Abstract
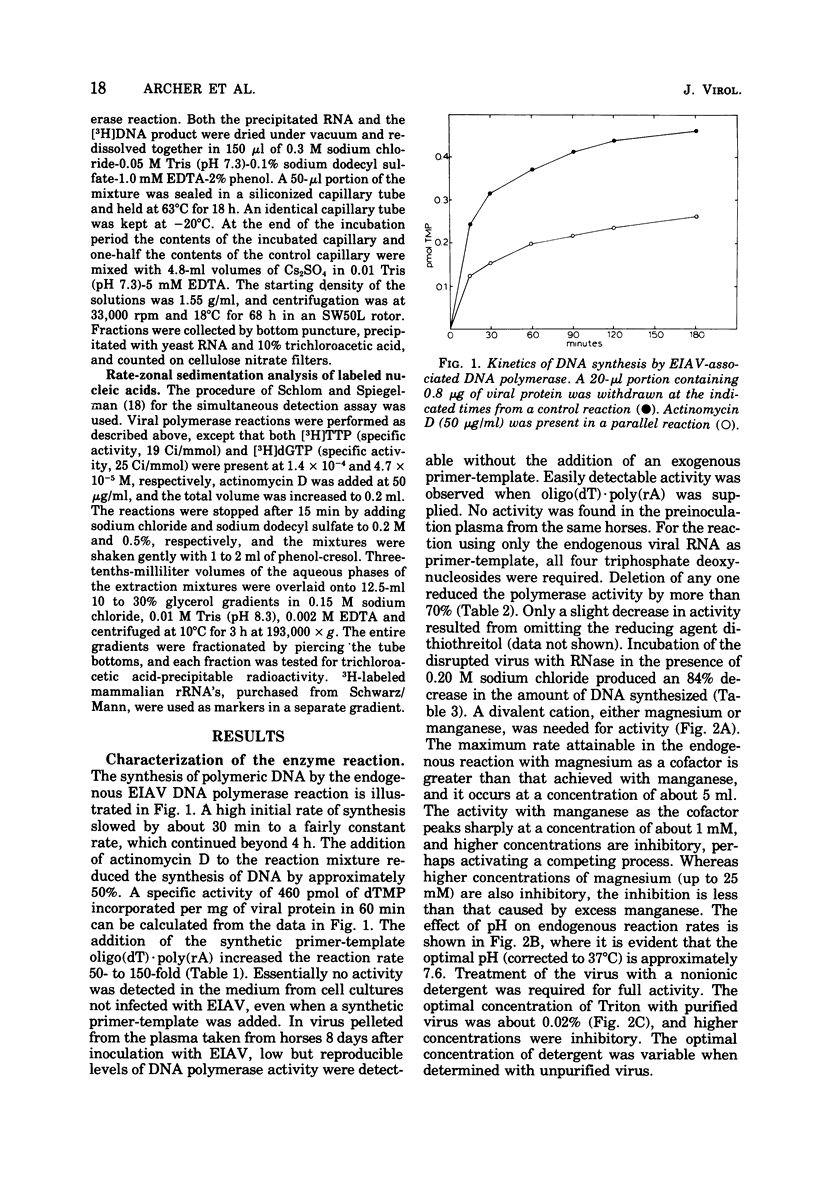
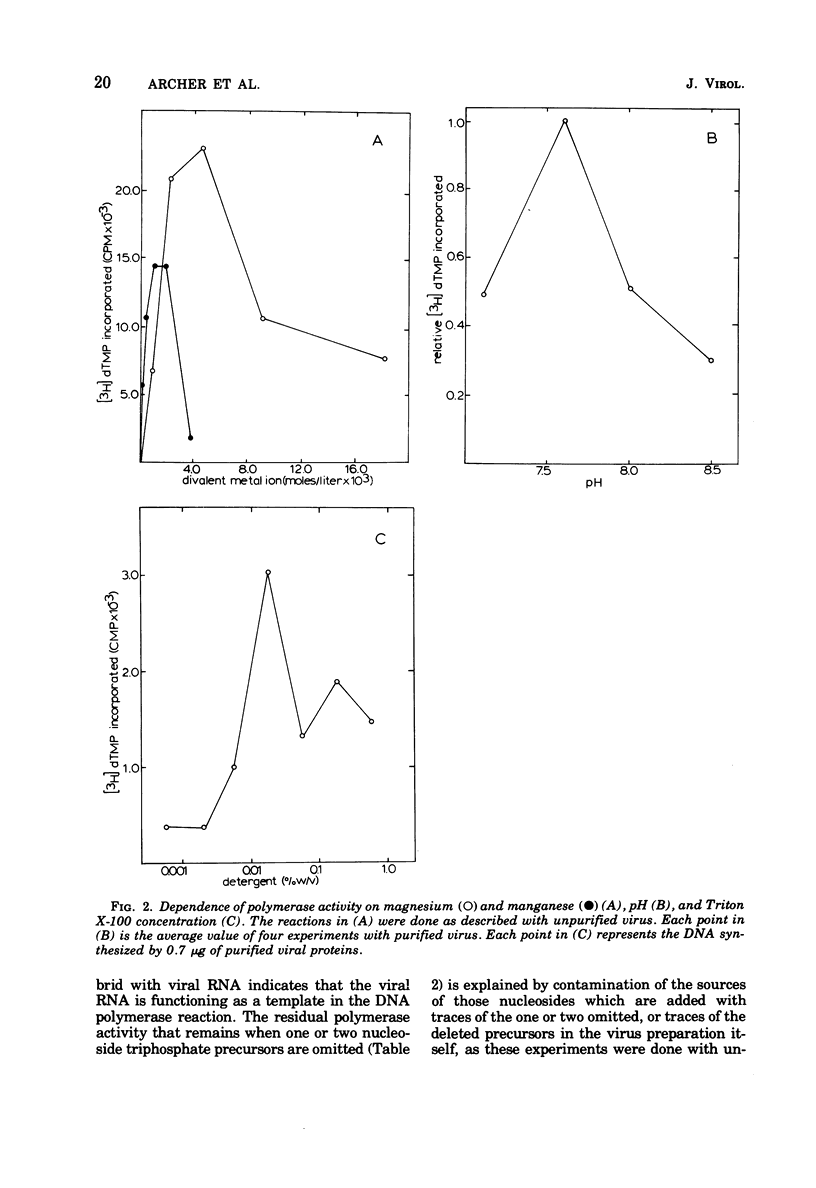
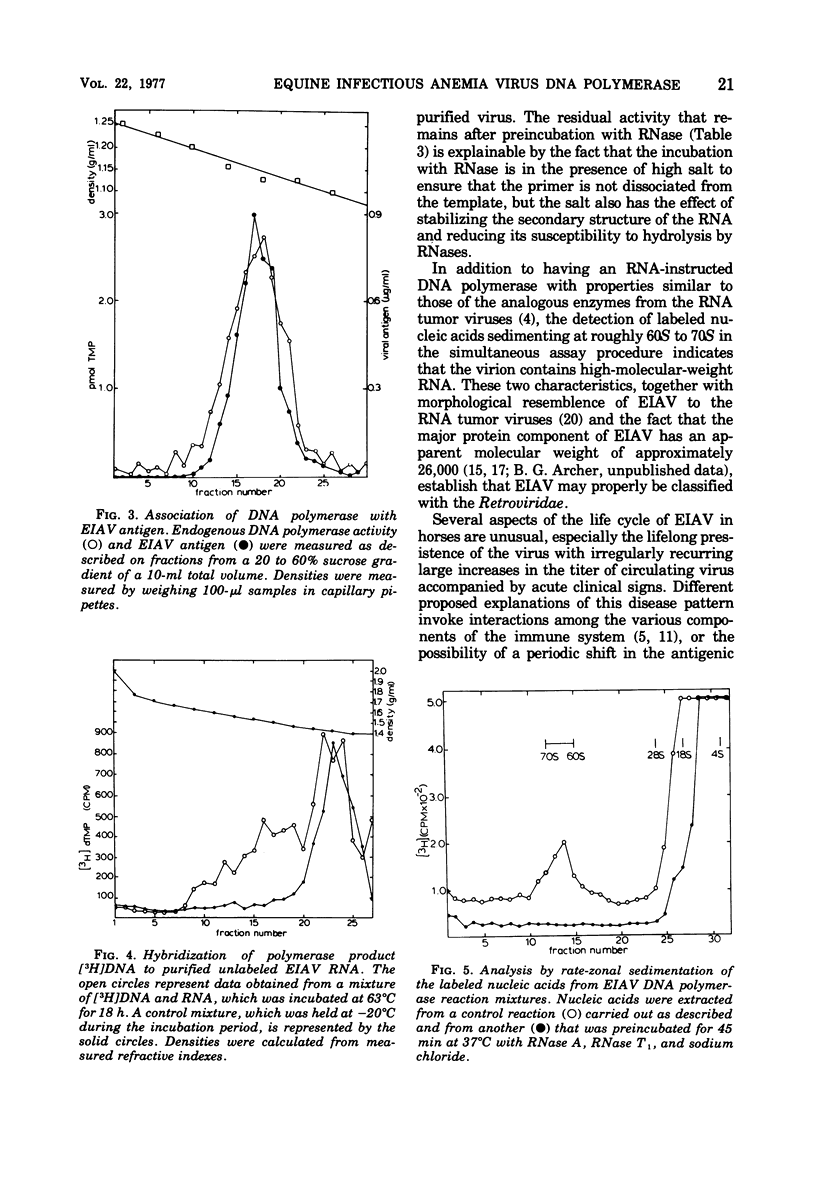
Equine infectious anemia (EIAV) is shown to have an associated RNA-instructed DNA polymerase similar in its cofactor requirements and reaction conditions to the RNA tumor virus DNA polymerases. Demonstrating this DNA polymerase activity requires a critical concentration of a nonionic detergent, all four deoxyribonucleoside triphosphates, and a divalent metal ion. The reaction is sensitive to RNase, and a substantial fraction of the FNA synthesized is complementary to viral RNA. The detection of a complex of tritium-labeled polymerase product DNA-template RNA, which sedimented at 60S to 70S, provided evidence that EIAV contains high-molecular-weight RNA. These results, obtained with both virus propagated in cell culture and virus from the serum of an experimentally infected horse, indicate that EIAV may properly be considered a member of the family Retroviridae. They may also be pertinent to the mechanism(s) of viral persistence and periodic recrudescence of disease in chronically infected horses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks K. L., Henson J. B., McGuire T. C. Immunologically mediated glomerulitis of horses. I. Pathogenesis in persistent infection by equine infectious anemia virus. Lab Invest. 1972 Jun;26(6):701–707. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Charman H. P., Bladen S., Gilden R. V., Coggins L. Equine infectious anemia virus: evidence favoring classification as a retravirus. J Virol. 1976 Sep;19(3):1073–1079. doi: 10.1128/jvi.19.3.1073-1079.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. The classification and nomenclature of viruses. Summary of results of meetings of the International Committee on Taxonomy of Viruses in Madrid, September 1975. Virology. 1976 Jun;71(2):371–378. doi: 10.1016/0042-6822(76)90364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41(1):1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- Kono Y. Viremia and immunological responses in horses infected with equine infectious anemia virus. Natl Inst Anim Health Q (Tokyo) 1969 Spring;9(1):1–9. [PubMed] [Google Scholar]
- Kono Y., Yoshino T., Fukanaga Y. Growth characteristics of equine infectious anemia virus in horse leukocyte cultures. Brief report. Arch Gesamte Virusforsch. 1970;30(2):252–256. doi: 10.1007/BF01250196. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Barnett D., Becvar C. S. Production of equine infectious anemia antigen in a persistently infected cell line. Arch Gesamte Virusforsch. 1973;42(4):361–370. doi: 10.1007/BF01250717. [DOI] [PubMed] [Google Scholar]
- Matheka H. D., Coggins L., Shively J. N., Norcross N. L. Purification and characterization of equine infectious anemia virus. Arch Virol. 1976;51(1-2):107–114. doi: 10.1007/BF01317839. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B. Equine infectious anemia: detection of infections virus-antibody complexes in the serum. Immunol Commun. 1972;1(6):545–551. doi: 10.3109/08820137209022963. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Henson J. B., Burger D. Complement (C'3)-coated red blood cells following infection with the virus of equine infectious anemia. J Immunol. 1969 Aug;103(2):293–299. [PubMed] [Google Scholar]
- Nakajima H., Norcross N. L., Coggins L. Demonstration of antigenic identity between purified equine infectious anemia virus and an antigen extracted from infected horse spleen. Infect Immun. 1972 Sep;6(3):416–417. doi: 10.1128/iai.6.3.416-417.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Tanaka S., Ushimi C. Physicochemical studies of equine infectious anemia virus. IV. Determination of the nucleic acid type in the virus. Arch Gesamte Virusforsch. 1970;31(3):273–280. doi: 10.1007/BF01253762. [DOI] [PubMed] [Google Scholar]
- Norcross N. L., Coggins L. Characterization of an equine infectious anemia antigen extracted from infected horse spleen tissue. Infect Immun. 1971 Nov;4(5):528–531. doi: 10.1128/iai.4.5.528-531.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Aaronson S. A., Todaro G. J. DNA synthesis by RNA-containing tumor viruses. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1034–1041. doi: 10.1073/pnas.67.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M., Nakajima H., Ito Y. Electron microscopy of equine infectious anemia virus. J Virol. 1969 Oct;4(4):521–527. doi: 10.1128/jvi.4.4.521-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



