Abstract
Background:
Although Faecalibacterium prausnitzii is a major bacterium in the intestine of adults, which is known to have anti-inflammatory effects, the development in infants or the response to prebiotics remains unclear.
Methods:
The counts of F. prausnitzii in the feces were examined by real-time polymerase chain reaction (PCR). Fecal samples were obtained from 65 atopic dermatitis (AD) infants who participated in a randomized controlled clinical trial to investigate the therapeutic effect of kestose, the smallest fructooligosaccharide.
Results:
Although the F. prausnitzii count was undetectable level in most 0- to 1-y-old infants, the count reached a level comparable to that in adults in 2- to 5-y-old infants. The bacterial number increased about 10-fold by oral administration of kestose every day for 12 wk in the younger infants, but not so much in the older infants. This bacterial increase was significantly correlated with an improvement in the AD symptoms in the older infants.
Conclusion:
The F. prausnitzii population in the intestine reaches a level comparable to that in adult at approximately 2 y of age. Kestose efficiently stimulates the growth of this bacterium in the intestine, which might lead to an improvement in AD symptoms in infants.
A prebiotic is defined as a nondigestive food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of the beneficial bacteria in intestine, thereby improving host health (1). Many of the current successes in prebiotic research have been achieved using nondigestible poly- or oligosaccharides. Among them, the most widely used are fructans including both short-chain fructooligosaccharides (FOS; degree of polymerization 2–9) and long-chain inulin (degree of polymerization 10–60).
Thus far, many prebiotic studies have focused on the stimulation of Bifidobacterium. A recent in vitro study using minimal medium broth by Scott et al. (2) showed that few of the Bifidobacterium species were able to grow on long chain inulin, while most of these species were capable of growing on FOS. Additionally, they suggested that the chain length of FOS is crucial in determining its fermentability among various kinds of FOS because most Bifidobacterium species grew better on low molecular weight FOS than on high molecular weight FOS. We previously demonstrated that the advantage of low molecular weight FOS for stimulating bifidobacterial growth in an in vitro study (3). Namely, kestose, the smallest FOS (glucose-fructose-fructose), exerted a far greater growth-stimulating activity on several species of Bifidobacterium than nystose, the next smallest FOS [glucose-fructose-fructose-fructose]. These results indicated kestose to be the most efficient FOS to simulate the growth of bifidobacteria.
Atopic dermatitis (AD) is a common chronic skin disease in infants. Improved hygiene and decreased exposure of the immune system to microorganisms in infants are considered to be possible environmental etiologies of AD. Among the known microbes, the commensal intestinal microbiota is thought to play an important role in the pathogenesis of AD (4). Because bifidobacteria, a predominant component of the intestinal microbiota in infants are presumed to exert a beneficial effect on AD, there have been attempts to administrate prebiotic oligosaccharides to increase and activate indigenous bifidobacteria in the gut of AD patients (5,6). In a previous study (7), we performed a small-scaled randomized controlled trial to assess the clinical effect of kestose on infants with AD, because kestose was revealed to be the most efficient FOS to stimulate bifidobacteria. While the symptoms of AD were significantly improved in the patients treated with kestose, no significant correlation was found between the improvement in the AD symptoms and the increase in the count of fecal bifidobacteria in the patients. These results suggested that a bacterium other than Bifidobacterium might be responsible for the improvement in the clinical symptoms in AD patients treated with kestose.
Faecalibacterium prausnitzii is one of the abundant bacteria in the intestinal microbiota in adults, accounting for 5–15% of the total bacterial population (8,9). However, it remains to be elucidated precisely when this bacterium initially colonizes the intestine of the infants after birth and how this bacterium expands in the intestinal microbiota thereafter. Accumulating evidence clearly has shown that this bacterium is a major component of the healthy human microbiota. F. prausnitzii is reported to be depleted in AD (10) as well as inflammatory bowel diseases (11). Moreover, this bacterium has been shown to exhibit anti-inflammatory effects by secretion of butyrate, a predominant short-chain fatty acid produced by gut microbiota (8). Thus, an enlargement of the indigenous F. prausnitzii population by prebiotics may be a promising method for the treatment of AD.
In the present study, we first examined the colonization of F. prausnitzii in the hosts ranging from early to late infancy who suffered from AD, using Bifidobacterium as a control because this genus is well known to be predominant at early infancy and remains a major component of the intestinal microbiota during all infancy (12). We then investigated the prebiotic effect of kestose on bacteria colonizing the intestine and analyzed the correlation between the changes in the count of the bacteria and the improvement in AD symptoms.
Results
Study Population
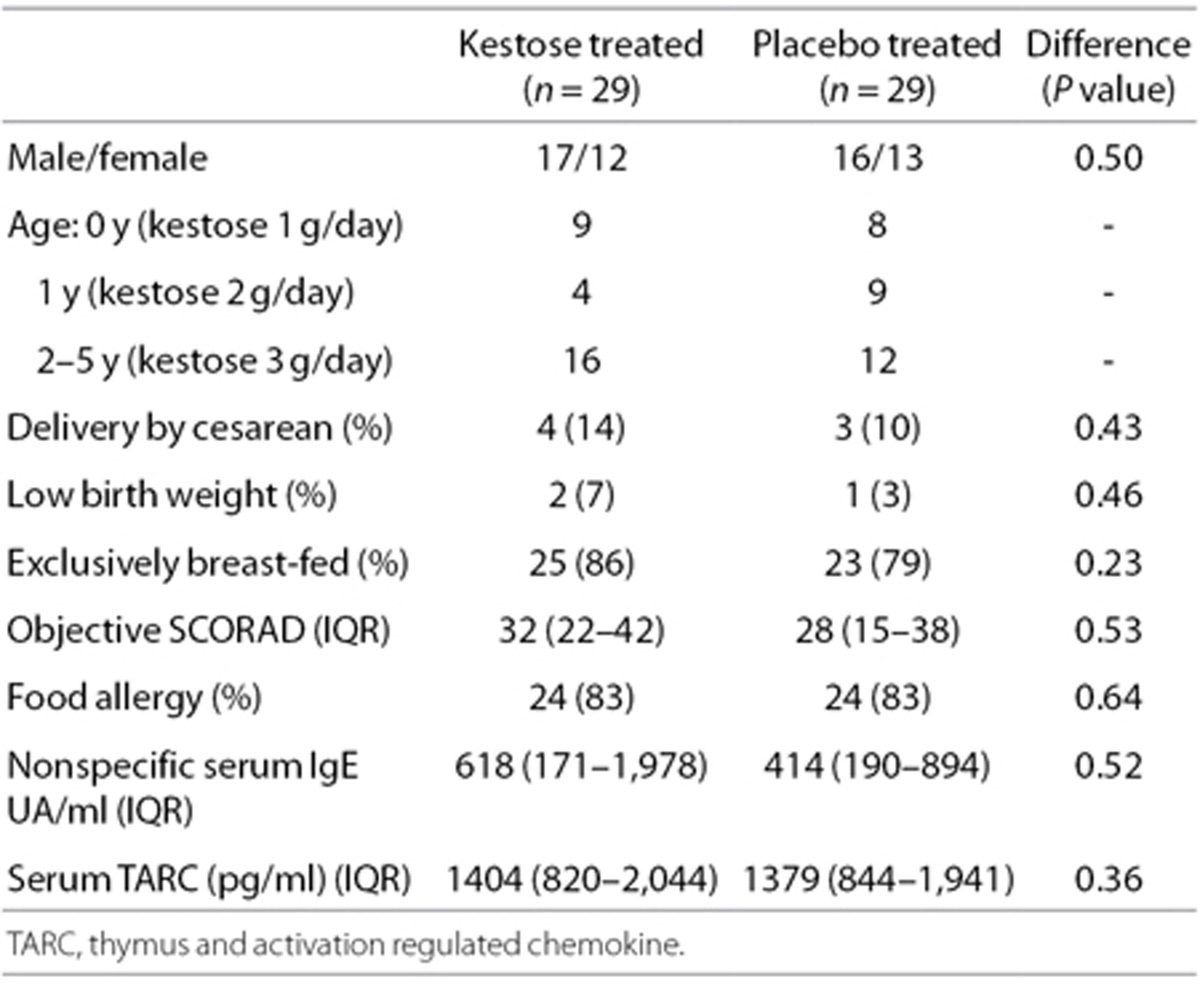
A total of 65 AD patients were enrolled, and 33 and 32 of the patients were randomly allocated to the kestose- and placebo-treated groups, respectively. Seven patients dropped out of the trial before the completion of the treatment because they moved out of the region during the trial. Compliance estimated by interviews with guardians was >90% for all subjects. Finally, 29 subjects in the kestose group and 29 subjects in the placebo were analyzed in the present study. The baseline characteristics and demographics of the subjects for the analyses are summarized in Table 1. No significant differences were found in any of the items.
Table 1. Baseline characteristics of the subjects.
The Relationship Between the Age and the Bacterial Count in the Infants
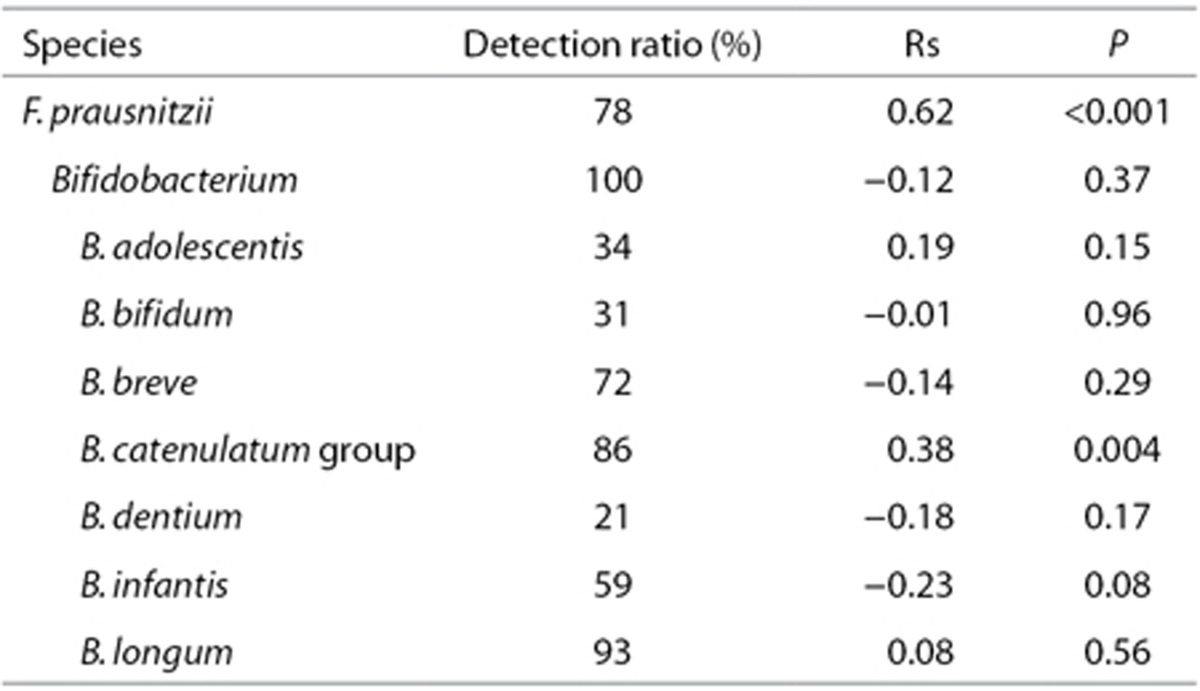
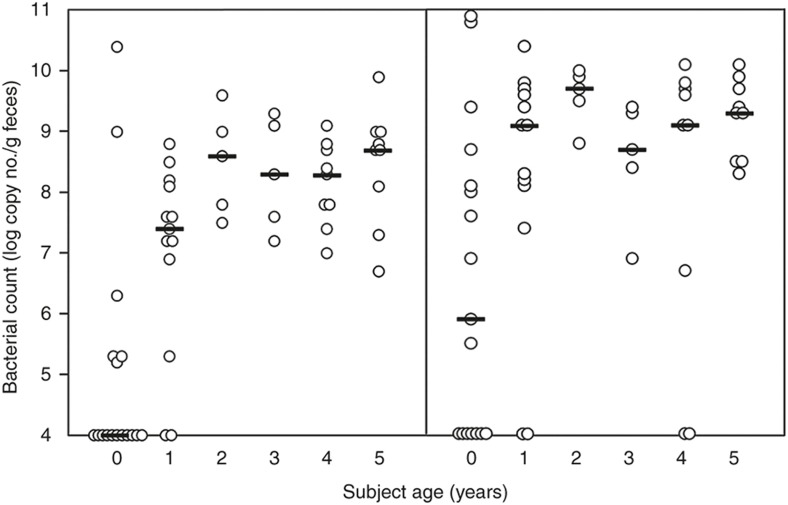
To investigate the development of F. prausnitzii and Bifidobacterium in the gut microbiota of the infants, we first analyzed the relationship between the subject age and the counts of these bacteria using the fecal samples from the subjects at the age of 0 to 5 y (Table 2). A significant positive correlation was found between subject age and the F. prausnitzii count (rs = 0.62, P < 0.001). The F. prausnitzii count was lower than the detectable level (105/g feces) in 13 of 17 subjects at the age of 0 y and 2 of 13 subjects at the age of 1 y (Figure 1, left subpanel). However the F. prausnitzii count was detectable in all infants at the ages of 2 to 5 y. The median of the bacterial count was also clearly lower in 0- to 1-y-old infants (<5.0 to 7.4) compared with those in 2- to 5-y-old infants (8.6 to 8.7). These results implied that the F. prausnitzii population expands from birth until 1 y of age and plateaus around 2 y of age in the gut microbiota. Regarding bifidobacteria, the B. catenulatum group exhibited a moderate, but significant correlation between subject age and the bacterial count (rs = 0.38, P = 0.004) but not the genus Bifidobacterium or other Bifidobacterium species (Table 2). The B. catenulateum group bacterial count was lower than the detectable level in 7 and 2 subjects at 0 and 1 y of age, respectively (Figure 1, right subpanel). The median count remained low at 0 y of age (5.9) but plateaued at 1 y of age (9.1). This finding suggests that the B. catenulatum group population expands from birth and plateaus in the gut microbiota at around 1 y of age.
Table 2. Correlation value between age and fecal bacterial number in the infants (at 0 wk = before the treatment).
Figure 1.
Bacterial count in the infants at different ages. Each circle represents the bacterial count of F. prausnitzii (left subpanel) or B. catenulatum group (right subpanel) in the feces obtained from a subject at 0 to 5 y of age. The counts are expressed as the logarithmic copy number per gram feces. Bars represent the median of the counts at each subject age.
Effect of Kestose on the Number of Fecal Bacteria
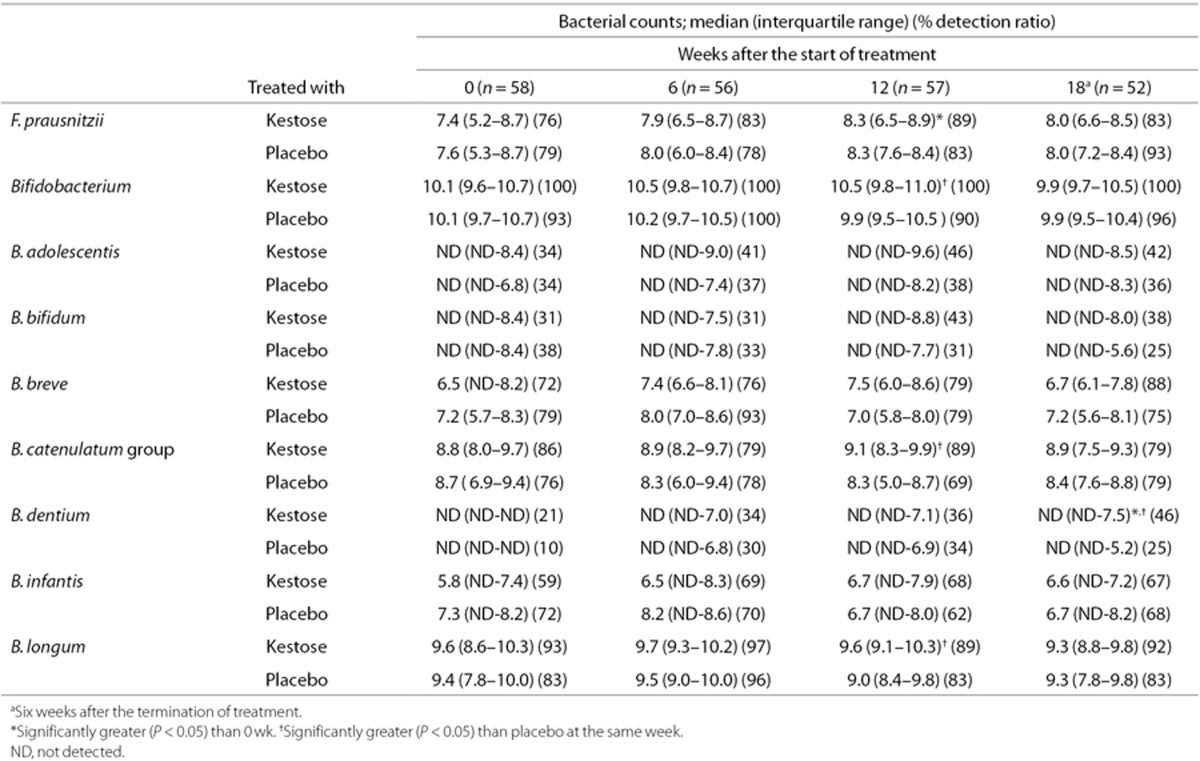
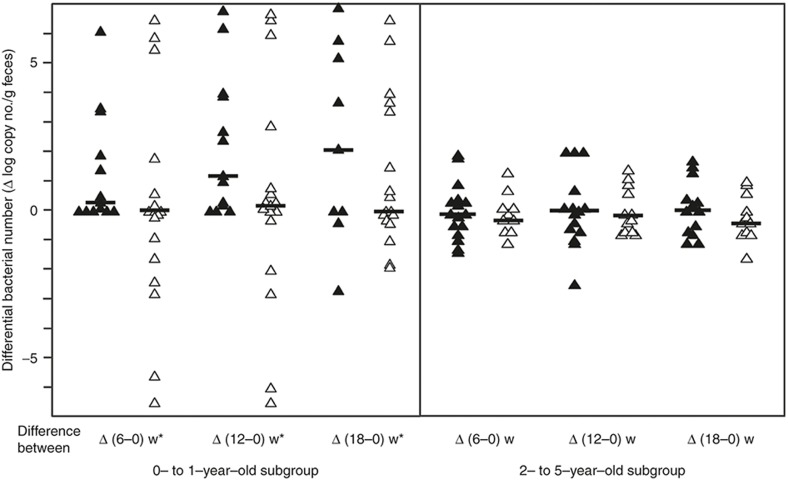
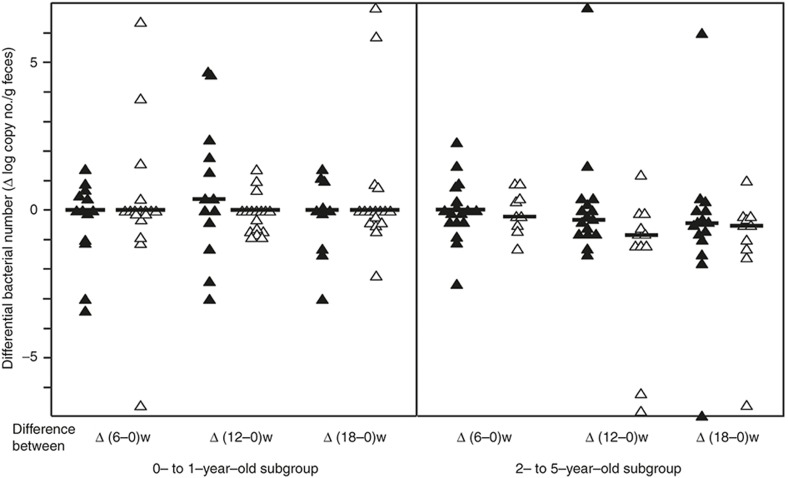
All subjects were treated with kestose or placebo daily for 12 wk and fecal samples were collected to measure the bacterial count at 0, 6, 12, and 18 wk after the start of the treatment (Table 3). In the kestose-treated group, the number of F. prausnitzii at 12 wk was about 10-fold higher than that at 0 wk, and the increase was statistically significant (P < 0.05). No such increase was observed in the placebo-treated group. Regarding bifidobacteria, the kestose group had a significantly higher Bifidobacterium count than the placebo group at 12 wk, a significantly higher B. catenulatum group count at 12 wk, a significantly higher B. dentium count at 18 wk and a significantly higher B. longum count at 12 wk. Next, to determine whether the stimulating effect of kestose on F. prausnitzii is age-dependent, the change in the bacterial count was compared between two subgroups including 0- to 1-y olds and 2- to 5-y olds. The basal count remained far lower in the former than in the latter subgroup (see Figure 1). In the 0- to 1-y-old subgroup, an exclusive increase, but no decrease, in the differential count was found in all subjects after kestose treatment at 6 and 12 wk, whereas both an increase and decrease in the count were observed after placebo treatment (Figure 2, left subpanel). Indeed, the median differential bacterial counts were considerably greater in the kestose group than in the placebo group, although the differences were not significant. Considering that a decrease was observed even in the kestose group at 18 wk, 6 wk after the termination of kestose treatment, the exclusive increase of the F. prausnitzii in this subgroup during kestose treatment may have been directly exerted by kestose itself. In the 2- to 5-y-old subgroup, however, an increase and decrease was observed in the kestose- and placebo-treated groups at 6 and 12 wk after the treatment. Thus, no significant difference in the differential count was observed between these groups while the median tended to be greater in the kestose group than in the placebo group (Figure 2, right subpanel). Regarding the distribution of the differential values in the placebo groups in this figure, the range of the differential bacterial counts was far wider in the 0- to 1-y-old subgroup (IQR: −1.63 to 3.44) than in the 2- to 5-y-old subgroup (IQR: −0.75 to 0.68). This finding suggests that the number of F. prausnitzii is physiologically increasing daily but greatly fluctuated in the gut microbiota of the hosts at 0 to 1 y of age. Regarding the B. catenulatum group, however, no age-dependent stimulating effect by kestose was observed. Both an increase and decrease in the differential bacterial count were observed in the 0- to 1-y-old and 2- to 5-y-old subgroups at 6, 12, and 18 wk after the treatment (Figure 3). Additinaly, there was no significant difference between the kestose- and placebo-treated groups at any time point after the start of the treatment.
Table 3. Effect of kestose on the number of fecal bacteria.
Figure 2.
Changes in the number of F. prausnitzii after the treatment in the subgroups of younger and older infants. Left and right subpanels show the subgroups composed of subjects at 0 to 1 y of age and 2 to 5 y of age, respectively. Each symbol represents the differential change in the number of bacteria in a subject at 6, 12, and 18 wk (= 6 wk after the finish of the treatment) after the start of the treatment. Filled and open symbols indicate the subjects treated with kestose and placebo, respectively. Bars represent the median of the differential bacterial numbers. *P < 0.05 value in the inter-group difference < 0.1
Figure 3.
Changes in the number of B. catenulatum group after the treatment in the subgroups of younger and older infants. Left and right subpanels show the subgroups composed of subjects at 0 to 1 y of age and 2 to 5 y of age, respectively. Each symbol represents the differential change in thenumber of bacteria in a subject at 6, 12, and 18 wk (= 6 wk after the finish of the treatment) after the start of the treatment. Filled and open symbols indicate the subjects treated with kestose and placebo, respectively. Bars represent the median of the differential bacterial numbers. *P < 0.05 value in the inter-group difference < 0.1.
Relationship Between the Differential Bacterial Count and the SCORAD Score
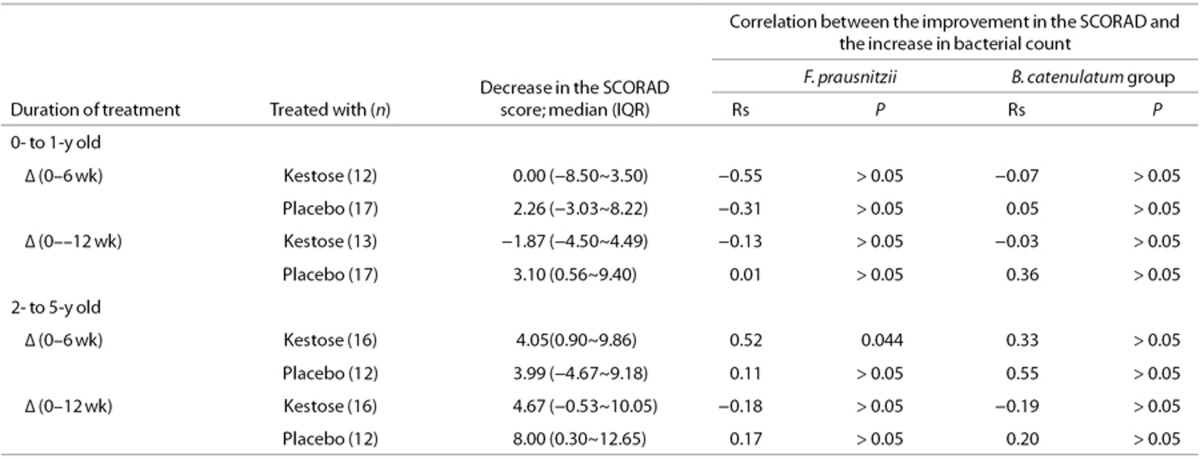
To determine whether the increase in the F. prausnitzii count by kestose was correlated with an improvement in the SCORAD score in the subgroup, this relationship was analyzed using Spearman's test (Table 4). In the 2- to 5-y-old subgroup, there was a significant positive correlation between an increase in the F. prausnitzii count and an improvement in the SCORAD score when subjects were treated with kestose for 6 wk (rs = 0.52, P = 0.044). However, no such positive correlation was observed in the 0- to 1-y-old subgroup treated with kestose, although there was a considerable increase in the F. prausnitzii count in this subgroup (see Figure 2). Additionally, there was no significant correlation between the changes in the B. catenulatum group count and the SCORAD score in any subgroup.
Table 4. Correlation between changes in the SCORAD score and the bacterial count in AD patients treated with kestose.
Discussion
The initial acquisition and subsequent succession of microbes colonizing the intestinal tract is marked in the infancy, during which feeding shifts from breast-feeding to weaning to the introduction of solid food. Cooperstock et al. (13) divided the development of intestinal microbiota in infants into four separate phases: phase I, the initial acquisition phase a few weeks after the birth; phase II, the remaining period of solely breast feeding around the first 6 mo; phase III, the time between the beginning of supplementation and the cessation of breast-feeding; and phase IV, the period of conversion to adult microbiota beginning after completion of weaning. In the present study, the development of F. prausnitzii and different Bifidobacterium species in the gut microbiota was investigated using fecal samples from infants suffering from AD. The analysis of the relationship between the subject age and the bacterial counts demonstrated a significant positive correlation in F. prausnitzii and the B. catenulatum group. A further analysis of the age-associated increase of F. prausnitzii revealed that this bacterium initially appeared at 0 y of age and plateaued at around 2 y of age. The bacterial count at plateau using the qPCR method was about 108 to 109 copies/g feces, which was comparable to the bacterial count in 45 healthy Japanese adults (mean age 48 y) examined by the same method in our previous study (9). A study conducted in Spain composed of 31 healthy adults (mean age 32 y) also showed that the median F. prausnitzii count was 6.5 × 108 copies/g feces (14). These studies thus indicated that the F. prausnitzii population in the gut microbiota in infants reaches that in adults at around 2 y of age. A previous study on the development of microbiota by the amount of short-chain fatty acids in the feces reported that butyrate levels in infants reached that in adult level at 9 to 18 mo of age (15). Considering that F. prausnitzii is the major bacterial source of butyrate in the intestine, our finding that the count of this bacterium became the adult level around 2 y old is nearly compatible to the result analyzed by such microbiota-associated characteristic as the amount of butyrate produced by the gut microbiota. According to the phases mentioned above, it is considered that this bacterial population reaches its full development in the intestine at around phase III. In a comparative study using atopic and healthy children who were 4 to 14 y of age, the number of fecal F. prausnitzii in atopic subjects was about one-third of that in healthy subjects (10). As the F. prausnitzii count in the present study was measured in AD patients at ages ranging from 0 to 5 y, the difference in the number between these AD patients and age-matched healthy infants remains to be examined.
Throughout the breast-feeding period (phase II), a predominance of bifidobacteria is generally already established and no more increase or rather a decrease in the bacterial count often follows after this period. In fact, six out of seven kinds of Bifidobacterium species examined in the present study exhibited no age-associated increase in the bacterial count during infancy. The B. catenulatum group, on the other hand, showed a significant age-associated increase similar to that of F. prausnitzii, although the bacterial count of the B. catenulateum group plateaued at about 1 y earlier than the F. prausnitzii count. In a study aimed to determine the influence of an obesity treatment program on the gut microbiota in adolescents (16), the counts of both the B. catenulatum group and the Clostridium leptum group which is phylogenetically closely related to F. prausnitzii were significantly higher in the high–weight loss group than in the low-weight loss group before and after intervention. Conversely, the counts of B. breve and B. bifidum, which showed no significant age-associated increase in the count in the present study, were significantly lower in the former group than in the latter group. These results suggested that there is a close association between the B. catenulatum group and F. prausnitzii with regard to nutritional and ecological requirements in the intestinal tract of the host, which are considerablly different from those required by the phase II-dominant Bifidobacterium species such as B. breve and B. bifidum.
Although modulation of indigenous F. prausnitzii abundance using probiotic strains of this bacterium might have therapeutic applications in inflammatory and allergic diseases, an extremely oxygen-sensitive characteristic of this bacterium renders industrial cultivation of this bacterium very difficult. Alternatively, the introduction of prebiotics to modulate the indigenous F. prausnitzii population in the intestine might be possible. Indeed, Ramirez-Farias et al. reported the effect of inulin on the number of F. prausnitzii in the gut (17). In their study, fecal samples obtained from 12 healthy adults who were given 10 g/day inulin for 16 d showed a significant increase in the abundance of the bacterium, however, the degree of increase was only about 50%. In the present study using infants, on the other hand, the degrees of increase in the count by 1~3 g/day kestose treatment were significantly greater that 3-fold and 10-fold at 6 and 12 wk, respectively, after the start of treatment.
Of note, there was a significant difference in the degree of kestose-induced increase in F. prausnitzii between the 0- to 1-y-old and 2- to 5-y-old subgroups in the present study. A greater increase in this bacterium was found in the younger subgroup after kestose treatment than in the older subgroup. In the younger subgroup, the primary source of nutrients was breast-feeding, while solid foods including dietary nondigestible carbohydrates were rarely consumed in the subgroup. As a result, bifidobacteria were dominant, while F. prausnitzii remained the minority in the microbiotas of these subjects. Thus, we speculate that kestose, a nondigestible FOS, exerted a marked stimulating effect on the growth of F. prausnitzii in younger infants because the intestinal environment was devoid of substrates to promote the growth of this bacterium. However, in the older subgroup, the nutritional source was primarily solid foods, which nearly led to a plateau in the number of F. prausnitzii; thus further intake of kestose exerted no marked stimulating effect on this bacterium. By contrast, no such difference in the stimulating effect of kestose on the B. catenulatum group count was observed between the younger and older subgroups, which had a low count in the younger subgroup. Moreover, in a clinical study to examine the effect of inulin in the gut microbiota (17), no increase was found in the B. catenulatum group, while a significant increase in other Bifidobacterium species, including B. adolescentis and B. bifidum, was observed. Taken together, fructans such as inulin and kestose might not be utilized so efficiently by the B. catenulateum group.
Allergies such as AD are dependent on T helper 2 (Th2)-derived immune responses characterized by the production of cytokines IL-4, -5, and -13, which promote the production of various mediators to induce allergic responses (18). The Foxp3+ regulatory T cell (Treg) is characterized by the production of IL-10, one of the major immunoregulatory cytokines, and considered to suppress the excessive activation of Th2 cells thereby ameliorating allergic responses. Some species of Clostridium, a dominant genus of commensal microbes in the gut, are known to induce Treg cells (19,20). Furthermore, Furusawa et al. (21) demonstrated that butyrate can induce the differentiation of Treg cells. Considering that F. prausnitzii is one of the main butyrate-producing bacteria in the intestinal microbiota of the human, it is reasonable that the kestose-induced improvement of the AD symptoms was correlated with the increased number of F. prausnitzii in the present study. Additionally in their study, acetate, the main short-chain fatty acid produced by bifidobacteria, could not induce Treg cell differentiation, suggesting a potential explanation for the lack of a correlation between the improvement in kestose-induced AD symptoms and the number of bifidobacteria observed in our previous clinical trial (7). However, in the present study, a significant correlation between the increase in the F. prausnitzii count and improvement in the AD symptoms was found only in the 2- to 5-y-old subgroup after 6-wk treatment with kestose but not in the 0- to 1-y-old subgroup in which a marked increase in the number of this bacterium was observed. One of the reasons for poor response to kestose in the younger subgroup may be that the basal count remained very low in this subgroup therefore even marked stimulation by kestose treatment could not increase the size of the F. prausnitzii population to an adequate level to have a significant effect on the host. However, in the older subgroup, the basal count was so great that even a small increase by kestose treatment might have resulted in a significant improvement in the AD symptoms.
In conclusion, while prebiotic oligosaccharides have thus far almost exclusively targeted bifidobacteria, the present study has demonstrated that kestose, the smallest FOS, was able to strongly activate F. prausnitzii, another major indigenous bacterium that has a beneficial effect and helps to maintain good health in infants.
Methods
Ethical Consideration
This study protocol was approved by the Committee on Ethical Practice in Tokai University School of Medicine. Written informed consent was obtained from all the patients' guardians.
Research Design
Fecal samples were collected from all subjects who participated in a randomized double-blind placebo-controlled trial named “A clinical study to investigate the therapeutic effect of 1-kestose on atopic dermatitis” which was carried out from October 2012 to December 2013 (Registered in UMIN-CTR www.umin.ac.jp/ctr TEST 000000488). In brief, the subjects were recruited from outpatient clinics in two hospitals in Fukuoka Prefecture, Japan. The inclusion criteria for the subjects were (i) children < 6 y of age and diagnosed with AD of which the severity grading of skin symptoms were Mild or Moderate by Japanese Dermatologist Association, (ii) no use of antibiotics or probiotic/prebiotic preparations during 1 mo before the start of the trial. Patients were randomly allocated to either the kestose- or placebo-treated group. The kestose-treated group was orally given 1 g (< 1 y old of age), 2 g (1 to 3 y old of age), or 3 g (4 to 5 y old of age) 1-kestose daily for 12 wk according to the protocol in the previous study (7). The 1-kestose lot used in the present study was supplied from B Food Science Co. Ltd., Tokyo, and contained >98% 1-kestose. The placebo-treated group was given maltose (glucose-glucose) in the same way as the kestose-treated group. Fecal sampling from the both groups was performed four times at 0, 6, 12, and 18 wk (=6 wk after the termination of the treatment). The fecal samples were immediately sent to the laboratory and frozen until analysis.
Clinical Evaluation
The clinical diagnosis of AD was made according to the criteria issued by the Japanese Dermatologist Association (22). In these criteria, the diagnosis of AD requires the presence of all three features including pruritus, typical morphology and distribution of eczematous dermatitis, and a chronically relapsing course. The severity grading of skin symptoms was rated using the following four-point scale: absence, mild, moderate, and severe. A clinical evaluation of the subjects before and after the treatment was performed using the Severity Scoring of Atopic Dermatitis (SCORAD) (23). The SCORAD index used in the present study includes objective items only (0–83 point scale).
DNA Extraction
Bacterial DNA was extracted from the feces using an Ultra Clean Soil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA) according to the manufacturer's instruction with some modifications (24,25). Briefly, feces (0.1 g) were added to a tube that contained lysozyme (25 mg/ml) and N-acetylmuramidase (0.3 mg/ml) and then incubated for 30 min at 37 °C for cell lysis. Next, the extracted DNA was purified using a High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions.
Real-Time Quantitative PCR
Real-time PCR amplification and detection were performed using an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster, CA). Bacterial DNA from the samples was subjected to real-time PCR assays. The primer set Fprau 07 (CCA TGA ATT GCC TTC AAA ACT GTT) and Fprau 02 (GAG CCT CAG CGT CAG TTG GT) described by Sokol et al. (11) was used to detect F. prausnitzii in the present study. Amplification and detection were carried out according to the method reported previously. Briefly, each reaction was performed in a final volume of 25 μl containing 12.5 μl SYBR GREEN Master Mix (Applied Biosystems) together with 0.2 μmol/l final concentration of each primer, and 10 μl of appropriate dilutions of DNA template. Amplifications were performed as follows: 95 °C for 10 min, to denature DNA and activate Ampli-Taq Gold Polymerase, followed by 40 cycles of 95 °C for 30 s and 60 °C for 1 min. The values of log10 cells/g feces for F. prausnitzii were calculated for each sample from the threshold cycle values using a constructed standard curve. The B. catenulatum group consists of B. catenulateum and B. pseudocatenulatum. The primer pairs used to detect these groups were BiCATg-1 (CGG ATG CTC CGA CTC CT) and BiCATg-2 (CGA AGG CTT GCT CCC GAT) (26). The primer pairs used to detect the genus Bifidobacterium and other species of Bifidobacterium including B. adolescentis, B. bifidum, B. breve, B. dentium, B. infantis, and B. longum were described by Matsuki et al. (26) For the detection of bifidobacteria, amplification was performed in a total volume of 25 μl, containing 12.5 μl SYBR GREEN Master Mix and 0.25 μl of each specific primer. The amplification program consisted of one cycle of 94 °C for 5 min, 40 cycles of 94 °C for 20 s, 55 °C for 30 s, and 72 °C for 1 min, followed by a final step of 94 °C for 15 s.
Statistics
All the values were initially examined to determine whether they had a normal distribution before the comparative analysis. The bacterial counts in the same group at different time points were first analyzed by the Friedman test. Then, the difference in the values was examined by the Wilcoxon signed-rank test. The Mann-Whitney U-test was applied to compare the values at the same time point or interval between the kestose and placebo groups. The relationship between the values stratified by the subject age and fecal bacterial count was analyzed by Spearman's correlation coefficient using the rank test. The relationship between the change in the SCORAD scores and the change in the bacterial count after the treatment was also analyzed by Spearman's test. All P-values were two-sided and considered to be significant at P < 0.05. The SPSS for Window version 23 software package (IBM, New York, NY) was used for all statistical analyses.
Statement of Financial Support
This work was supported by a Grant-in-Aid to Y.K. from the Japanese Society for the Promotion of Science and from the Research Fund of Tokai University School of Medicine.
Disclosure
The authors do not have any financial or other conflicts of interest to disclosure.
References
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995;125:1401–12. [DOI] [PubMed] [Google Scholar]
- Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol 2014;87:30–40. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Aiba Y, Takeda H, Fukumori Y, Koga Y Superiority of 1-kestose, the smallest fructo-oligosaccharide, to a synthetic mixture of fructo-oligosaccharides in the selective stimulating activity on bifidobacteria. Bioscience Microflora 2006;25:109–16. [Google Scholar]
- Kalliomäki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol 2003;3:15–20. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Narisawa Y, Arase S, et al. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol 2003;111:587–91. [DOI] [PubMed] [Google Scholar]
- Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child 2006;91:814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Kimura M, Takahashi H, et al. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin Exp Allergy 2009;39:1397–403. [DOI] [PubMed] [Google Scholar]
- Miquel S, Martín R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 2013;16:255–61. [DOI] [PubMed] [Google Scholar]
- Tsuda A, Suda W, Morita H, et al. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin Transl Gastroenterol 2015;6:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela M, Rampelli S, Turroni S, et al. Unbalance of intestinal microbiota in atopic children. BMC Microbiol 2012;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 2009;15:1183–9. [DOI] [PubMed] [Google Scholar]
- Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr 1999;69:1035S–45S. [DOI] [PubMed] [Google Scholar]
- Cooperstock MS, Zedd AJ. Intestinal flora of infants. In: Hentges DJ, ed. Human intestinal microflora in health and disease. New York: Academic Press 1983:79–99. [Google Scholar]
- Varela E, Manichanh C, Gallart M, et al. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther 2013;38:151–61. [DOI] [PubMed] [Google Scholar]
- Midtvedt AC, Midtvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr 1992;15:395–403. [DOI] [PubMed] [Google Scholar]
- Santacruz A, Marcos A, Wärnberg J, et al.; EVASYON Study Group. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009;17:1906–15. [DOI] [PubMed] [Google Scholar]
- Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 2009;101:541–50. [DOI] [PubMed] [Google Scholar]
- Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E. The hygiene hypothesis of atopic disease–an extended version. J Pediatr Gastroenterol Nutr 2004;38:378–88. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA 2010;107:12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013;500:232–6. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–50. [DOI] [PubMed] [Google Scholar]
- Tagami H. Japanese Dermatological Association Criteria for the diagnosis of atopic dermatitis. J Dermatol 1995;22:966–7. [Google Scholar]
- Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997;195:10–9. [DOI] [PubMed] [Google Scholar]
- Clement BG, Kitts CL. Isolating PCR-quality DNA from human feces with a soil DNA kit. Biotechniques 2000;28:640–2, 644, 646. [DOI] [PubMed] [Google Scholar]
- Gueimonde M, Sakata S, Kalliomäki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr 2006;42:166–70. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Watanabe K, Tanaka R, Fukuda M, Oyaizu H. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl Environ Microbiol 1999;65:4506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]