Abstract
Volatile compounds from smoked dry-cured ham were isolated by using headspace-solid phase microextraction and gas chromatography–mass spectrometry (GC–MS). Samples of biceps femoris were also evaluated for sensory physical and chemical characteristics. Eighty seven volatile aroma compounds of smoked dry-cured ham were identified. Chemical groups identified were aldehydes (35.6%), phenols (34.3%), alcohols (13.8%), terpenes (6.4%), aromatic hydrocarbons (2.6%), alkanes (2.2%), ketones (2.2%), esters (1.7%) and acids (0.7%). Except volatile compounds derived from lipolysis and proteolysis the second most abundant constituents were phenols that originate from smoking phase of the production process. The most abundant phenols were: 4-methylphenol, 3-methylphenol, 2-metoxy-4-methylphenol, 2-methylphenol, 2,6-dimethoxyphenol and 4-ethyl-2 methoxyphenol. Principal components analysis showed that NaCl and ash content positively correlated with the salty taste while fat content was correlated to marbling. Water content and aw value were negatively correlated with hardness of dry-cured ham while phenols were positively corelated with smoky aroma. Due to the different volatile composition and pronounced smoky aroma, smoked dry-cured ham can be distinguished from other types of dry-cured hams.
Keywords: Aroma compounds, Chemical composition, Gas chromatography–mass spectrometry (GC–MS), Dry-cured ham, Solid-phase microextraction (SPME), Sensory analysis
Introduction
Smoking is one of the oldest methods of food preservation, being an integral part of the curing process of many traditional products. Smoking adds desirable sensory properties to many foods and is widely applied in meat processing. As much as 40–60% of the total amounts of meat products are smoked (Sikorski and Kolakowski 2010). Smoked meat products, mainly from pork meat, have a huge impact on the economy of Mediterranean countries, where a great variety of smoked meat products are industrially or traditionally produced. Phenolic components of the smoke contribute to the flavour and aroma of the product. Smoke also provides a protective film on the surface of the smoked product, thus providing a barrier against development of rancidity. Combined chemical constituents of smoke together with heating and drying processes are responsible for bactericidal and bacteriostatic effects. In dry-cured meat products, smoking, combined with salting and partial dehydration, increases the shelf life, due to surface drying and deposition onto the surface of antioxidant and antimicrobial compounds (Martuscelli et al. 2009). Dry-cured hams are manufactured in many countries, but production is mainly located in the Mediterranean area. There is a great variety of dry-cured hams in this area, some of the most important being Spanish Iberian and Serrano, Italian Parma and San Daniele, and French Bayonne hams.
The aroma is perhaps the most important quality parameter and it is markedly affected by the raw material and the production process. In the case of dry-cured hams, the aroma is due to the presence of many volatile compounds, most of them produced by chemical and enzymatic mechanisms during the post-mortem process (Flores et al. 1997). The main biochemical reactions involved in the generation of these compounds are lipolysis and proteolysis that produce a wide range of volatiles and precursors (Toldrá 1998). Also, the development of moulds during pre-maturation and ripening is considered, within certain limits, natural, and they are believed to contribute to the colour and sensory characteristics of the final product (Comi and Iacumin 2013). So, an understanding the aroma of dry-cured ham should include the identification and quantification of its volatiles.
The volatile composition of dry-cured hams has been investigated for European hams but little information is available for smoked dry-cured ham.
Smoking also modifies colour, texture, aroma and flavour and thus improves the overall sensory acceptability of foods. According to the study of Pham et al. (2008), the smoked dry-cured ham products received higher consumer acceptability scores than non-smoked ones. So, the aim of this research was to determine physico-chemical and sensory characteristics of smoked dry-cured ham as well as the volatile flavour compounds using headspace solid phase microextraction (HS-SPME) and gas chromatography-mass spectrometry (GC–MS).
Materials and methods
Traditional production process
Traditional Dalmatian smoked dry-cured ham is an autochthonous top quality product produced in southern part of Croatia–Dalmatia. Dalmatian smoked ham is usually processed with pelvic bones, skin and subcutaneous fatty tissue. It is produced from Duroc × (Yorksire × Landrace) pig breed. It is prepared according to the traditional processing procedures without any additives such as nitrites or ascorbic acid. Traditional processing of Dalmatian smoked ham starts in December, when the raw ham is placed in sea salt at a temperature of 10–12 °C. After 12 days, the ham is drained off and smoked for 5 days and then pressed (for removal of water) under a constant pressure of cca 0.1 kg cm−2 for 2 days. Thereafter, the ham is smoked with dry hornbeam wood for 20 days and pressured again under a constant pressure of cca 0.17 kg cm−2. The ham is then dried under normal environmental conditions (cold and dry north-eastern wind is important) with occasionally smoking until March, and left for further drying until May. The relative humidity (60–70%) during the processing depends on the climatic conditions. The ham is further moved to a cellar for ripening at mild temperatures (12–15 °C) until consumption. The ripening period (before consumption) is usually 12–24 months (Jerković et al. 2007).
Samples
Samples of biceps femoris of traditional Dalmatian smoked dry-cured ham were obtained from 9 different manufacturers at International dry-cured ham fair in Tinjan, Croatia. Dry-cured hams were ripened for 12–18 months. Samples of biceps femoris from each manufacturer were analyzed for volatile compounds and physico-chemical analysis to see the real situation of Dalmatian smoked dry-cured ham on the Croatian market. Samples of biceps femoris were vacuum-packed and stored at −20 °C until further analysis.
Chemical composition analysis
Fat, protein and ash contents were estimated according to methods recommended by AOAC (1999). Moisture content and sodium chloride were determined in the biceps femoris according to AOAC methods (1984). Two replicates of each sample were analysed and the mean value was used in the data analyses. Water activity of the biceps femoris was determined with a precision multi-function measuring instrument, Testo 650 (Testo Inc., New York, USA). Two replicates of each sample were analysed and the mean value was used in the data analyses.
Lipid oxidation by the TBARS test
Oxidation of lipids was assessed by the thiobarbituric acid (TBA) assay which is based on the reaction between TBA and malondialdehyde (MDA) and the production of a coloured pigment, the concentration of which is calculated by measuring the absorbance at 538 nm on three replicates of each sample (Lemon 1975). The spectrophotometer was a Helios β (Spectronic Unicam, Cambridge, UK). TBARS values were expressed as mg of malondialdehyde equivalents/kg dry-cured ham.
Colour instrumental measurement
Colour measurements were carried out with a Minolta CM-700d (Osaka, Japan) spectrophotometer with target mask CM-A178 (diameter 8 mm). The L* (lightness), a* (redness), and b* (yellowness) colour was measured (CIE, Commission Internationale de l’Eclairage 1976). Before analysis spectrophotometer was calibrated with White Calibration Cap CM-A177. Each sample of biceps femoris was analysed in ten replicates, avoiding regions with excess fat to achieve representative measurements of the lean colour.
Analysis of volatile compounds
Analyses were carried out by extraction of volatile compounds above the samples on SPME fibre and their tentative characterization on GC/MS by the method as described by Marušić et al. (2011). 30 g of biceps femoris muscle slices from dry-cured ham was ground with a commercial grinder. Then dry-cured ham homogenates were prepared by dispersing 5 g of minced muscle slices with 25 mL of distilled water saturated with NaCl in a commercial blender. Ten millilitres of this mixture was placed into 20 mL vials tightly capped with a PTFE septum. A magnetic stirrer was placed into the homogenates for stirring during extraction.
A SPME fibre coated with 2 cm of 50/30 μm DVB/Carboxen/PDMS (Supelco, Bellefonte, PA, USA) was conditioned for 2 min at 240 °C prior to extraction and placed above the sample mixture. Triplicate 20 mL vials were placed in a water bath at 40 °C and extracted for 180 min with stirring. After extraction the SPME fibre was immediately injected to 6890 N gas chromatograph coupled to a 5975i mass selective detector (Agilent Technologies, Santa Clara, CA, USA). Capillary column DB-5 ms 30 m × 0.25 mm, film thickness 0.25 μm (Agilent Technologies, Santa Clara, CA, USA) was used with helium as a carrier gas at 1.0 mL/min flow rate. The temperature of the injector, used in the splitless mode, was 230 °C and desorption time was 2 min. Temperature programme was at 40 °C, isothermal for 10 min, then rising to 200 °C at a rate of 5 °C/min and then raising to 250 °C at a rate of 20 °C/min. Final temperature was held for 5 min. The transfer line temperature was maintained at 280 °C. The mass spectra were obtained at 70 eV with a rate of 1 scan/s over the m/z range of 50–450. An in-house mixture of C8–C20 n-alkanes was run under the same chromatographic conditions to calculate the retention indices (RI) of detected compounds. AMDIS 3.2 program version 2.62 was used for identification of components using NIST 2005 version 2.0 spectral library (NIST, Gaithersburg, MD, USA) as well as comparison of obtained retention indices with literature values. The mean value of SPME–GC–MS replicates was calculated and used in the data analysis. Data is expressed as percentage of the total area of identified peaks.
Sensory analysis
Dalmatian smoked dry-cured hams from nine producers were assessed by eight trained panellists who were selected and trained in accordance with international standard described in Marušić et al. (2014).
Nine traits related to sensory characteristics of dry-cured hams (Table 4) were evaluated by the quantitative- descriptive analysis method. Sensory attributes evaluated were: odour intensity, red colour, fat colour, marbling, salty taste, sweet taste, smoky aroma, hardness and overall acceptability. Sensory attributes were assessed with a 10 point intensity line scale, where 0 = not detected and 9 = extremely strong. All the samples, slices of 1.5 mm thickness, were evaluated at 20–22 °C in sensory panel rooms. About 50 mL of water and 20 g of unsalted bread were provided to assessors between successive ham samples. Samples were individually labelled and were randomly served one at a time. All hams were evaluated in slices from the same anatomical area. In each sensory session, panellists evaluated 2 samples and the sensory evaluation consisted of nine sessions (each sample was evaluated two times).
Table 4.
Sensory attributes of Dalmatian smoked dry-cured ham
| Code | Sensory attributes | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | Odour intensity | 4.9 ± 0.5 | 4.4 ± 1.8 | 4.6 ± 0.4 | 4.3 ± 1.7 | 4.2 ± 0.2 | 6.7 ± 1.4 | 5.6 ± 0.2 | 4.3 ± 1.0 | 5.2 ± 0.1 |
| A2 | Red colour | 4.5 ± 0.7 | 4.8 ± 0.6 | 4.8 ± 0.4 | 3.9 ± 0.6 | 5.2 ± 1.6 | 5.6 ± 1.6 | 5.6 ± 0.2 | 4.5 ± 0.7 | 4.4 ± 0.8 |
| A3 | Fat colour | 5.1 ± 0.6 | 4.6 ± 0.3 | 5.7 ± 0.1 | 4.7 ± 0.2 | 5.7 ± 0.3 | 5.1 ± 0.2 | 6.1 ± 0.2 | 5.8 ± 0.1 | 5.4 ± 1.0 |
| A4 | Marbling | 5.5 ± 0.8ab | 6.6 ± 0.6b | 4.8 ± 0.1ab | 4.4 ± 0.3ab | 4.5 ± 0.6ab | 4.9 ± 1.0ab | 3.6 ± 0.1a | 4.0 ± 0.1a | 5.3 ± 0.7ab |
| A5 | Salty taste | 5.5 ± 0.6 | 5.4 ± 0.3 | 5.5 ± 0.1 | 6.4 ± 0.2 | 5.4 ± 0.2 | 6.4 ± 0.4 | 5.2 ± 0.2 | 5.9 ± 0.1 | 5.5 ± 0.5 |
| A6 | Sweet taste | 3.1 ± 0.8ab | 1.4 ± 0.1a | 3.4 ± 0.1b | 2.2 ± 0.3ab | 1.6 ± 0.1ab | 1.5 ± 0.3ab | 3.2 ± 0.9ab | 1.6 ± 0.3ab | 3.3 ± 0.6a |
| A7 | Smoky aroma | 3.2 ± 0.8 | 2.1 ± 1.6 | 5.0 ± 1.4 | 5.4 ± 0.8 | 2.9 ± 0.1 | 4.4 ± 0.2 | 4.6 ± 0.1 | 6.0 ± 1.2 | 4.1 ± 0.4 |
| A8 | Hardness | 4.7 ± 0.2 | 3.7 ± 1.8 | 4.5 ± 0.4 | 4.6 ± 0.5 | 4.2 ± 0.2 | 4.8 ± 0.1 | 4.4 ± 0.3 | 4.5 ± 0.3 | 6.1 ± 0.1 |
| A9 | Overall acceptability | 8.1 ± 0.3 | 7.3 ± 0.1 | 7.9 ± 0.4 | 7.5 ± 0.1 | 7.7 ± 0.2 | 7.6 ± 0.3 | 7.8 ± 0.4 | 7.7 ± 0.3 | 7.1 ± 0.1 |
* Results are expressed as mean ± SE. Different letters (a, b) within a same row indicate statistical significant difference (P < 0.05)
Statistical analyses
Data from physical, chemical and sensory analysis as well as volatile compounds of Dalmatian smoked dry-cured ham was analysed by one-way ANOVA (SPSS 12.0 computer programme) using the nine manufacturers as main factor, with the detected differences being tested by the Tukey post hoc test. Tukey test was used when the ANOVA showed a significant effect. Statistical significance was determined at P ≤ 0.05. The results were subjected to principal component analysis (PCA; STATISTICA software 10.0) in order to interpret sensory attributes, volatile compounds and physical–chemical parameters.
Results and discussion
Physical and chemical analysis
There are various factors (pig breed, animal production practices, production methods) which produce considerable differences in qualitative and quantitative aspects of fat, protein levels, presence of salt (sodium), etc., that are responsible for the sensory and nutritional characteristics of hams (Jiménez-Colmenero et al. 2010). Results of physical and chemical analysis in the biceps femoris muscle of Dalmatian smoked dry-cured ham are shown in Table 1. Differences between nine producers for raw hams (raw ham weight, different breed), ripening period (12–18 months) and smoking phase (duration and intensity) of smoked dry-cured ham production has resulted in different physical and chemical composition. The average water content in the Dalmatian smoked dry-cured hams ranged from 37.2 to 48.2 g/100 g which was similar with values for Spanish Iberian (49.0 g/100 g) and Serrano (48.5 g/100 g) dry cured hams (Carrapiso and García 2008) produced with skin and subcutaneous fat. Italian dry-cured hams like Parma and San Daniele had little higher water content (54.1–61.8 g/100 g) (Laureati et al. 2014). In contrast to this Mediterranean dry-cured hams Istrian dry-cured ham has the lowest water content (37.9–41.0 g/100 g) because it is produced without pig’s skin and subcutaneous adipose tissue.
Table 1.
Physico-chemical parameters (mean ± standard error) in the biceps femoris muscle in Dalmatian smoked dry-cured ham from 9 different producers
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | |
|---|---|---|---|---|---|---|---|---|---|
| Water (g/100 g) | 43.3 ± 0.5c | 45.6 ± 0.5b | 44.8 ± 0.6b | 41.3 ± 0.3c | 46.3 ± 0.6ab | 40.0 ± 0.3cd | 46.1 ± 0.2ab | 48.2 ± 0.6a | 37.2 ± 0.2d |
| Protein (g/100 g) | 27.8 ± 0.2e | 27.5 ± 0.0e | 32.4 ± 0.1cd | 36.8 ± 0.1b | 32.9 ± 0.2bcd | 40.2 ± 0.5a | 27.9 ± 1.2e | 30.9 ± 0.4de | 35.2 ± 0.0bc |
| Fat (g/100 g) | 17.5 ± 0.5a | 15.2 ± 1.4bc | 12.5 ± 0.2bcd | 10.9 ± 0.2cd | 13.0 ± 0.1bcd | 11.6 ± 0.0cd | 14.1 ± 0.1bcd | 12.0 ± 0.1cd | 16.9 ± 0.4ab |
| Ash (g/100 g) | 7.2 ± 0.0de | 8.7 ± 0.1bc | 6.9 ± 0.3de | 10.0 ± 0.1ab | 6.8 ± 0.1e | 9.3 ± 0.1abc | 8.2 ± 0.0cd | 8.9 ± 0.1abc | 10.2 ± 0.2a |
| NaCl (%) | 7.5 ± 0.0 | 8.5 ± 0.2 | 7.6 ± 0.0 | 9.3 ± 0.4 | 7.5 ± 0.0 | 8.8 ± 0.2 | 8.2 ± 0.1 | 9.1 ± 0.1 | 9.8 ± 0.2 |
| mg MDA/kg sample | 0.4 ± 0.0d | 0.8 ± 0.0e | 0.3 ± 0.0c | 0.2 ± 0.0a | 0.4 ± 0.0d | 0.2 ± 0.0b | 0.7 ± 0.0e | 0.4 ± 0.0d | 0.8 ± 0.0e |
| aw | 0.83 ± 0.0ab | 0.83 ± 0.0ab | 0.84 ± 0.0a | 0.85 ± 0.0a | 0.85 ± 0.0a | 0.83 ± 0.0ab | 0.85 ± 0.0a | 0.84 ± 0.0a | 0.78 ± 0.0b |
* Different letters (a–f) within a same row indicate statistical significant difference (P < 0.05)
Dry-cured ham is an excellent source of high-biological-value proteins because it contains essential amino acids in appropriate ratios. Dalmatian smoked dry-cured ham showed protein content of 27.9–42.2 g/100 g which was relatively high and consistent with protein content in other types of dry-cured ham (30 g/100 g) depending on the extent of drying and the fat content (Toldrá 2002).
Different types of hams had varied fat content. The difference may be due to use of different breed of pig and feed composition. Fat content in Dalmatian smoked dry-cured ham varied from different producers and was 9.6–17.5 g/100 g. Fat content of Spanish Serrano dry- cured ham was 12.0 g/100 g (Gilles 2009) while Italian Parma (18.4 g/100 g) and San Daniele (23.0 g/100 g) dry-cured hams showed little higher fat values (D’Evoli et al. 2009). Average ash content of Dalmatian smoked dry-cured ham was 6.8–10.2 g/100 g arising from the high salt content. Salt content of Dalmatian smoked dry-cured ham was 7.5–9.8% which was higher than salt content reported for other types of dry cured ham like Spanish Iberian ham had 4.0–5.9% (Martín et al. 1998), French Bayonne ham showed 5.4–7.7% (Santé-Lhoutellier et al. 2012) and Italian Parma and San Daniele ham had 4.5–6.9% (Laureati et al. 2014). Based on these results producers are now decreasing salt content in Dalmatian smoked dry-cured ham (unpublished data) to have more uniform product with the highest salt content of 7.5% which now has Protected Geographical Indication-PGI. Lower salt in meat products is preferred, because a high proportion of NaCl is associated with cardiovascular diseases (Andrés et al. 2004). Salt has many positive effects on the dry-cured ham; microbiological stability by reducing water activity, a pleasant savoury taste and partial solubility and cohesiveness of myofibrillar proteins, it is essential to reduce the proportion of NaCl without affecting the drying process because the salt is an important inhibitor of the most muscle proteases (Armenteros et al. 2012).
Biceps femoris of Dalmatian smoked dry-cured ham had a water activity of 0.78–0.85. Similar values were found for Iberian dry-cured ham (0.86–0.88) (Carrapiso and García 2008). Water activity is the most important for controlling spoilage. The growth of most bacteria (aw < 0.91) and moulds (aw < 0.80) is reduced at low water activity. Dry-cured ham with the low water activity can be kept at room temperature.
These results were used as a guide for getting Protected Geographical Indication-PGI and today Dalmatian smoked dry-cured ham which has PGI must have 40–55% content of water, water activity <0.93 and NaCl content 4.5–7.5%.
The TBARS value was 0.2–0.8 mg MDA/kg sample. Results were comparable with results of Iberian dry-cured ham (0.4–0.5 mg MDA/kg sample) (Andrés et al. 2004).
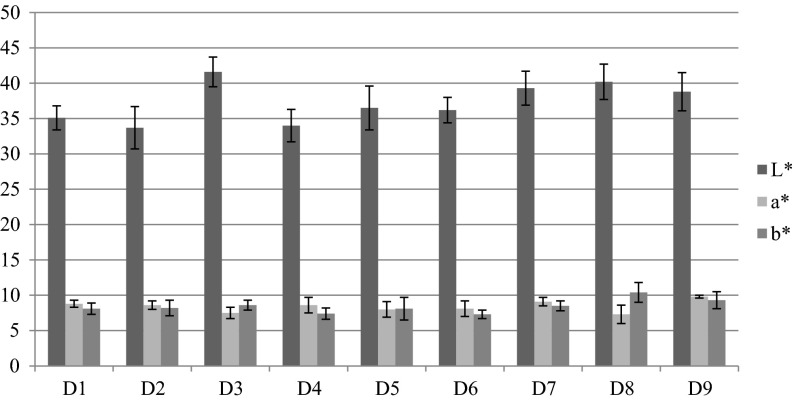
The formation of colour in smoked products was due to chemical and physical actions that occured during the smoking process. Among these, the most important were adhesion of smoke colouring compounds, polymerization and oxidation of the smoke components (e.g. phenols, aldehydes) and the reaction of compounds in the smoke with proteins, particularly between smoke carbonyl groups and amino groups of proteins on the food surface. Colour parameters (L* a* b* values) of Dalmatian smoked dry-cured ham were also evaluated (Fig. 1). L* value was 33.7–41.6 which was little higher than L* values in Spanish (34.8–38.8) (Pérez-Alvarez et al. 1998) and Italian (37.9–38.0) (Laureati et al. 2014) dry-cured hams. Higher L* value could be due to smoking phase of production process which affected colour of dry-cured ham so smoked dry-cured hams were darker than non-smoked. a* values of Dalmatian smoked dry-cured ham was lower (7.3–9.8) than values of biceps femoris reported for Spanish Iberian and Serrano (16.6–18.9) and Italian Parma and San Daniele (15.9–17.7) dry-cured hams. Spanish dry-cured ham had intense red colour (higher a* values) than Dalmatian smoked dry-cured ham because of addition of nitrates and nitrites in these types of dry-cured hams. The use of nitrites seems to improve the colour (higher intensity of the red colour and brightness in the lean and lower dryness) and the odour (intensity and typical odour of cured meats attributes) of the final product (Toldrá et al. 2009). b* values for Dalmatian smoked dry-cured ham was 7.3–10.4 which was similar to the values reported for Spanish and Italian dry-cured hams.
Fig. 1.
Colour (L*a*b* values) of the biceps femoris muscle in Dalmatian smoked dry-cured ham from 9 different producers (D1–D9)
Analysis of volatile compounds
A total of 87 volatile compounds were found in headspace of Dalmatian smoked dry-cured ham (Table 2). Chemical groups identified were aldehydes (35.6%), phenols (34.3%), alcohols (13.8%), terpenes (6.4%), aromatic hydrocarbons (2.6%), alkanes (2.2%), ketones (2.2%), esters (1.7%) and acids (0.7%) (Table 3). Aldehydes and phenols were the major group of compounds. Based on variations in physical and chemical composition, the difference in the composition of the volatile compounds from nine different producers of Dalmatian smoked dry-cured hams were expected. For example, samples with higher NaCl content had lower content of aldehydes (D4, D8 and D9); longer smoking phase resulted in higher content of phenols (D3, D4, and D8).
Table 2.
Contents of volatile compounds extracted in Dalmatian smoked dry-cured ham from 9 different producers (percentage of the total area)
| Volatile compound | RI | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | Identification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||||
| 3-Methylbutanal | 700 | 0.7 ± 0.1 | 0.8 ± 0.3 | 0.3 ± 0.0 | 0.7 ± 0.0 | 0.4 ± 0.0 | 0.9 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 1.0 ± 0.0 | MS, RI |
| Hexanal | 804 | 6.7 ± 0.0d | 11.1 ± 0.4f | 1.5 ± 0.0a | 1.7 ± 0.1a | 8.8 ± 0.3e | 3.9 ± 0.1bc | 2.3 ± 0.1ab | 3.3 ± 0.2ab | 5.3 ± 0.4cd | MS, RI |
| Heptanal | 904 | 2.2 ± 0.1c | 4.4 ± 0.2d | 0.7 ± 0.1a | 0.7 ± 0.0a | 4.3 ± 0.1d | 1.7 ± 0.1bc | 1.1 ± 0.0ab | 1.3 ± 0.0ab | 1.1 ± 0.1ab | MS, RI |
| Benzaldehyde | 967 | 2.6 ± 1.3 | 6.3 ± 0.6 | 3.7 ± 0.1 | 3.9 ± 0.3 | 5.5 ± 0.3 | 7.1 ± 0.0 | 3.7 ± 0.2 | 5.2 ± 0.3 | 6.5 ± 0.2 | MS, RI |
| 2.4-Heptadienal | 997 | 0.9 ± 0.0 | 0.8 ± 0.1 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.9 ± 0.1 | 0.7 ± 0.1 | MS, RI |
| Octanal | 1005 | 3.6 ± 0.3ab | 9.4 ± 0.1d | 3.8 ± 0.0ab | 3.4 ± 0.0a | 8.5 ± 0.0d | 6.7 ± 0.1c | 4.8 ± 0.1b | 3.6 ± 0.2ab | 3.0 ± 0.0a | MS, RI |
| Unknown aldehyde | 1063 | 2.2 ± 0.2 | 2.5 ± 0.3 | 1.0 ± 0.0 | 1.3 ± 0.1 | 2.3 ± 0.0 | 1.6 ± 0.0 | 1.5 ± 0.1 | 1.1 ± 0.0 | 1.9 ± 0.3 | MS, RI |
| Nonanal | 1106 | 4.2 ± 0.0a | 8.6 ± 2.0b | 5.2 ± 0.6ab | 3.2 ± 0.0a | 8.2 ± 0.0b | 6.1 ± 0.4ab | 6.8 ± 0.3ab | 3.3 ± 0.4a | 4.9 ± 0.1ab | MS, RI |
| 2-Nonenal | 1162 | 1.3 ± 0.1 | 1.5 ± 0.4 | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.8 ± 0.4 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | MS, RI |
| Decanal | 1206 | 0.7 ± 0.0d | 0.4 ± 0.0abc | 0.5 ± 0.1abc | 0.2 ± 0.0a | 0.4 ± 0.0abc | 0.6 ± 0.1bc | 0.4 ± 0.0abc | 0.3 ± 0.0ab | 0.5 ± 0.0abc | MS, RI |
| 2.4-Nonadienal | 1216 | 0.6 ± 0.0c | 0.3 ± 0.0abc | 0.3 ± 0.0ab | 0.5 ± 0.0bc | 0.4 ± 0.0bc | 0.4 ± 0.0abc | 0.8 ± 0.0d | 0.1 ± 0.0a | 0.4 ± 0.0bc | MS, RI |
| Undecanal | 1319 | 1.7 ± 0.3 | 1.0 ± 0.6 | 0.8 ± 0.0 | 0.7 ± 0.0 | 2.2 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.0 | 0.7 ± 0.0 | 1.4 ± 0.0 | MS, RI |
| 2E-Decanal | 1264 | 2.0 ± 0.3 | 2.8 ± 0.8 | 1.1 ± 0.0 | 0.5 ± 0.0 | 2.3 ± 0.0 | 1.9 ± 0.1 | 3.1 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.0 | MS, RI |
| Benzeneacetaldehyde | 1268 | 0.2 ± 0.0a | 0.3 ± 0.0a | 0.3 ± 0.0a | 5.4 ± 0.4b | 0.3 ± 0.0a | 0.3 ± 0.0a | 0.3 ± 0.0a | 0.2 ± 0.0a | 0.3 ± 0.0a | MS, RI |
| Decadienal | 1294 | 0.7 ± 0.0b | 0.4 ± 0.2ab | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.4 ± 0.0ab | 0.1 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| Tetradecanal | 1613 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | MS, RI |
| Pentadecanal | 1715 | 0.2 ± 0.0abc | 0.2 ± 0.0bc | 0.2 ± 0.0abc | 0.1 ± 0.0ab | 0.3 ± 0.0c | 0.2 ± 0.0abc | 0.2 ± 0.0abc | 0.2 ± 0.0abc | 0.1 ± 0.0a | MS, RI |
| Hexadekanal | 1818 | 3.0 ± 0.1ab | 4.5 ± 0.7ab | 3.7 ± 0.3ab | 2.3 ± 0.1a | 5.4 ± 0.0b | 3.8 ± 0.2ab | 3.4 ± 0.0ab | 3.5 ± 0.3ab | 1.9 ± 0.1a | MS, RI |
| Unknown aldehyde | 1824 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | MS, RI |
| Octadecanal | 1924 | 0.3 ± 0.0ab | 0.4 ± 0.0b | 0.3 ± 0.0ab | 0.2 ± 0.0a | 0.6 ± 0.0c | 0.3 ± 0.0ab | 0.2 ± 0.0ab | 0.3 ± 0.0ab | 0.2 ± 0.0a | MS, RI |
| Heptadecanal | 2015 | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.2 ± 0.0b | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.1 ± 0.0a | MS, RI |
| Phenols | |||||||||||
| 2-Methylphenol | 1058 | 4.2 ± 0.1c | 1.0 ± 0.1a | 4.6 ± 0.1c | 4.6 ± 0.0c | 1.5 ± 0.0a | 3.0 ± 0.1b | 6.1 ± 0.1d | 4.1 ± 0.3bc | 3.5 ± 0.0bc | MS, RI |
| 3-Methylphenol (m-cresol) | 1079 | 8.0 ± 0.2d | 2.4 ± 0.4ab | 10.2 ± 0.1e | 8.8 ± 0.1de | 3.3 ± 0.0b | 6.1 ± 0.1c | 1.4 ± 0.0a | 8.0 ± 0.4d | 8.3 ± 0.1d | MS, RI |
| 4-Methylphenol | 1085 | 5.1 ± 0.1b | 4.1 ± 0.6b | 10.4 ± 0.2de | 9.8 ± 0.0cd | 6.6 ± 0.0bc | 8.5 ± 0.6cd | 0.0 ± 0.0a | 13.1 ± 0.7e | 8.6 ± 0.3cd | MS, RI |
| 2-Methoxyphenol (guaiacol) | 1090 | 0.4 ± 0.0ab | 0.2 ± 0.0a | 0.4 ± 0.0bc | 0.5 ± 0.0bc | 0.3 ± 0.0ab | 0.4 ± 0.0abc | 1.1 ± 0.0d | 0.9 ± 0.0d | 0.6 ± 0.0c | MS, RI |
| Phenol | 1137 | 0.6 ± 0.0bcd | 0.2 ± 0.0a | 0.6 ± 0.0bcd | 0.7 ± 0.0d | 0.2 ± 0.0a | 0.3 ± 0.0ab | 0.4 ± 0.0abc | 0.6 ± 0.0cd | 0.6 ± 0.0bcd | MS, RI |
| 2.3-Dimethylphenol | 1146 | 0.7 ± 0.1bc | 0.2 ± 0.0a | 0.3 ± 0.0a | 1.2 ± 0.0d | 0.3 ± 0.0ab | 0.3 ± 0.0a | 0.7 ± 0.0c | 1.6 ± 0.0e | 0.6 ± 0.1abc | MS, RI |
| 2.5-Dimethylphenol | 1149 | 0.9 ± 0.0bc | 0.2 ± 0.0a | 1.2 ± 0.0c | 1.4 ± 0.1d | 0.4 ± 0.0a | 0.5 ± 0.1ab | 1.5 ± 0.1d | 0.9 ± 0.1bc | 0.9 ± 0.0bc | MS, RI |
| 3-Ethylphenol | 1167 | 0.5 ± 0.0abc | 0.3 ± 0.0ab | 0.9 ± 0.0d | 0.7 ± 0.0cd | 0.3 ± 0.1ab | 0.3 ± 0.0a | 0.9 ± 0.0d | 0.6 ± 0.0bc | 0.5 ± 0.0abc | MS, RI |
| p-Methylguaiacol | 1176 | 1.1 ± 0.0 | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.2 | 1.1 ± 0.1 | MS, RI |
| 2-Metoxy-5-methylphenol | 1183 | 1.7 ± 0.0 | 1.5 ± 0.0 | 1.6 ± 0.0 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.8 ± 0.0 | 1.2 ± 0.2 | 1.9 ± 0.0 | MS, RI |
| 2-Metoxy-4-methylphenol | 1188 | 3.9 ± 0.2cd | 1.5 ± 0.2ab | 7.6 ± 0.0e | 9.3 ± 0.3f | 2.5 ± 0.0bc | 4.0 ± 0.1cd | 0.0 ± 0.0a | 7.1 ± 0.4e | 5.4 ± 0.0d | MS, RI |
| 3.4-Dimethylphenol | 1191 | 0.7 ± 0.0b | 0.2 ± 0.0a | 0.4 ± 0.0ab | 0.7 ± 0.0ab | 0.2 ± 0.0ab | 0.2 ± 0.0ab | 12.9 ± 0.1c | 0.4 ± 0.0ab | 0.5 ± 0.0ab | MS, RI |
| 2.3.6-Trimethylphenol | 1231 | 0.2 ± 0.0a | 0.0 ± 0.0a | 0.3 ± 0.0a | 1.7 ± 0.1b | 0.0 ± 0.0a | 0.2 ± 0.0a | 0.3 ± 0.1a | 0.2 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| 4-Ethyl-2-methoxyphenol | 1273 | 1.6 ± 0.1bc | 0.5 ± 0.0ab | 3.2 ± 0.0d | 0.2 ± 0.0a | 0.7 ± 0.0ab | 1.3 ± 0.2abc | 4.6 ± 0.3e | 2.7 ± 0.3cd | 2.4 ± 0.1cd | MS, RI |
| 2-Methylpropylphenol | 1316 | 0.6 ± 0.0c | 0.3 ± 0.1ab | 0.4 ± 0.0ab | 0.4 ± 0.0abc | 0.5 ± 0.0abc | 0.3 ± 0.0a | 0.7 ± 0.0bc | 0.3 ± 0.0ab | 0.4 ± 0.0ab | MS, RI |
| 2.6-Dimethoxyphenol | 1346 | 2.6 ± 0.0b | 0.4 ± 0.0a | 3.5 ± 0.0cd | 0.7 ± 0.0a | 0.1 ± 0.0a | 4.9 ± 0.0e | 4.4 ± 0.1de | 2.9 ± 0.2bc | 2.5 ± 0.1b | MS, RI |
| Alcohols | |||||||||||
| 1-Pentanol | 775 | 0.5 ± 0.0b | 0.9 ± 0.0c | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.6 ± 0.0b | 0.2 ± 0.0a | 0.1 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| 2-Furanmethanol | 857 | 0.6 ± 0.1abc | 0.1 ± 0.0a | 1.4 ± 0.0d | 1.1 ± 0.0cd | 0.4 ± 0.1ab | 1.0 ± 0.1bcd | 1.2 ± 0.0cd | 1.2 ± 0.0cd | 0.5 ± 0.2abc | MS, RI |
| 1-Hexanol | 875 | 0.1 ± 0.0 | 0.9 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | MS, RI |
| 1-Butoxy-2-propanol | 950 | 0.1 ± 0.0ab | 0.2 ± 0.0a | 0.1 ± 0.0ab | 0.1 ± 0.0ab | 0.1 ± 0.0ab | 0.2 ± 0.0ab | 0.1 ± 0.0ab | 0.2 ± 0.0b | 0.1 ± 0.0ab | MS, RI |
| Unknown alcohol | 957 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.3 | 0.1 ± 0.0 | 0.2 ± 0.1 | MS, RI |
| 1-Octen-3-ol | 987 | 8.8 ± 0.3ab | 8.1 ± 0.4ab | 9.0 ± 0.3ab | 6.6 ± 0.0a | 10.0 ± 0.4b | 8.6 ± 0.4ab | 8.5 ± 0.0ab | 8.2 ± 0.1ab | 8.0 ± 0.2ab | MS, RI |
| Benzylalcohol | 1037 | 1.0 ± 0.0ab | 0.9 ± 0.2a | 3.9 ± 0.2d | 2.0 ± 0.0bc | 0.7 ± 0.0a | 1.2 ± 0.0ab | 2.6 ± 0.0c | 2.9 ± 0.0cd | 3.1 ± 0.3cd | MS, RI |
| 1-Octenol | 1074 | 0.5 ± 0.0ab | 0.3 ± 0.0ab | 0.3 ± 0.0ab | 0.4 ± 0.0a | 0.5 ± 0.0b | 0.5 ± 0.0ab | 0.5 ± 0.0ab | 1.0 ± 0.2ab | 0.7 ± 0.0ab | MS, RI |
| Phenylethyl alcohol | 1113 | 0.4 ± 0.0abc | 0.8 ± 0.1bc | 0.1 ± 0.0a | 0.8 ± 0.0bc | 0.6 ± 0.0abc | 0.6 ± 0.0abc | 0.9 ± 0.0bc | 1.0 ± 0.0c | 0.3 ± 0.2ab | MS, RI |
| Dodecanol | 1255 | 0.2 ± 0.0a | 0.3 ± 0.0b | 0.1 ± 0.0a | 1.2 ± 0.0c | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.2 ± 0.0a | 0.1 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| Tetradecanol | 1484 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | MS, RI |
| Octadekanol | 1997 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | MS, RI |
| Terpenes | |||||||||||
| α-Thujene | 934 | 0.1 ± 0.0ab | 0.1 ± 0.0cd | 0.1 ± 0.0ad | 0.1 ± 0.0a | 0.1 ± 0.0bcd | 0.1 ± 0.0abc | 0.2 ± 0.0d | 0.0 ± 0.0a | 0.0 ± 0.0a | MS, RI |
| Sabinene | 979 | 0.8 ± 0.0b | 1.5 ± 0.1c | 0.2 ± 0.0a | 0.2 ± 0.0a | 1.7 ± 0.1c | 0.4 ± 0.0ab | 0.2 ± 0.0a | 0.4 ± 0.0ab | 0.4 ± 0.0ab | MS, RI |
| β-Pinene | 983 | 0.5 ± 0.0cde | 0.5 ± 0.0def | 0.2 ± 0.0ab | 0.3 ± 0.0abc | 0.7 ± 0.0ef | 0.7 ± 0.0f | 0.4 ± 0.0bcd | 0.4 ± 0.0bcd | 0.2 ± 0.0a | MS, RI |
| β-Myrcene | 995 | 0.6 ± 0.0b | 0.8 ± 0.1b | 0.7 ± 0.0b | 0.6 ± 0.0b | 0.0 ± 0.0a | 0.8 ± 0.0b | 0.9 ± 0.0b | 0.9 ± 0.0b | 0.7 ± 0.0b | MS, RI |
| o-Cymene | 1025 | 0.3 ± 0.1a | 0.2 ± 0.0a | 0.6 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.7 ± 0.0ab | 0.4 ± 0.1a | 1.3 ± 0.2b | 0.6 ± 0.0a | MS, RI |
| d-Limonene | 1032 | 1.2 ± 0.0bc | 1.1 ± 0.3bc | 0.3 ± 0.1ab | 0.3 ± 0.0a | 1.8 ± 0.0c | 0.8 ± 0.0ab | 0.4 ± 0.1ab | 0.4 ± 0.0ab | 0.5 ± 0.0ab | MS, RI |
| Linalool | 1100 | 0.4 ± 0.0ab | 0.4 ± 0.0ab | 0.3 ± 0.0a | 0.3 ± 0.0a | 0.4 ± 0.0a | 0.6 ± 0.0b | 0.5 ± 0.1ab | 0.4 ± 0.0a | 0.7 ± 0.0b | MS, RI |
| β-Fenchyl alcohol | 1195 | 0.3 ± 0.0c | 0.3 ± 0.0c | 0.2 ± 0.0bc | 0.1 ± 0.0b | 0.3 ± 0.0bc | 0.3 ± 0.0bc | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.3 ± 0.0c | MS, RI |
| Piperidine | 1197 | 0.2 ± 0.0a | 0.3 ± 0.0a | 0.1 ± 0.0a | 0.5 ± 0.0b | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.3 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| d-Elemene | 1338 | 0.3 ± 0.0a | 0.3 ± 0.0a | 0.1 ± 0.0a | 5.5 ± 0.2b | 0.3 ± 0.0a | 0.1 ± 0.0a | 0.2 ± 0.0a | 0.1 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| α-Copaene | 1377 | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.6 ± 0.0b | 0.1 ± 0.0a | 0.3 ± 0.0a | 0.2 ± 0.0a | 0.1 ± 0.0a | 0.3 ± 0.0a | MS, RI |
| Italicene | 1396 | 0.5 ± 0.0bcd | 0.3 ± 0.0abc | 0.2 ± 0.0ab | 0.5 ± 0.0cd | 0.2 ± 0.0a | 0.4 ± 0.0abcd | 0.4 ± 0.0abcd | 0.4 ± 0.0abcd | 0.6 ± 0.0d | MS, RI |
| β-Carryophyllene | 1422 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.7 ± 0.4 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | MS, RI |
| α-Guaiene | 1440 | 0.7 ± 0.1c | 0.1 ± 0.0a | 0.8 ± 0.0cd | 0.1 ± 0.0ab | 0.1 ± 0.0ab | 1.0 ± 0.1d | 0.8 ± 0.0cd | 0.4 ± 0.0bc | 0.7 ± 0.0cd | MS, RI |
| α-Carryophyllene | 1459 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | MS, RI |
| β-Selinene | 1490 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | MS, RI |
| β-Bisabolene | 1511 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | MS, RI |
| Aromatic hydrocarbons | |||||||||||
| 1.2-Dimetoxybenzene | 1151 | 1.3 ± 0.0de | 0.4 ± 0.0ab | 1.1 ± 0.0cde | 1.5 ± 0.1e | 0.2 ± 0.2ab | 0.6 ± 0.0bc | 0.0 ± 0.0a | 1.0 ± 0.1cde | 1.0 ± 0.0cd | MS, RI |
| 2.4-Dimetoxytoluene | 1237 | 0.8 ± 0.0cd | 0.4 ± 0.0b | 0.0 ± 0.0a | 0.6 ± 0.0bc | 0.0 ± 0.0a | 0.6 ± 0.0bc | 1.0 ± 0.0de | 1.2 ± 0.0e | 1.1 ± 0.0de | MS, RI |
| 3.4-Dimetoxytoluene | 1241 | 0.4 ± 0.0c | 0.2 ± 0.0a | 0.3 ± 0.0bc | 0.1 ± 0.0a | 0.2 ± 0.0a | 0.3 ± 0.0ab | 0.8 ± 0.0d | 0.5 ± 0.0c | 0.4 ± 0.0bc | MS, RI |
| 1.2.3-Trimetoxybenzene | 1306 | 0.6 ± 0.0bc | 0.2 ± 0.1a | 0.4 ± 0.0abc | 0.6 ± 0.0c | 0.3 ± 0.0ab | 0.2 ± 0.0a | 0.4 ± 0.0abc | 0.6 ± 0.0c | 0.6 ± 0.0bc | MS, RI |
| 1.2.4-Trimetoxybenzene | 1336 | 0.1 ± 0.0b | 0.0 ± 0.0a | 0.1 ± 0.0b | 0.2 ± 0.0b | 0.1 ± 0.0b | 0.1 ± 0.0b | 0.2 ± 0.0b | 0.1 ± 0.0b | 0.2 ± 0.0b | MS, RI |
| g-Nonalactone | 1362 | 0.3 ± 0.0bc | 0.3 ± 0.0bc | 0.3 ± 0.0bc | 0.4 ± 0.0c | 0.0 ± 0.0a | 0.1 ± 0.0c | 0.3 ± 0.0bc | 0.2 ± 0.0ab | 0.3 ± 0.0bc | MS, RI |
| Alkanes | |||||||||||
| Heptane | 720 | 1.0 ± 0.1 | 0.8 ± 0.4 | 0.0 ± 0.0 | 0.3 ± 0.0 | 1.2 ± 0.1 | 0.5 ± 0.0 | 0.3 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | MS, RI |
| Dodecane | 1201 | 0.3 ± 0.0ab | 0.2 ± 0.0a | 0.5 ± 0.0bc | 0.3 ± 0.0ab | 0.3 ± 0.1ab | 0.6 ± 0.0c | 0.3 ± 0.0ab | 0.4 ± 0.0abc | 0.6 ± 0.0c | MS, RI |
| Tridecane | 1300 | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.1 ± 0.0a | 1.1 ± 0.1b | 0.1 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.1 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| Tetradecane | 1400 | 0.3 ± 0.0ab | 0.3 ± 0.0ab | 0.4 ± 0.0abc | 0.2 ± 0.0a | 0.3 ± 0.0ab | 0.5 ± 0.0bc | 0.3 ± 0.0ab | 0.3 ± 0.0ab | 0.7 ± 0.0c | MS, RI |
| Pentadecane | 1502 | 0.7 ± 0.1 | 0.8 ± 0.0 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.8 ± 0.1 | 0.3 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.1 | MS, RI |
| Hexadecane | 1600 | 0.2 ± 0.0ab | 0.3 ± 0.0b | 0.1 ± 0.0ab | 0.1 ± 0.0ab | 0.2 ± 0.0ab | 0.2 ± 0.0ab | 0.1 ± 0.0ab | 0.1 ± 0.0a | 0.2 ± 0.0ab | MS, RI |
| Ketones | |||||||||||
| 2-Heptanone | 892 | 0.3 ± 0.0a | 0.9 ± 0.2b | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.4 ± 0.0a | 0.3 ± 0.0a | 0.2 ± 0.0a | 0.3 ± 0.0a | 0.4 ± 0.0a | MS, RI |
| 2.3-Octadienone | 991 | 1.1 ± 0.0 | 1.2 ± 0.2 | 0.9 ± 0.0 | 0.7 ± 0.2 | 1.6 ± 0.0 | 1.3 ± 0.3 | 0.8 ± 0.0 | 1.3 ± 0.1 | 1.5 ± 0.0 | MS, RI |
| 2-Nonanone | 1093 | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.7 ± 0.0b | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.3 ± 0.0a | 1.3 ± 0.0c | 0.3 ± 0.0a | 0.2 ± 0.0a | MS, RI |
| 2.5-Cyclohexadiene-1.4-dione | 1462 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.1 | MS, RI |
| Esters | |||||||||||
| Hexyl hexanoate | 1352 | 0.3 ± 0.0abc | 0.2 ± 0.0a | 0.4 ± 0.0abc | 0.6 ± 0.0c | 0.2 ± 0.0a | 0.2 ± 0.0ab | 0.5 ± 0.0bc | 0.3 ± 0.0abc | 0.3 ± 0.1abc | MS, RI |
| Isohexyl hexanoate | 1371 | 1.5 ± 0.1cd | 0.4 ± 0.0a | 2.1 ± 0.1de | 0.2 ± 0.0a | 1.3 ± 0.2bc | 0.6 ± 0.0ab | 2.4 ± 0.2e | 0.3 ± 0.0a | 2.5 ± 0.1e | MS, RI |
| Dodecenyl acetate | 1475 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | MS, RI |
| Acids | |||||||||||
| Octanoic acid | 1179 | 0.7 ± 0.0bc | 1.2 ± 0.1d | 0.6 ± 0.0bc | 0.0 ± 0.0a | 0.8 ± 0.0bc | 0.9 ± 0.1cd | 0.5 ± 0.0b | 0.5 ± 0.0bc | 0.7 ± 0.0bc | MS, RI |
| Hexadecanoic acid | 1964 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | MS, RI |
* Results are expressed as mean ± SE. Different letters (a–f) within a same row indicate statistical significant difference (P < 0.05). Compounds stated in bold are significant compounds within class
Table 3.
Chemical groups of volatile compounds extracted in Dalmatian smoked dry-cured ham from 9 different producers (percentage of the total area)
| Chemical group | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | Average |
|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | 34.3 ± 0.0cd | 56.3 ± 1.1e | 25.0 ± 0.8a | 25.9 ± 0.6ab | 52.3 ± 0.2e | 38.3 ± 0.7d | 32.0 ± 0.8c | 25.9 ± 0.2ab | 30.8 ± 0.5bc | 35.6 ± 0.5 |
| Phenols | 32.6 ± 0.7b | 13.8 ± 1.4a | 46.3 ± 0.8d | 43.1 ± 0.9cd | 19.2 ± 0.3a | 32.3 ± 0.4b | 37.7 ± 0.6bc | 45.8 ± 0.0d | 37.8 ± 0.3bc | 34.3 ± 0.6 |
| Alcohols | 12.9 ± 0.5 | 13.0 ± 0.2 | 15.2 ± 0.5 | 12.8 ± 0.1 | 13.6 ± 0.4 | 13.4 ± 0.6 | 14.7 ± 0.2 | 15.3 ± 0.5 | 13.7 ± 0.0 | 13.8 ± 0.4 |
| Terpenes | 6.5 ± 0.2bc | 6.5 ± 0.2bc | 4.5 ± 0.1a | 10.4 ± 0.2d | 6.4 ± 0.2bc | 6.9 ± 0.3c | 5.3 ± 0.1abc | 5.2 ± 0.1ab | 5.7 ± 0.0abc | 6.4 ± 0.2 |
| Aromatic hydrocarbons | 3.5 ± 0.2d | 1.5 ± 0.0ab | 2.2 ± 0.0bc | 3.5 ± 0.1d | 0.8 ± 0.0a | 2.1 ± 0.1bc | 2.6 ± 0.1cd | 3.6 ± 0.1d | 3.5 ± 0.1d | 2.6 ± 0.1 |
| Alkanes | 2.6 ± 0.3 | 2.5 ± 0.4 | 1.8 ± 0.2 | 2.4 ± 0.0 | 2.5 ± 0.0 | 2.8 ± 0.0 | 1.5 ± 0.0 | 1.3 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 |
| Ketones | 2.1 ± 0.1ab | 2.8 ± 0.3b | 1.9 ± 0.0ab | 1.1 ± 0.2a | 2.5 ± 0.1b | 2.2 ± 0.3ab | 2.6 ± 0.0b | 2.0 ± 0.1ab | 2.5 ± 0.0b | 2.2 ± 0.1 |
| Esters | 2.1 ± 0.0bc | 0.7 ± 0.0a | 2.7 ± 0.1cd | 0.8 ± 0.0a | 1.7 ± 0.0b | 1.0 ± 0.1a | 3.1 ± 0.2d | 0.7 ± 0.0a | 3.0 ± 0.0d | 1.7 ± 0.0 |
| Acids | 0.8 ± 0.0b | 1.3 ± 0.1c | 0.6 ± 0.0b | 0.1 ± 0.0a | 0.8 ± 0.0bc | 1.0 ± 0.1bc | 0.5 ± 0.0b | 0.6 ± 0.0b | 0.8 ± 0.0bc | 0.7 ± 0.0 |
* Results are expressed as mean ± SE. Different letters (a–e) within a same row indicate statistical significant difference (P < 0.05)
Lipid oxidation is a major factor that has an impact on the quality and acceptability of meat products (Morrissey et al. 1998). Secondary products of lipid oxidation and major contributors to the flavour of Iberian ham were aldehydes (Ruiz et al. 2002). Aldehydes were the most abundant group of compounds (35.6%) in Dalmatian smoked dry-cured ham. This was comparable to the results of other European dry-cured ham where aldehydes were also the most abundant group of compounds like in San Daniele (31.5%) (Gaspardo et al. 2008) and Istrian (51.4%) (Marušić et al. 2014). The most abundant aldehyde in Dalmatian smoked dry-cured ham was nonanal (3.2–8.6%) followed by octanal (3.0–9.4%), benzaldehyde (2.6–7.1%) hexanal (1.5–11.1%) and hexadecanal (1.9–5.4%) (Table 2). Because aldehydes had a low odour threshold value that contributed significantly to the flavour of dry-cured ham even when present in small amounts. Nonanal comes from the oxidation of oleic acid that is the most abundant unsaturated fatty acid in hams (Pham et al. 2008) and contributes to flavour with sweet and fruity aroma (Nunes et al. 2008). The most abundant saturated aldehydes in Iberian dry-cured ham were octanal, nonanal and hexanal (García-González et al. 2013) which is similar with the results of this study. In general, saturated aldehydes contributed to aroma with sensory descriptors such as green/grassy (hexanal), hamlike/fatty (heptanal) and meat-like/fruity (octanal) although with different intensity depending on the breed (Tjener and Stahnke 2007).
Oxidative deamination via Strecker degradation is a reaction where branched aldehydes are formed. Strecker degradation of amino acids includes oxidative deamination and decarboxylation of α-amino acids in the presence of volatile aldehyde (Sabio et al. 1998). Branched aldehydes present in Dalmatian smoked dry-cured ham were 3-methylbutanal (0.3–1.0%) which was a result of proteolysis and had a flavour associated with nutty, cheese aroma, generally salty flavour notes (Hinrichsen and Pedersen 1995). Except 3-methylbutanal in Dalmatian smoked dry-cured ham 2,4-heptadienal (0.5–0.9%) and 2,4-nonadienal (0.1–0.8%) were also found. In addition to the branched aldehydes, the reaction between amino acids also produced aromatic aldehydes such as benzaldehyde, although the latter can also be formed during lipid oxidation. It contributes substantially to dry-cured ham aroma with a bitter almond sensory note. Benzaldehyde (2.6–7.1%) was one of the most abundant aldehyde in Dalmatian smoked dry-cured ham. This compound has been found in Iberian hams at a very high concentration (García-González et al. 2013). 2-Methylbutanal as well as 3-methylbutanal is found to be the largest contributors to the flavour of Spanish and Italian dry-cured hams (Marušić et al. 2014).
Phenols were the second most abundant group of compounds in the Dalmatian smoked dry-cured ham (34.3%). Phenols and phenolic derivated volatiles were formed primarily due to pyrolysis and oxidation of lignin, at comparatively low temperature (200–400 °C) (Sikorski and Kolakowski 2010). Cold smoking (15–25 °C) is mainly used for flavour and to extend shelf-life due to the antioxidant and antimicrobial effects of smoke compounds. Phenolic compounds adsorbed by the food during processing are mainly responsible for the unique aroma and taste of smoked products. The most important phenolic compounds of smoke were reported to be guaiacol, eugenol, 4-methyl guaiacol, phenol, 2,6-dimetoxyphenol, 4-ethylguaiacol and o-, p- and m-cresol. In Dalmatian smoked dry-cured ham 16 phenols were found. Methoxyphenols are components of great importance for smoke flavour and for their preserving and antioxidant effect. Metoxyphenols and phenols have pungent, cresolic, heavy, burnt and smoky notes (Guillén and Manzanos 2002). The most abundant phenols were: 4-methylphenol (0.0–10.4%), 3-methylphenol (1.4–10.2%), 2-metoxy-4-methylphenol (0.0–9.3%), 2-methylphenol (1.0–6.1%), 2,6-dimethoxyphenol (0.1–4.9%), 4-ethyl-2-methoxyphenol (0.2–4.6%) and 3,4-dimethylphenol (0.2–12.9%). Jerković et al. (2007) found seven phenols in Dalmatian smoked dry-cured ham guaiacol, phenol, o-cresol, m-cresol, 2,5-xylenol, 2,6-xylenol and 2,6-dimethoxyphenol. These compound were also found in this research among others like 4-methylphenol; 2,3-dimethylphenol; 3-ethylphenol etc. (Table 2). SPME extraction showed better extraction of phenols compared to other extraction methods (SE, SDE and NPTD). Phenols have low threshold value so their impact on the flavour of Dalmatian smoked dry-cured ham was significant. Phenols like 4-methyl-2-methoxyphenol, 4-ethyl-2-methoxyphenol, 2-methoxyphenol, 2,6-dimethoxyphenol and 2-furanmethanol characterized American dry-cured ham (Pham et al. 2008). They have sweet, smoky and savory flavours and were also found in this research. Phenols are not only the most classical smoke components but can also originate from the added spices like eugenol (4-allyl-2-metoxyphenol) found in Istrian dry-cured ham that originates from the added spices (pepper, bay leaves and rosemary) (Marušić et al. 2014). The smoky taste was a result of the sensory properties of smoke constituents, mainly numerous phenols and carbonyl compounds, as well as various products of the interactions with proteins and lipids. Some results of experiments point to the crucial role of the fraction of smoke condensates containing guaiacol and its four derivatives, eugenol, phenol, 3 cresols, 4-ethylphenol, 3 xylenols, tyglic acid, and 4 carbonyl compounds (Sikorski and Kolakowski 2010).
Alcohols represented 13.8% of the total area of identified compounds. The most abundant alcohols were: 1-octene-3-ol (6.6–10.0%), benzyl alcohol (0.7–3.9%), phenylethyl alcohol (0.1–1.0%) and 1-octenol (0.3–1.0%). The identified alcohols, linear and branched, are among the main lipid oxidation products. The methyl branched alcohols can also be derived from the Strecker degradation of amino acids. It is known that branched alcohols originate from microbial degradation of the respective branched aldehydes (Muriel et al. 2004). Thus, the formation and release of branched alcohols is affected by the salting conditions due to the antimicrobial activity of NaCl. Thus, a higher production of branched alcohols is observed when NaCl is partially replaced by other formulations.
Alcohols contribute to ham flavour with herbaceous, woody and fatty notes. Because of the high odour threshold of some alcohols (2-propanol, ethanol, 2-methyl-3-buten-2-ol, 2-mehyl propanol, 2-butanol, and nonanol) their impact on aroma has been considered minor. However, the low odour threshold of 1-octen-3-ol indicated that it contributed strong mushroom aroma to almost all the hams. The concentration of this compound significantly arose as the amount of curing salt increases, which explained the differences between samples (García-González et al. 2013). Therefore, 1-octene-3-ol was the most abundant alcohol in Dalmatian smoked dry-cured ham due to the high salt content. Wood smoke contained several aliphatic and aromatic alcohols, including methanol, ethanol, allylalcohol, n-amylalcohol, benzyl alcohol, and phenylethyl alcohol (Sikorski and Kolakowski 2010). So, benzyl alcohol and phenylethyl alcohol found in Dalmatian smoked dry-cured ham were a result of production these types of dry-cured hams during smoking.
1-octene-3-ol, 1-penten-3-ol and pentanol are the most abundant compounds in Corsican dry-cured hams while the branched alcohols (2-methylpropanol, 2- and 3-methylbutanol) and ethanol in French Bayonne and Spanish Serrano dry-cured hams. Italian San Daniele dry-cured ham was characterized with ethanol, isobutanol, 1-propanol and 1-penten-3-ol (Gaspardo et al. 2008).
3-Methyl-1-butanol was by far the most abundant alcohol in Iberian dry-cured ham compared to other breeds. The high concentration of 3-methyl-1-butanol can be due to the activity of the microorganisms present in the ham. Another odour compound whose concentration was higher in Iberian hams was hexanol, which contributed to fruity-green odour perception. Hexanol was also found in Dalmatian smoked dry-cured ham.
Terpenes were resulted from animal feedstuffs, but they mainly varied with spices in dry-cured ham production. Terpenes like α-pinene, β-caryophyllene, 3-carene, limonene, and β-pinene is a result of addition of black pepper like in the production of Bayonne and Corsican hams (Hinrichsen and Pedersen 1995). The most abundant terpenes in Dalmatian smoked dry-cured ham were: d-elemene (0.1–5.5%), d-limonene (0.3–1.8%), β-myrcene (0.0–0.9%), sabinene (0.2–1.7%) and α-guaiene (0.1–0.8%). Terpenes were found in large content (16.4–16.5) in the Istrian dry-cured ham and are derived from the added spices (Marušić et al. 2014).
Ketones present in Dalmatian smoked dry-cured ham were 2,3-octadienone (0.7–1.5%), 2-nonanone (0.2–1.3%), 2,5-cyclohexadiene-1,4-dione (0.2–0.5%) and 2-heptanone (0.1–0.9%). Methyl ketones are produced by lipid oxidation, by means of autoxidation or beta-oxidation of fatty acids. These compounds contribute to dry-cured ham aroma and they are considered as responsible for ham fatty aromas associated with cooked meat and the blue cheese sensory note (García-González et al. 2013). Two methyl ketones (2-heptanone and 2-nonanone) were also present in the Dalmatian smoked dry-cured. The most abundant ketone in French and Spanish dry-cured hams was 2-propanone. Dirinck et al. (1997) had already reported that 2-propanone had the highest concentration among the ketones identified in Iberian dry-cured ham. In general, the concentration of methyl ketones is higher in Iberian hams. Octen-3-one is a remarkable ketone since its very low odour threshold allows contributing to ham aroma, with floral/fresh sensory note, and also distinguishing Iberian from non Iberian hams, the latter having higher concentrations. 2,3-octadienone found in Istrian dry-cured ham was also found in this research.
Esters are found in dry-cured hams at the end of the maturation process and it seems that NaCl concentration affects the ester production through the activation of esterases (Armenteros et al. 2012). Esters can be formed from the interaction of free fatty acids and alcohols by lipid oxidation in the intramuscular tissue so that the higher the content of alcohols, the higher the concentration of esters (Sabio et al. 1998). In Dalmatian smoked dry-cured ham only three esters hexyl hexanoate, isohexyl hexanoate and dodecenyl acetate were found as a result of the antimicrobial activity of sodium chloride to the long curing period (Gaspardo et al. 2008).
Relationship between physico-chemical analysis, volatile compounds and sensory attributes
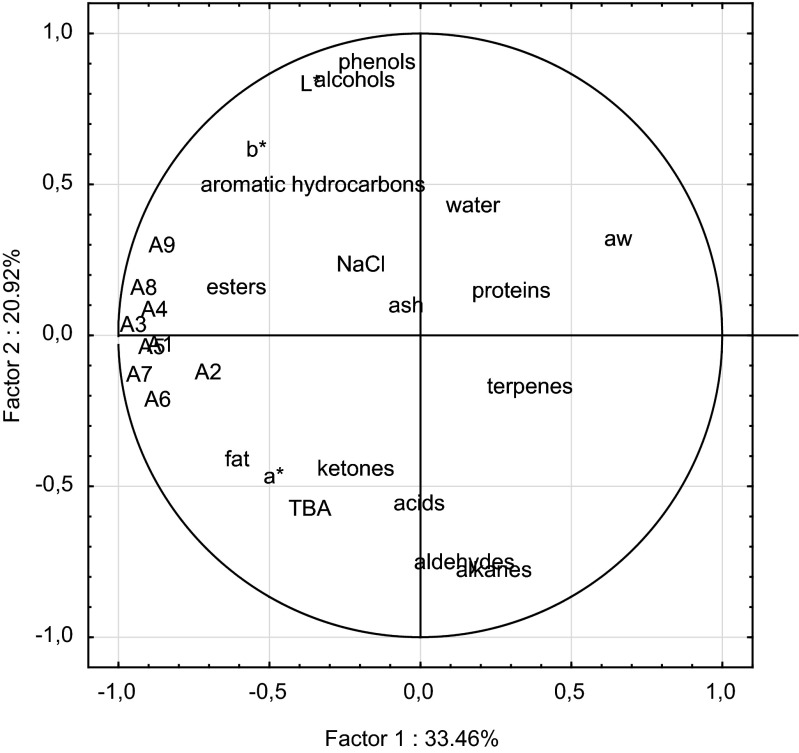
In Table 4 sensory attributes of Dalmatian smoked dry-cured ham are shown. Dalmatian smoked smoked dry-cured hams have high overall acceptability with pronounced smoky aroma. Principal component analysis (PCA) was performed to analyse the whole sensory assessment and to determine the relationship between physico-chemical and sensory parameters and volatile compounds. Data used for PCA analysis was obtained from the physico-chemical, and sensory characterisation as well as chemical groups of volatile compounds of muscle biceps femoris from Dalmatian smoked dry-cured hams. Results are shown in Fig. 2. The two principal components account for 33.46 and 20.92% of the variance, respectively, (54.38% in total).
Fig. 2.
Plot of principal component analysis of the physico–chemical parameters, volatile compounds and sensory attributes of Dalmatian smoked dry-cured ham (codes are in Table 4)
Red colour of the dry-cured ham as well as fat colour were positively correlated with a* value (redness). Water content and aw value were negatively correlated with hardness of dry- cured ham which is to expected; the lower water content results with higher hardness. NaCl content as well and ash content positively correlated with the salty taste. Fat content was in correlation with marbling which means that a larger proportion of fat affects marbling of dry-cured ham. Sweet taste and the presence of esters were positively correlated. Esters have fruity notes especially those that arise from short chain acids (higher concentration of esters, more pronounced sweetness of the products) (Marušić et al. 2014). Phenols corelated with smoky aroma of dry-cured ham. Phenolic compounds are mainly responsible for the unique aroma and taste of smoked products. Phenolic components of the smoke contributed significantly to the aroma of the product.
Conclusion
In all, 87 volatile aroma compounds of Dalmatian smoked dry-cured ham were found. Chemical groups identified were aldehydes (35.6%), phenols (34.3%), alcohols (13.8%), terpenes (6.4%), aromatic hydrocarbons (2.6%), alkanes (2.2%), ketones (2.2%), esters (1.7%) and acids (0.7%). Aldehydes and phenols were the major group of compounds. Except volatile compounds derived from lipolysis and proteolysis the second most abundant constituents were phenols that originate from smoking phase of the production process. The most abundant phenols were: 4-methylphenol (0.0–10.4%), 3-methylphenol (1.4–10.2%), 2-metoxy-4-methylphenol (0.0–9.3%), 2-methylphenol (1.0–6.1%), 2,6-dimethoxyphenol (0.1–4.9%), 4-ethyl-2-methoxyphenol (0.2–4.6%) and 3,4-dimethylphenol (0.2–12.9%). Principal components analysis showed that NaCl and ash content positively correlated with the salty taste while fat content was in correlation with marbling. Water content and aw value were negatively correlated with hardness of dry-cured ham while phenols were positively corelated with smoky aroma. Due to the different volatile composition, Dalmatian smoked dry-cured ham can be distinguished from other types of dry-cured hams.
Future studies regarding sensory, volatile and nutritional characteristics in smoked dry-cured ham compared with other types of dry-cured ham will be carried out.
Acknowledgements
The authors would like to thank the “Producers association of the Istrian dry cured ham” and the International fair of dry cured hams (www.isap.hr) for the samples of Dalmatian smoked dry-cured ham.
References
- Andrés AI, Cava R, Ventanas J, Thovar V, Ruiz J. Sensory characteristics of Iberian ham: influence of salt content and processing conditions. Meat Sci. 2004;68:45–51. doi: 10.1016/j.meatsci.2003.08.019. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. Washington, DC: Association of Official Analytical Chemists; 1984. [Google Scholar]
- AOAC (1999) Meat and meat products, vol. II. In: Cunniff P (ed). Official method 950.46 moisture in meat; official method 991.36 fat (crude) in meat and meat products; official method 981.10 crude protein in meat; official method 920.153 ash in meat. Official methods of analysis of the AOAC International, Gaithersburg, MD, USA, pp 1–15
- Armenteros M, Toldrá F, Aristoy MC, Ventanas J, Estevez M. Effect of the partial replacement of sodium chloride by other salts on the formation of volatile compounds during ripening of dry-cured ham. J Agric Food Chem. 2012;60:7607–7615. doi: 10.1021/jf3013772. [DOI] [PubMed] [Google Scholar]
- Carrapiso AI, García C. Effect of the Iberian pig line on dry-cured ham characteristics. Meat Sci. 2008;80:529–534. doi: 10.1016/j.meatsci.2008.02.004. [DOI] [PubMed] [Google Scholar]
- CIE, Commission Internationale de l’Eclairage (1976) Official recommendations on uniform colour spaces, colour differences equations and metric colour terms. Paris, France
- Comi G, Iacumin L. Ecology of moulds during the pre-ripening and ripening of San Daniele dry cured ham. Food Res Int. 2013;54:1113–1119. doi: 10.1016/j.foodres.2013.01.031. [DOI] [Google Scholar]
- D’Evoli L, Lucarini M, Nicoli S, Aguzzi A, Gabrielli P, Lombardi-Boccia G (2009) Nutritional profile of traditional Italian hams. In Proceeding of 5th world congress of dry-cured ham, Aracena, Spain, 6–8 May 2009
- Dirinck P, Opstaele FV, Vandendriessche F. Flavour differences between northern and southern European cured hams. Food Chem. 1997;59:511–521. doi: 10.1016/S0308-8146(97)00012-5. [DOI] [Google Scholar]
- Flores M, Grimm CC, Toldrá F, Spanier AM. Correlations of sensory and volatile compounds of Spanish Serrano dry-cured hams as a function of two processing times. J Agric Food Chem. 1997;45:2178–2186. doi: 10.1021/jf960862c. [DOI] [Google Scholar]
- García-González DL, Aparicio R, Aparicio-Ruiz R. Volatile and amino acid profiling of dry-cured hams from different swine breeds and processing methods. Molecules. 2013;18:3927–3947. doi: 10.3390/molecules18043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspardo B, Procida G, Toso B, Stefanon B. Determination of volatile compounds in San Daniele ham using headspace GC–MS. Meat Sci. 2008;80:204–209. doi: 10.1016/j.meatsci.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Gilles G. Dry cured ham quality as related to lipid quality of raw material and lipid changes during processing: a review. Grasas Aceites. 2009;60:297–307. doi: 10.3989/gya.130908. [DOI] [Google Scholar]
- Guillén MD, Manzanos MJ. Study of the volatile composition of an aqueous oak smoke preparation. Food Chem. 2002;79(3):283–292. doi: 10.1016/S0308-8146(02)00141-3. [DOI] [Google Scholar]
- Hinrichsen LL, Pedersen SB. Relationship among flavour, volatile compounds, chemical changes, and microflora in Italian-type dry-cured ham during processing. J Agric Food Chem. 1995;43:2932–2940. doi: 10.1021/jf00059a030. [DOI] [Google Scholar]
- Jerković I, Mastelić J, Tartaglia S. A study of volatile flavour substances in Dalmatian traditional smoked ham: impact of dry-curing and frying. Food Chem. 2007;104:1030–1039. doi: 10.1016/j.foodchem.2007.01.013. [DOI] [Google Scholar]
- Jiménez-Colmenero F, Ventanas J, Toldrá F. Nutritional composition of dry-cured ham and its role in a healthy diet. Meat Sci. 2010;84:585–593. doi: 10.1016/j.meatsci.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Laureati M, Buratti S, Giovanelli G, Corazzin M, Lo Fiego DP, Pagliarini E. Characterization and differentiation of Italian Parma, San Daniele and Toscano dry-cured hams: a multi-disciplinary approach. Meat Sci. 2014;96:288–294. doi: 10.1016/j.meatsci.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Lemon DW (1975) An improved TBA test for rancidity. New series. Circular no. 51. Halifax, Nova Scotia, Halifax Laboratory
- Martín L, Córdoba JJ, Antequera T, Timón ML, Ventanas J. Effects of salt and temperature on proteolysis during ripening of Iberian ham. Meat Sci. 1998;49:145–153. doi: 10.1016/S0309-1740(97)00129-0. [DOI] [PubMed] [Google Scholar]
- Martuscelli M, Pittia P, Casamassima LM, Manetta AC, Lupieri L, Neri L. Effect of intensity of smoking treatment on the free amino acids and biogenic amines occurrence in dry cured ham. Food Chem. 2009;116:955–962. doi: 10.1016/j.foodchem.2009.03.061. [DOI] [Google Scholar]
- Marušić N, Petrović M, Vidaček S, Petrak T, Medić H. Characterization of traditional Istrian dry-cured ham by means of physical and chemical analyses and volatile compounds. Meat Sci. 2011;88:786–790. doi: 10.1016/j.meatsci.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Marušić N, Vidaček S, Janči T, Petrak T, Medić H. Determination of volatile compounds and quality parameters of traditional Istrian dry-cured ham. Meat Sci. 2014;96:1409–1416. doi: 10.1016/j.meatsci.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Morrissey PA, Sheehy PJA, Galvin K, Kerry JP, Buckley DJ. Lipid stability in meat and meat products. Meat Sci. 1998;49:73–86. doi: 10.1016/S0309-1740(98)90039-0. [DOI] [PubMed] [Google Scholar]
- Muriel E, Antequera T, Petron MJ, Andres AI, Ruiz J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004;68:391–400. doi: 10.1016/j.meatsci.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Nunes C, Coimbra MA, Saraiva J, Rocha MS. Study of the volatile components of a candied plum and estimation of their contribution to the aroma. Food Chem. 2008;111(4):897–905. doi: 10.1016/j.foodchem.2008.05.003. [DOI] [Google Scholar]
- Pérez-Alvarez JA, Sayas-Barberá ME, Fernández-López J, Gago-Gago MA, Pagán-Moreno MJ, Aranda-Catalá V. Chemical and colour characteristics of spanish dry-cured ham at the end of the aging process. J Muscle Foods. 1998;10:195–201. doi: 10.1111/j.1745-4573.1999.tb00395.x. [DOI] [Google Scholar]
- Pham AJ, Schilling MW, Mikel WB, Williams JB, Martin JM, Coggins PC. Relationships between sensory descriptors, consumer acceptability and volatile flavor compounds of American dry-cured ham. Meat Sci. 2008;80:728–737. doi: 10.1016/j.meatsci.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Muriel E, Ventanas J (2002) The flavour of Iberian ham. In: Toldrá F (ed) Research advances in the quality of meat and meat products. Research Signpost, Trivandrum, India, pp 289–309
- Sabio E, Vidal-Aragon MC, Bernalte MJ, Gata JL. Volatile compounds present in six types of dry-cured ham from south European countries. Food Chem. 1998;61:493–503. doi: 10.1016/S0308-8146(97)00079-4. [DOI] [Google Scholar]
- Santé-Lhoutellier V, Robert N, Martin JF, Gou P, Hortós M, Arnau J, Diestre A, Candek-Potokar M. PRKAG3 and CAST genetic polymorphisms and quality traits of dry-cured hams–II. Associations in French dry-cured ham Jambon de Bayonne and their dependence on salt reduction. Meat Sci. 2012;92:354–359. doi: 10.1016/j.meatsci.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Sikorski ZE, Kolakowski E. Smoling. In: Toldrá F, editor. Handbook of meat processing. Iowa: Blackwell Publishing; 2010. pp. 231–245. [Google Scholar]
- Tjener K, Stahnke LH. Flavor. In: Toldrá F, editor. Handbook of fermented meat and poultry. Iowa: Blackwell Publishing; 2007. pp. 227–239. [Google Scholar]
- Toldrá F. Proteolysis and lypolisis in flavour development of dry-cured meat products. Meat Sci. 1998;49:101–110. doi: 10.1016/S0309-1740(98)90041-9. [DOI] [PubMed] [Google Scholar]
- Toldrá F. Dry-cured meat products. Iowa: Wiley-Blackwell; 2002. pp. 27–62. [Google Scholar]
- Toldrá F, Aristoy MC, Flores M. Relevance of nitrate and nitrite in dry-cured ham and their effects on aroma development. Grasas Aceites. 2009;60(3):291–296. doi: 10.3989/gya.130708. [DOI] [Google Scholar]