measured properties.
Fig. 1.
Principal component Analysis Loading plot a showing an overview of relations amongst different properties of common Indian fruits and vegetables; and score plot b showing similarities and differences amongst them on the basis of the measured properties. TPC Total phenolic content, TFC total flavonoid content, TDF total dietary fibre, SDF soluble dietary fibre, IDF insoluble dietary fibre
Results and discussions
Colour properties
The colour parameters of fruit peels as well as pulps and different vegetables are shown in Supplementary Table S1. The L* value of different fruits and vegetables ranged from 36.2 to 54.7 and 21.1 to 47.6, respectively. L* value was observed to be higher for fruit peels than their pulps. The lowest value for L* was observed for black carrot, possibly due to the presence of high amounts of dark coloured pigments. The a* values ranged between −2.2 and 14.0 for different fruits and between −10.0 and 22.4 for different vegetables (Supplementary Table S1). A variable trend of a* and b* values was observed for fruit peels and their pulps. All fruits (except grapes) and vegetables (except bitter gourd and spinach) showed positive values of a* (redness). The natural pigments present in fruits and vegetables are responsible for their colour. These pigments include carotenoids, chlorophylls, flavonoids, anthocyanins and betalains imparting yellow–orange, green, yellow, red–blue and red colour, respectively. The differences in a* values could be due to varying amounts of chlorophylls and carotenoids. All fruits and vegetables showed positive b* values, ranging from 0.3 to 42.0 for different fruits and 0.3 to 31.2 for vegetables, indicating a predominance of yellowness. Zielinski et al. (2014) reported L*, a* and b* values ranging from 2.1 to 80.4, −8.5 to 30.3 and 0.3 to 49.2, respectively for 19 Brazilian frozen fruit pulps, while ranging from 43.0 to 57.0, −1.56 to 15.7 and 7.08 to 22.7 for different fruits and vegetables was also reported (Krokida et al. 2001).
Chemical composition
Proximate composition of different fruit peels as well as pulps and vegetables is shown in Supplementary Table S2. Ash content ranged from 2.6 to 5.2 g/100 g for different fruits, while it ranged from 3.2 to 4.3 g/100 g for different vegetables. Protein content ranged from 1.4 to 2.4 g/100 g for different fruits, while it ranged from 1.1 to 3.3 g/100 g for different vegetables. All fruits and vegetables had a low fat content (0.45 to 1.19 g/100 g). L*, a* and b* values of different fruits and vegetables were related positively with ash and fat content, but negatively with protein content (Fig. 1a).
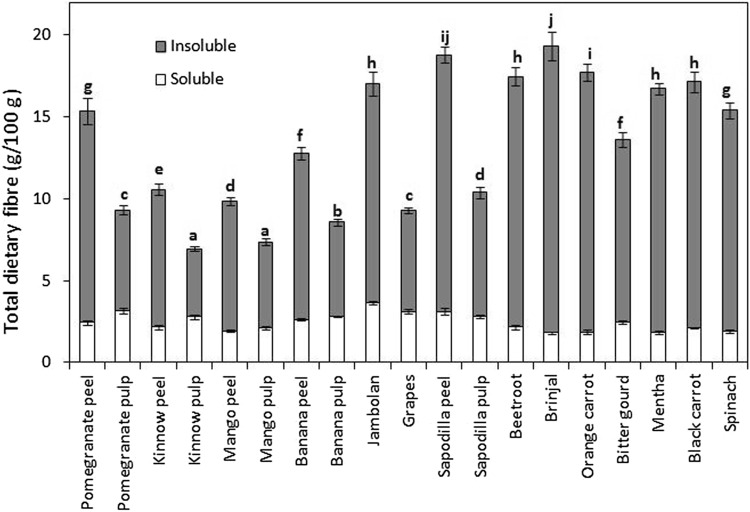
Dietary fibre includes a mixture of many carbohydrate polymers present in plants (such as cellulose, resistant starch, pectin, gums, inulin and hemicelluloses) that may be associated with lignin and other non-carbohydrate components (such as saponins and waxes). The soluble, insoluble and total dietary fibre content of different fruits and vegetables are presented in Fig. 2. All fruits and vegetables had higher content of insoluble than soluble dietary fibre. The results were consistent with those of Ramulu and Rao (2003) for different Indian fruits. It was further observed that fruit peels had higher total dietary fibre content than the respective pulps, being the highest for sapodilla. Overall, vegetables contained higher amount of insoluble but lower of soluble fibre than different fruits. Total dietary fibre is categorized into soluble and insoluble on the basis of its solubility in the human digestive system. Insoluble dietary fibre mainly includes cellulose, whereas pectins mainly represent soluble dietary fibre in fruits and vegetables. Dietary fibre is known to have many health benefits and 25–30 g/day of fibre intake is recommended for an individual (Singh et al. 2016c). PCA revealed that total dietary fibre content of different fruits and vegetables was positively related with the insoluble dietary fibre content but negatively with the soluble dietary fibre content (Fig. 1a).
Fig. 2.
Total dietary fibre (insoluble and soluble) contents of common Indian fruits and vegetables
Mineral composition of different fruits and vegetables is shown in Table 1. The concentration of macroelements K, Mg, Na and Ca ranged from 135 to 444, 43 to 147, 31 to 138 and 52 to 320 mg/100 g, respectively for fruits, while from 357 to 819, 71 to 182, 15 to 182 and 25 to 180 mg/100 g, respectively for vegetables. The concentration of microelements Cu, Fe, Mn and Zn ranged from 0.06 to 0.76, 0.21 to 4.02, 0.03 to 0.69 and 0.13 to 1.51 mg/100 g, respectively for fruits, while from 0.11 to 0.70, 4.16 to 9.49, 0.39 to 4.96 and 0.55 to 2.75 mg/100 g, respectively for vegetables. Fruit peels had significantly high concentrations of different minerals (Table 1) suggesting that these can be utilized for the production of nutraceuticals. It was also observed that different vegetables had higher content of various minerals as compared to fruits. Among these, black carrot, orange carrot, bitter gourd and mentha showed the highest concentration of K, Na, Mg and Ca, respectively, while brinjal, orange carrot, mentha and bitter gourd showed the highest concentration of Cu, Fe, Mn and Zn, respectively. The results were in agreement of Sanchez-Castillo et al. (1998) who also reported that vegetables were better sources of minerals than fruits. Martinez-Ballesta et al. (2010) reported an iron content ranging from 0.1 to 3 mg/100 g in various fruits and vegetables. Szefer and Grembecka (2006) reported the presence of manganese (ranging between 0.01 and 0.67 mg/100 g) and zinc (ranging between 0.02 and 0.6 mg/100 g) in different fruits and vegetables. PCA revealed that fruits and vegetables with higher content of protein accumulated higher amounts of Cu, K, Mn, Mg and Na (Fig. 1a). The physical and chemical characteristics of fruits and vegetables were reported to be influenced by several factors including varietal differences, soil types, weather conditions and time of harvest as well as post-harvest handling (de Souza et al. 2012).
Table 1.
Mineral composition of common Indian fruits and vegetables
| Fruit/vegetable | Portion | K (mg/100 g) | Mg (mg/100 g) | Na (mg/100 g) | Ca (mg/100 g) | Cu (mg/100 g) | Fe (mg/100 g) | Mn (mg/100 g) | Zn (mg/100 g) |
|---|---|---|---|---|---|---|---|---|---|
| Fruits | |||||||||
| Pomegranate | Peel | 295 ± 14d | 64 ± 5cd | 127 ± 11g | 105 ± 2e | 0.19 ± 0.01c | 2.71 ± 0.04e | 0.23 ± 0.01bc | 0.13 ± 0.00a |
| Pulp | 363 ± 18e | 79 ± 7f | 102 ± 9f | 170 ± 8f | 0.19 ± 0.00c | 2.87 ± 0.01f | 0.04 ± 0.00a | 0.74 ± 0.03d | |
| Kinnow | Peel | 135 ± 6a | 78 ± 2ef | 43 ± 5c | 320 ± 7g | 0.21 ± 0.01d | 0.21 ± 0 00a | 0.33 ± 0.02d | 0.95 ± 0.01e |
| Pulp | 300 ± 7d | 73 ± 5d | 138 ± 8h | 55 ± 2b | 0.66 ± 0.02h | 3.48 ± 0.08f | 0.54 ± 0.02f | 1.51 ± 0.07g | |
| Mango | Peel | 444 ± 13g | 117 ± 6g | 107 ± 10f | 60 ± 0b | 0.21 ± 0.01d | 3.19 ± 0.10f | 0.25 ± 0.03c | 0.98 ± 0.07e |
| Pulp | 185 ± 11b | 18 ± 2a | 48 ± 3c | 105 ± 3e | 0.06 ± 0.01a | 1.91 ± 0.10d | 0.03 ± 0.01a | 0.48 ± 0.06c | |
| Banana | Peel | 337 ± 7e | 48 ± 1c | 41 ± 3bc | 55 ± 4b | 0.18 ± 0.00c | 2.43 ± 0.06e | 0.17 ± 0.00b | 0.24 ± 0.04ab |
| Pulp | 382 ± 15f | 82 ± 2f | 31 ± 2b | 52 ± 2b | 0.24 ± 0.01d | 1.98 ± 0.07d | 0.09 ± 0.00a | 0.34 ± 0.01b | |
| Jambolan | Whole | 220 ± 9c | 147 ± 1h | 136 ± 6gh | 170 ± 10f | 0.76 ± 0.03i | 4.02 ± 0.14g | 0.69 ± 0.05g | 1.47 ± 0.10g |
| Grapes | Whole | 225 ± 14c | 43 ± 2b | 35 ± 4b | 52 ± 8b | 0.24 ± 0.00d | 0.86 ± 0.11b | 0.22 ± 0.01bc | 0.52 ± 0.03c |
| Sapodilla | Peel | 235 ± 16c | 57 ± 3c | 42 ± 6bc | 65 ± 3c | 0.18 ± 0.01c | 1.24 ± 0.02c | 0.20 ± 0.00b | 0.54 ± 0.03c |
| Pulp | 191 ± 7b | 40 ± 0b | 48 ± 5c | 58 ± 4b | 0.27 ± 0.00de | 0.95 ± 0.06b | 0.28 ± 0.02c | 0.15 ± 0.03a | |
| Vegetables | |||||||||
| Beetroot | Whole | 819 ± 27j | 182 ± 3i | 182 ± 9i | 55 ± 3b | 0.40 ± 0.01g | 4.85 ± 0.13h | 2.44 ± 0.08 k | 2.75 ± 0.16j |
| Brinjal | Whole | 438 ± 21g | 115 ± 2g | 86 ± 2e | 85 ± 4d | 0.70 ± 0.04h | 4.78 ± 0.09h | 0.82 ± 0.06h | 1.65 ± 0.04h |
| Orange carrot | Whole | 357 ± 19e | 71 ± 3d | 126 ± 7g | 25 ± 0a | 0.37 ± 0.02f | 9.28 ± 0.26j | 0.94 ± 0.01i | 1.28 ± 0.07f |
| Bitter gourd | Whole | 526 ± 18h | 153 ± 6h | 72 ± 3d | 110 ± 3e | 0.35 ± 0.01f | 8.08 ± 0.19i | 1.18 ± 0.01j | 2.00 ± 0.11i |
| Mentha | Whole | 514 ± 33h | 78 ± 1ef | 46 ± 3c | 180 ± 10f | 0.17 ± 0.02c | 4.92 ± 0.22h | 4.96 ± 0.05l | 0.87 ± 0.02e |
| Black carrot | Whole | 632 ± 17j | 80 ± 3f | 15 ± 2a | 175 ± 9f | 0.11 ± 0.00b | 4.16 ± 0.18g | 0.39 ± 0.00e | 1.49 ± 0.07g |
| Spinach | Whole | 579 ± 12i | 81 ± 6f | 84 ± 9de | 98 ± 5e | 0.28 ± 0.01e | 9.49 ± 0.37j | 0.53 ± 0.01f | 0.55 ± 0.01c |
Whole, deseeded edible fruit/vegetable portion
Values are mean ± SD. n = 3
Means with same letter in a column did not differ significantly (P > 0.05)
Total phenolics, flavonoids and antioxidant activity
Polyphenols are a major group of phytochemicals ubiquitously distributed in all higher plants and are sub-classified into two main groups: the phenolics and the flavonoids (Singh et al. 2016a). Folin-Ciocalteu reagent colorimetric method is a widely used method for the estimation of TPC (Granato et al. 2010). The benefits of using this method is the low cost and simplicity, but it cannot be used for quantifying the individual polyphenols. TPC of different fruits and vegetables is shown in Table 2. TPC ranged from 354.9 to 1639.7 mg GAE/100 g for different fruits, while it ranged from 179.3 to 1028.6 mg GAE/100 g for different vegetables. Fruit peels had higher TPC than the respective pulps, indicating greater accumulation of phenolic compounds in peels than pulps (Pande and Akoh 2009). Amongst fruits, pomegranate showed the highest TPC for both peel and pulp and the lowest was observed for kinnow; while black carrot showed the highest and orange carrot showed the lowest TPC amongst different vegetables. Alasalvar et al. (2001) also reported higher TPC for black carrots as compared to orange or yellow cultivars. Fu et al. (2011) reported a TPC ranging from 11.9 to 585.5 mg GAE/g for different Chinese fruits. TPC varying between 368 and 1750 mg GAE/100 g has been reported for six different fruit residues previously (Babbar et al. 2011). Flavonoids and their derivatives (flavones, flavonols, flavanols, flavanones, isoflavones and anthocyanidins) constitute the largest group of phenolic compounds. TFC of different fruits and vegetables is shown in Table 2. TFC ranged from 206.1 to 1085.2 mg QE/100 g for different fruits, while it ranged from 80.5 to 647.5 mg QE/100 g for different vegetables. TFC of fruit peels was greater than respective pulps. Amongst fruits, pomegranate showed the highest TFC for both peel and pulp, while the lowest was observed for kinnow peels and banana pulp. Black carrot showed the highest, while bitter gourd showed the lowest TFC amongst different vegetables. The synthesis of flavonoids has been reported to be stimulated by sunlight; therefore, these are concentrated in the outer portions of fruits (Haminiuk et al. 2012).
Table 2.
Total phenolic and total flavonoid content of common Indian fruits and vegetables
| Fruit/vegetable | Portion | TPC (mg GAE/100 g) | TFC (mg QE/100 g) |
|---|---|---|---|
| Fruits | |||
| Pomegranate | Peel | 1639.7 ± 30.7m | 1085.2 ± 19.8k |
| Pulp | 671.1 ± 18.5g | 473.9 ± 22.7f | |
| Kinnow | Peel | 762.3 ± 20.0h | 442.8 ± 15.1f |
| Pulp | 354.9 ± 12.5d | 261.3 ± 21.5d | |
| Mango | Peel | 944.4 ± 27.2i | 712.8 ± 29.2i |
| Pulp | 440.6 ± 16.6e | 389.1 ± 15.3e | |
| Banana | Peel | 1049.2 ± 21.4j | 715.0 ± 18.1i |
| Pulp | 362.4 ± 17.2d | 206.1 ± 21.8c | |
| Jambolan | Whole | 1090.7 ± 38.1k | 894.2 ± 23.6j |
| Grapes | Whole | 538.6 ± 22.0f | 214.5 ± 17.4c |
| Sapodilla | Peel | 1151.4 ± 32.3l | 564.5. ± 30.5g |
| Pulp | 413.9 ± 19.9e | 208.3 ± 14.5c | |
| Vegetables | |||
| Beetroot | Whole | 909.5 ± 37.4i | 221.6 ± 26.8c |
| Brinjal | Whole | 292.3 ± 12.7c | 224.2 ± 34.3c |
| Orange carrot | Whole | 179.3 ± 9.7a | 121.9 ± 8.7b |
| Bitter gourd | Whole | 226.5 ± 10.5b | 80.5 ± 9.8a |
| Mentha | Whole | 428.2 ± 19.6e | 146.2 ± 24.2b |
| Black carrot | Whole | 1028.6 ± 23.4j | 647.5 ± 10.2h |
| Spinach | Whole | 233.5 ± 20.2b | 108.7 ± 8.3a |
Whole, deseeded edible fruit/vegetable portion; TPC, total phenolic content; TFC, total flavonoid content
Values are mean ± SD. n = 3
Means with same letter in a column did not differ significantly (P > 0.05)
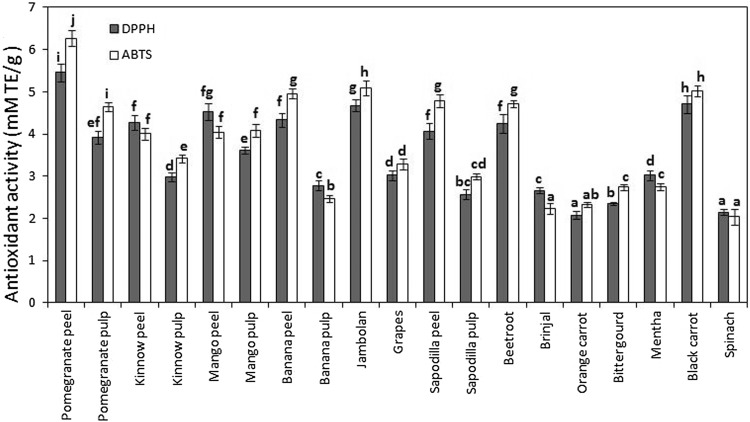
The determination of individual ingredients does not represent the true antioxidant activity of foods owing to the possible interactions between these components and the food matrix (Singh et al. 2016b). Therefore, the antioxidant activity of different fruits and vegetables was evaluated using DPPH and ABTS assays to get a clear picture of the antioxidant activity. DPPH (used in lipophilic systems) and ABTS (used in both aqueous and lipophilic systems) assays are based on the evaluation of free radical scavenging activities of samples by measuring the colour degradation (Singh et al. 2016d). The antioxidant activity of different fruits and vegetables is shown in Fig. 3. DPPH is a stable free radical (maximum absorbance at 515 nm), which is scavenged from purple to yellow after it accepts an electron or a proton to become a stable diamagnetic molecule when antioxidants are encountered (Singh et al. 2015). DPPH antioxidant activity ranged between 2.6 and 5.5 mM TE/g for different fruits, while it ranged from 2.1 to 4.7 mM TE/g for different vegetables. For fruits, the highest DPPH scavenging activity was observed for pomegranate peel and pulp, while the lowest was observed for sapodilla peel and pulp. Amongst vegetables, the highest DPPH scavenging activity was observed for black carrot, while the lowest was observed for orange carrot. ABTS antioxidant assay involved the oxidation of ABTS radical, forming an intensely-green coloured nitrogen-centred ABTS cation. ABTS antioxidant activity ranged between 3.0 and 6.3 mM TE/g for different fruits, while it ranged from 2.0 to 5.0 mM TE/g for different vegetables. For fruits, the highest ABTS scavenging activity was observed for pomegranate peel and pulp, while the lowest was observed for kinnow and banana for peels and pulps, respectively. Among vegetables, the highest ABTS scavenging activity was observed for black carrot, while the lowest was observed for spinach. The high values of scavenging activities for black carrot and beetroot among vegetables were mainly due to anthocyanins and betalains, respectively, which have been reported to exhibit strong antioxidant activities (Alasalvar et al. 2001). The in vitro antioxidant activity measurements might produce valuable data (when properly performed) for screening antioxidants in vivo. PCA revealed positive relations amongst TPC, TFC, DPPH and ABTS antioxidant activities (Fig. 1a), corroborating that phenolic compounds were mainly responsible for the antioxidant activities of fruits and vegetables. In general, the overall values of ABTS scavenging activity were comparatively higher than DPPH for most fruits and vegetables. This might be due to the presence of coloured compounds (such as anthocyanins) in fruits and vegetables that had an overlapping spectrum with DPPH, thus interfered with the measurements (Moo-Huchin et al. 2014). PCA analysis revealed differences between various fruits and vegetables in terms of the measured properties as the former were located at right of the plot (except jambolan), while the latter were located on the left of the score plot (Fig. 1b).
Fig. 3.
Antioxidant activity (DPPH and ABTS inhibition) of common Indian fruits and vegetables (Dark bars = DPPH; White bars = ABTS)
HPLC analysis
Reverse phase HPLC is one of the main techniques that are used for the separation of polyphenols, having mobile phase more polar than the stationary phase. Polyphenols are identified as well as quantified using a gradient elution in the HPLC method instead of isocratic mode, owing to their chemical complexity as well as similarity (Merken and Beecher 2000). The retention times of polyphenols are higher for less polar molecules (such as kaempferol and quercetin); while these are lower for polar molecules (such as protocatechuic acid and gallic acid) that elute easily. HPLC analysis revealed the presence of a number of phenolic compounds in different fruits portions and vegetables. The phenolic compounds identified were gallic acid, protocatechuic acid, catechin, caffeic acid, ferulic acid, sinapic acid, quercetin, resveratrol and kaempferol, which eluted at the retention time of 16.9, 25.1, 34.3, 43.3, 61.8, 63.4, 86.3, 90.7 and 93.1 min, respectively. Gallic and protocatechuic acid are hydroxybenzoic acids; caffeic, ferulic and sinapic acid are hydroxycinnamic acids; catechin is a flavan-3-ol; quercetin and kaempferol are flavonols; while resveratrol is stilbenoid. The content of these polyphenols in different fruit portions and vegetables is shown in Table 3, while the chromatograms are shown in Supplementary Figure S1. Fruit peels had higher amounts of polyphenols, evaluated in this study than the respective pulps. Gallic acid was present in all fruits (except kinnow) and in most vegetables (except brinjal, orange carrot and spinach). Beetroot and black carrot had higher content of gallic acid as compared to other vegetables. Gallic acid has been reported to be commonly present in tropical fruits (Shahidi and Naczk 2003). Protocatechuic acid was observed in kinnow peel and banana pulp only. Catechin was present in most fruits, except kinnow and jambolan. Pomegranate peel and pulp had the highest amount of catechin, followed by banana and sapodilla. Caffeic acid was observed in all fruits except sapodilla; however, it was not present in mango and pomegranate pulps. Manach et al. (2004) reported that caffeic acid represented more than three-fourths of the total content of hydroxycinnamic acids in many fruits. Sinapic acid was present in pomegranate, kinnow, jambolan and grapes, while resveratrol was observed in pomegranate, banana, grapes, mentha and spinach only. Protocatechuic acid was not present in any of the vegetables evaluated and catechin was observed in bitter gourd only, while caffeic acid was present in all vegetables except spinach. Quercetin was present in most fruits except kinnow, while it was observed in beetroot, black carrot and spinach only for vegetables. On the other hand, kaempferol was present in kinnow, mango, sapodilla and brinjal only. These polyphenols have been previously reported in pomegranate (Li et al. 2015), mango (Ribeiro et al. 2008), banana (Russell et al. 2009), jambolan (Singh et al. 2016d), beetroot (Wruss et al. 2015) and black carrot (Kammerer et al. 2004). Gallic acid, p-coumaric acid, catechin, ferulic acid, caffeic acid and tannic acid have been previously reported in bitter gourd (Kubola and Siriamornpun 2008). Quercetin and myricetin derivatives were reported by Singh et al. (2009) in brinjal. Chu et al. (2000) reported myricetin and quercetin as the main polyphenols in spinach.
Table 3.
Contents of various polyphenols (mg/100 g) in common Indian fruits and vegetables
| Fruit/vegetable | Portion | Gallic acid | Protocatechuic acid | Catechin | Caffeic acid | Ferulic acid | Sinapic acid | Quercetin | Resveratrol | Kaempferol |
|---|---|---|---|---|---|---|---|---|---|---|
| Fruits | ||||||||||
| Pomegranate | Peel | 52.3 ± 0.1l | ND | 76.5 ± 0.4i | 22.2 ± 0.3f | ND | 30.7 ± 0.3e | ND | ND | ND |
| Pulp | 18.4 ± 0.2g | ND | 18.9 ± 0.3f | ND | ND | 16.5 ± 0.2c | 8.7 ± 0.2d | 19.9 ± 0.2e | ND | |
| Kinnow | Peel | ND | 17.7 ± 0.1b | ND | 50.2 ± 0.4j | 33.9 ± 0.2e | 20.6 ± 0.3d | ND | ND | ND |
| Pulp | ND | ND | ND | 16.8 ± 0.1e | 12.3 ± 0.2b | 14.4 ± 0.1b | ND | ND | 14.2 ± 0.1c | |
| Mango | Peel | 20.7 ± 0.2h | ND | 11.1 ± 0.1d | 7.4 ± 0.0b | 16.6 ± 0.3c | ND | 29.1 ± 0.1i | ND | 37.3 ± 0.2e |
| Pulp | 9.1 ± 0.0c | ND | 6.4 ± 0.1b | ND | 10.2 ± 0.1a | ND | 12.4 ± 0.1f | ND | 9.1 ± 0.2b | |
| Banana | Peel | 44.2 ± 0.3k | ND | 60.1 ± 0.2h | 67.6 ± 0.3 k | 61.7 ± 0.4f | ND | 43.5 ± 0.2j | 38.8 ± 0.1f | ND |
| Pulp | 13.3 ± 0.1e | 3 4 ± 0.0a | 14.9 ± 0.1e | 7.5 ± 0.0b | 16.6 ± 0.2c | ND | ND | 5.6 ± 0.1a | ND | |
| Jambolan | Whole | 18.1 ± 0.2g | ND | ND | 30.5 ± 0.1i | ND | 20.3 ± 0.2d | 15.8 ± 0.3h | ND | ND |
| Grapes | Whole | 6.6 ± 0.1a | ND | 6.8 ± 0.0b | 4.7 ± 0.1a | ND | 3.2 ± 0.0a | 3.5 ± 0.1a | 8.4 ± 0.2b | 6.9 ± 0.1a |
| Sapodilla | Peel | 27.5 ± 0.0j | ND | 43.3 ± 0.2g | ND | ND | ND | 49.1 ± 0.3k | ND | 51.2 ± 0.2f |
| Pulp | 10.2 ± 0.1d | ND | 8.1 ± 0.0c | ND | ND | ND | 5.4 ± 0.1b | ND | ND | |
| Vegetables | ||||||||||
| Beetroot | Whole | 25.7 ± 0.2j | ND | ND | 22.6 ± 0.4f | ND | ND | 6.3 ± 0.0c | ND | ND |
| Brinjal | Whole | ND | ND | ND | 7.9 ± 0.1c | ND | ND | ND | ND | 16.6 ± 0.2d |
| Orange carrot | Whole | ND | ND | ND | 23.2 ± 0.2g | 12.1 ± 0.0b | ND | ND | ND | ND |
| Bitter gourd | Whole | 13.9 ± 0.1f | ND | 5.3 ± 0.0a | 8.2 ± 0.1c | ND | ND | ND | ND | ND |
| Mentha | Whole | 7.1 ± 0.0b | ND | ND | 12.8 ± 0.2d | ND | ND | ND | 9.4 ± 0.0c | ND |
| Black carrot | Whole | 24.2 ± 0.2i | ND | ND | 24.2 ± 0.3h | 21.8 ± 0.1d | ND | 10.5 ± 0.1e | ND | ND |
| Spinach | Whole | ND | ND | ND | ND | ND | ND | 14.0 ± 0.2g | 19.3 ± 0.1d | ND |
Whole, deseeded edible fruit/vegetable portion; ND, not detected
Values are mean ± SD. n = 3
Means with same letter in a column did not differ significantly (P > 0.05)
Conclusion
The present study provided useful information on the chemical composition and amounts of health promoting constituents (dietary fibre and polyphenols) from different fruits, their peels and vegetables, which are essential for the understanding of their nutraceutical potential and future application in the food industry. Different fruits and vegetable varied in chemical composition and antioxidant activities. In general, the fruits and vegetables were good source of essential minerals and bioactive constituents, while fruit peels were superior to pulps in this regard. Moreover, the antioxidant activity of fruits and vegetables was dependant on the content of polyphenols.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors acknowledge UPE-UGC, New Delhi for financial assistance.
References
- Alasalvar C, Grigor JM, Zhang D, Quantick PC, Shahidi F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J Agric Food Chem. 2001;49:1410–1416. doi: 10.1021/jf000595h. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of AOAC international. 17. Gaithersburg: AOAC International; 2000. [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80:561–566. doi: 10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#. [DOI] [Google Scholar]
- de Souza VR, Pereira PAP, Queiroz F, Borges SV, Carneiro JDDS. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012;134:381–386. doi: 10.1016/j.foodchem.2012.02.191. [DOI] [Google Scholar]
- Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem. 2011;124:411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- Faller ALK, Fialho EFNU. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J Food Compost Anal. 2010;23:561–568. doi: 10.1016/j.jfca.2010.01.003. [DOI] [Google Scholar]
- FAOSTAT (2012) Statistical database of the food and agriculture organization of the United Nations. http://faostat.fao.org/
- Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, Li HB. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Fujita A, Borges K, Correia R, de Melo Franco BDG, Genovese MI. Impact of spouted bed drying on bioactive compounds, antimicrobial and antioxidant activities of commercial frozen pulp of camu–camu (Myrciaria dubia Mc. Vaugh) Food Res Int. 2013;54:495–500. doi: 10.1016/j.foodres.2013.07.025. [DOI] [Google Scholar]
- Granato D, Katayama F, Castro I. Assessing the association between phenolic compounds and the antioxidant activity of Brazilian red wines using chemometrics. LWT-Food Sci Technol. 2010;43:1542–1549. doi: 10.1016/j.lwt.2010.05.031. [DOI] [Google Scholar]
- Haminiuk CW, Maciel GM, Plata-Oviedo MS, Peralta RM. Phenolic compounds in fruits—an overview. Int J Food Sci Technol. 2012;47:2023–2044. doi: 10.1111/j.1365-2621.2012.03067.x. [DOI] [Google Scholar]
- Heimler D, Vignolini P, Dini MG, Romani A. Rapid tests to assess the antioxidant activity of phaseolus vulgaris L. dry beans. J Agric Food Chem. 2005;53:3053–3056. doi: 10.1021/jf049001r. [DOI] [PubMed] [Google Scholar]
- Kammerer D, Carle R, Schieber A. Characterization of phenolic acids in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:1331–1340. doi: 10.1002/rcm.1496. [DOI] [PubMed] [Google Scholar]
- Kang J, Xie C, Li Z, Nagarajan S, Schauss AG, Wu T, Wu X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011;128:152–157. doi: 10.1016/j.foodchem.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Krokida MK, Maroulis ZB, Saravacos GD. The effect of the method of drying on the colour of dehydrated products. Int J Food Sci Technol. 2001;36:53–59. doi: 10.1046/j.1365-2621.2001.00426.x. [DOI] [Google Scholar]
- Kubola J, Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008;110:881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Li J, He X, Li M, Zhao W, Liu L, Kong X. Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem. 2015;176:7–11. doi: 10.1016/j.foodchem.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Makris DP, Boskou G, Andrikopoulos NK. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Comp Anal. 2007;20:125–132. doi: 10.1016/j.jfca.2006.04.010. [DOI] [Google Scholar]
- Malviya S, Jha A, Hettiarachchy N. Antioxidant and antibacterial potential of pomegranate peel extracts. J Food Sci Technol. 2014;51:4132–4137. doi: 10.1007/s13197-013-0956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Martinez-Ballesta M, Dominguez-Perles R, Moreno D, Muries B, Alcaraz-Lopez C, Bastías E, García-Viguera C, Carvajal M. Minerals in plant food: effect of agricultural practices and role in human health. A review. Agron Sustain Dev. 2010;30:295–309. doi: 10.1051/agro/2009022. [DOI] [Google Scholar]
- Merken HM, Beecher GR. Measurement of food flavonoids by high-performance liquid chromatography: a review. J Agric Food Chem. 2000;48:577–599. doi: 10.1021/jf990872o. [DOI] [PubMed] [Google Scholar]
- Moo-Huchin VM, Estrada-Mota I, Estrada-León R, Cuevas-Glory L, Ortiz-Vázquez E, y Vargas MDLV, Betancur-Ancona D, Sauri-Duch E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014;152:508–515. doi: 10.1016/j.foodchem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Ozgen M, Reese RN, Tulio AZ, Scheerens JC, Miller AR. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agr Food Chem. 2006;54:1151–1157. doi: 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]
- Pande G, Akoh CC. Antioxidant capacity and lipid characterization of six Georgia-grown pomegranate cultivars. J Agr Food Chem. 2009;57:9427–9436. doi: 10.1021/jf901880p. [DOI] [PubMed] [Google Scholar]
- Rai DR, Chadha S, Kaur MP, Jaiswal P, Patil RT. Biochemical, microbiological and physiological changes in Jamun (Syzyium cumini L.) kept for long term storage under modified atmosphere packaging. J Food Sci Technol. 2011;48:357–365. doi: 10.1007/s13197-011-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramulu P, Rao PU. Total, insoluble and soluble dietary fiber contents of Indian fruits. J Food Comp Anal. 2003;16:677–685. doi: 10.1016/S0889-1575(03)00095-4. [DOI] [Google Scholar]
- Ribeiro SMR, Barbosa LCA, Queiroz JH, Knödler M, Schieber A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 2008;110:620–626. doi: 10.1016/j.foodchem.2008.02.067. [DOI] [Google Scholar]
- Russell WR, Labat A, Scobbie L, Duncan GJ, Duthie GG. Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chem. 2009;115:100–104. doi: 10.1016/j.foodchem.2008.11.086. [DOI] [Google Scholar]
- Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- Sanchez-Castillo CP, Dewey PJ, Aguirre A, Lara JJ, Vaca R, de la Barra PL, Ortiz M, Escamilla I, James WPT. The mineral content of Mexican fruits and vegetables. J Food Comp Anal. 1998;11:340–356. doi: 10.1006/jfca.1998.0598. [DOI] [Google Scholar]
- Shahidi F, Naczk M. Phenolics in food and nutraceuticals. Boca Raton: CRC Press; 2003. [Google Scholar]
- Singh AP, Luthria D, Wilson T, Vorsa N, Singh V, Banuelos GS, Pasakdee S. Polyphenols content and antioxidant capacity of eggplant pulp. Food Chem. 2009;114:955–961. doi: 10.1016/j.foodchem.2008.10.048. [DOI] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten-free eggless rice muffins. Int J Food Sci Technol. 2015;50:1190–1197. doi: 10.1111/ijfs.12764. [DOI] [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016;206:1–11. doi: 10.1016/j.foodchem.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N, Singh B. Physicochemical characterisation of corn extrudates prepared with varying levels of beetroot (Beta vulgaris) at different extrusion temperatures. Int J Food Sci Technol. 2016;51:911–919. doi: 10.1111/ijfs.13051. [DOI] [Google Scholar]
- Singh JP, Kaur A, Singh N. Development of eggless gluten-free rice muffins utilizing black carrot dietary fibre concentrate and xanthan gum. J Food Sci Technol. 2016;53:1269–1278. doi: 10.1007/s13197-015-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, Arora DS. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci Technol. 2016;65:1025–1030. doi: 10.1016/j.lwt.2015.09.038. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Method Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Szefer P, Grembecka M (2006) 7 Mineral Components in Food Crops, Beverages, Luxury Food, Spices, and Dietary Food. Miner Compon Foods pp 231–322
- Wruss J, Waldenberger G, Huemer S, Uygun P, Lanzerstorfer P, Müller U, Höglinger O, Weghuber J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J Food Comp Anal. 2015;42:46–55. doi: 10.1016/j.jfca.2015.03.005. [DOI] [Google Scholar]
- Zielinski AAF, Ávila S, Ito V, Nogueira A, Wosiacki G, Haminiuk CWI. The association between chromaticity, phenolics, carotenoids, and in vitro antioxidant activity of frozen fruit pulp in Brazil: an application of chemometrics. J Food Sci. 2014;79:C510–C516. doi: 10.1111/1750-3841.12389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.