Abstract
Introduction
With more than 150 million chronically infected people, hepatitis C virus (HCV) remains a substantial global health burden. Direct-acting antivirals have dramatically improved viral cure. However, limited access to therapy, late stage detection of infection and re-infection following cure illustrate the need for a vaccine for global control of infection. Vaccines with induction of neutralizing antibodies (nAbs) have been shown to protect successfully against infections by multiple viruses and are currently developed for HCV.
Areas covered
Here we review the progress towards the development of vaccines aiming to confer protection against chronic HCV infection by inducing broadly nAbs. The understanding or viral immune evasion in infected patients, the development of novel model systems and the recent structural characterization of viral envelope glycoprotein E2 has markedly advanced our understanding of the molecular mechanisms of virus neutralization with the concomitant development of several vaccine candidates.
Expert commentary
While HCV vaccine development remains challenged by the high viral diversity and immune evasion, marked progress in HCV research has advanced vaccine development. Several vaccine candidates have shown robust induction of nAbs in animal models and humans. Randomized clinical trials are the next step to assess their clinical efficacy for protection against chronic infection.
Keywords: Hepatitis C, vaccine, immunity, antibodies
1. Introduction
With more than 150 million people infected worldwide, hepatitis C virus (HCV) infection remains a major global health concern. Following a generally asymptomatic initial infection, a large majority of patients develop chronic hepatitis and are subsequently at risk to progress to liver cirrhosis and hepatocellular carcinoma (HCC) within twenty years.[1] Various parameters may accelerate disease progression, such as alcohol consumption and human immunodeficiency virus (HIV) co-infection.[1] Twenty-five years after the discovery of HCV, the development of a prophylactic vaccine remains a major challenge.[2–7] This is largely due to the high mutation rate of HCV and numerous other strategies employed by the virus to escape host immune responses.[4]
Recent approval of direct-acting antivirals (DAAs) has greatly improved the outcome for treated patients, with cure now possible for the majority of cases.[8] Despite being highly effective, DAA treatments present important drawbacks, one being limited access due too high cost.[9] The current price of a DAA treatment course reaches thousands of dollars, while it is estimated that approximately 80% of HCV-infected individuals are living in low- and middle-income countries.[10] Even with production of generics, it remains unclear whether these will be accessible for these populations.[10] Importantly, in the absence of screening programs, a large proportion of chronic HCV carriers are unaware that they are infected and do not seek DAA treatment, thus potentially transmitting the infection.[9] Additionally, DAAs are less effective when patients are diagnosed at late stages, such as during hepatic decompensation or liver transplantation (LT).[8, 11, 12] Moreover, recent studies demonstrate that HCC risks persists following viral once liver fibrosis is established.[9, 13]. Moreover, even a DAA capable of curing 100% of HCV-infected patients will not be able to protect patients from newly acquired infection. Re-infection following HCV cure remains possible – a major challenge in injection drug users.[14] These limitations underscore the need for a protective vaccine that can be employed in parallel with new treatments.
While the high viral diversity may preclude to develop a prophylactic vaccine, protecting all vaccines against HCV infection, modeling studies estimate that even a vaccine with a lower efficacy will have a substantial impact on HCV prevalence and incidence, especially in high risk populations,[15, 16] as well as important economic benefit.[17] It is of interest to note that it has been suggested, that even a partially effective vaccine would continue to have a substantial benefit even in the setting of highly effective treatments.[16] Collectively, these modeling approaches suggest that both an effective antiviral therapy as well as an efficient vaccine will be required to fully control HCV spread and eradicate viral infection on a global level.[18] Furthermore, a parallel approach of treatment and vaccination appears to be in particular of relevance for populations at high risk for HCV infection such as intravenous drug abusers.
While vaccines inducing protective neutralizing antibodies (nAbs) have been successfully developed for many viral infections including hepatitis B,[19] poliovirus,[20] or human papillomavirus,[21] the development of a B cell vaccine with induction of neutralizing antibodies protecting against HCV infection has been a challenge[4]. B cell vaccines exert their major protective effect through the induction of cross-neutralizing antibodies.
The choice of an appropriate HCV immunogen is critical to anticipate and prevent viral escape[4]. NAbs are essential components of host immune responses elicited following HCV infection and several studies have demonstrated that nAbs clearly play a role for protection against HCV chronic infection.[22] NAbs are considered to mainly interfere with HCV entry which is a complex multistep process consisting of interactions between the viral envelope glycoproteins (GPs) E1/E2 and host cell entry factors.[23] Thus, HCV GPs play a major role in the initiation of hepatocyte infection and are the main target of nAbs. Several vaccine candidates evaluated in clinical trials are based on eliciting strong humoral immune responses by using HCV envelope proteins as immunogens (Table 1).[4, 24] A key aspect of this approach is the inclusion of highly conserved epitopes to overcome viral diversity and prevent viral evasion. Furthermore, consideration of highly conserved conformational epitopes for vaccine design is essential to deliver an antigen truly representative of neutralizing epitopes that are recognized in infected humans.[25, 26]
Table 1. B cell vaccine candidates in preclinical studies in chimpanzees or clinical trials in humans.
Summary of the different HCV vaccine candidates in preclinical or clinical development with description of the strategy employed and the main results obtained.
| Strategy employed | Animal model/Patient | Results | References |
|---|---|---|---|
| Genotype 1a Recombinant E1E2 proteins |
Chimpanzees |
|
[90] [91] |
| Genotype 1a DNA encoding E2 protein |
Chimpanzees |
|
[106] |
| HCV-like particles derived from recombinant HCV structural proteins (core, E1 and E2) +/− AS01B adjuvant |
Chimpanzees |
|
[111] |
| E1 recombinant protein aluminum hydroxide adjuvant |
Phase I Healthy volunteers |
|
[98] |
| Genotype 1a Recombinant E1E2 proteins MF59 adjuvant |
Phase I Healthy volunteers |
|
[93] [94] [95] |
| DNA encoding core E1E2 proteins (CIGB-230) | Phase I Treatment-experienced genotype 1b infected patients |
|
[108] |
| Genotype 1a Recombinant E1E2 proteins MF59 adjuvant +/− pegIFN/RBV |
Phase Ib Treatment-experienced genotype 1a/1b infected patients |
|
[97] |
nAbs, neutralizing antibodies; pegIFN/RBV, pegylated interferon ribavirin; SVR, sustained virological response
Two recent studies delineating the crystallographic structure of the HCV E2 core domain in complex with antigen-binding fragments of two distinct antibodies have greatly improved the understanding of HCV envelope glycoprotein (GP) structure. These structures are a major advance toward the utilization of conserved epitopes for rational vaccine design.[27, 28] Aiming to address the progress and challenges of vaccine development, here we review the molecular mechanisms of virus neutralization, the impact of nAbs for control of viral infection in patients and provide an overview on vaccine candidates inducing nAbs which are in preclinical and clinical development.
2. Molecular mechanisms and targets of virus neutralization
E1 and E2 envelope glycoproteins are the natural targets of the protective antibody response. Since E2 interacts with the HCV co-receptors, scavenger receptor class B type 1 (SRB1)[29] and the tetraspanin CD81[30] during virus entry, the majority of nAbs are directed against E2. There is some evidence that the E1E2 heterodimer, not E2 alone, interacts with a third co-receptor, the tight junction protein claudin-1.[31] A major region mediating virus neutralization is the hypervariable region 1 (HVR1), encompassing the first 27 residues of E2.[32, 33] Antibodies to HVR1 are isolate-specific and over time will select viral quasispecies that are not recognized by these antibodies.[34] This region also blocks access of other broadly neutralizing antibodies to their cognate epitopes[35, 36] and the binding of antibodies to HVR1 was recently shown to interfere with the binding of broadly neutralizing antibodies to E2 by steric hindrance.[37] Taken together, we believe that vaccine strategies should avoid the generation of HVR antibodies.
Antibodies with broad neutralizing activities mainly target conformational epitopes on E2 (reviewed in [22]), with some directed against the E1E2 heterodimer.[38] Based on cross-competition analyses and epitope mapping of broadly neutralizing human monoclonal antibodies (HMAbs) that were isolated from B cells of chronically infected patients, at least five clusters of overlapping conformational epitopes have been identified. Three clusters, designated as antigenic domains B, C and D, are composed of conformational epitopes on E2,[39–41] and two clusters, designated as antigenic regions (AR) 4 and 5, are composed of conformational epitopes on the E1E2 heterodimer.[38] It should be noted that the AR3 cluster substantially overlaps with antigenic domain B, as identified by cross-competition and epitope mapping studies.[42] Interestingly, AR5 overlaps with an antigenic domain C HMAb, CBH-7, although the latter antibody binds to E2 and does not require E1.[38, 39] A sixth cluster of broadly nAbs to overlapping linear epitopes are located immediately downstream of HVR1, designated as antigenic domain E, encompassing amino acids 412-423, that have been defined mainly by murine MAbs.[43–46]. It should be noted that neutralizing murine MAbs, typically generated via immunization with recombinant E2 protein, have also been mapped to regions encompassing domains B, C and D but they tend to be antibodies to linear epitopes[22, 47] with decreased breadth of neutralization against different HCV genotypes[44]. However, some neutralizing murine MAbs that recognize conformational epitopes on E2 have been described.[44, 48]
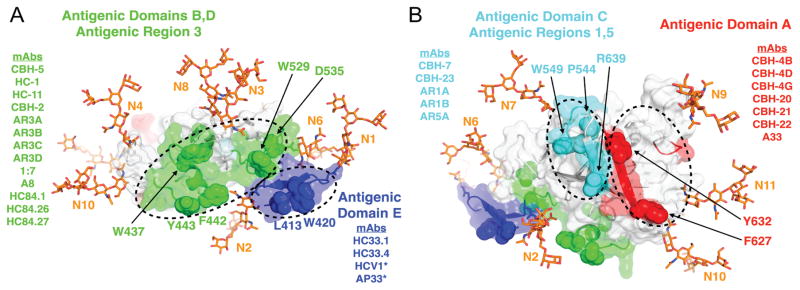
Epitope mapping by alanine substitution studies identified two E2 residues, Gly530 and Asp535, that are required for binding by the majority of currently known domain B HMAbs.[49] Residues Gly523 and Trp529 are also required for some, but not all, of these antibodies. Importantly, Trp529, Gly530 and Asp535 participate in the interaction of E2 with CD81.[50] Thus, antigenic domain B HMAbs exert broad neutralizing activities by competing with CD81 for binding to conserved residues on E2 that are necessary for this step in virus entry. Similarly, three critical residues form a core region for antigenic domain D epitopes at Leu441, Phe442 and Tyr443.[41] Leu441 and Tyr443 are absolutely conserved among all known HCV isolates.[51] Phe442, however, is only 84% conserved (LANL database). The domain D region is also involved in interaction with CD81. For AR4- and AR5-specific epitopes that require the E1E2 heterodimer, their associated antibodies do not mediate neutralization by inhibiting E2 interaction with CD81, but instead potentially block E1E2 engagement with a different co-receptor or conformational changes in the E1E2 structure during virus entry.[38] With the elucidation of the E2 core structure,[27, 28] it became clear that the vast majority of nAbs, encompassing antigenic domain B and D, and AR3, engage the neutralizing face of E2 (Fig. 1A). However, critical residues for antigenic domain C, which mediates virus neutralization, are located on the “non-neutralizing” face, along with a cluster of conformational epitopes that do not mediate virus neutralization, designated as antigenic domain A (Fig. 1B).
Figure 1. Antibody binding supersites on the E2 surface.
Antigenic domains and antigenic regions are grouped into four supersites (colored green, blue, cyan and red, circled by dashed lines), based on previous binding competition and alanine scanning studies, shown on A) the neutralizing antibody face (CD81-binding region) and B) the non-neutralizing face of E2. Key binding residues shared by multiple antibodies are labeled and shown in space fill, and representative mAbs are shown for each supersite. The E2 structure is from the isolate H77 E2 core crystal structure[27, 28]. N-terminal residues (aa 412-420) missing from the crystal structure were modeled using Rosetta[113] and the crystal structure of HCV1 bound to aa 412-423.[114] Glycan structures were added using the Glyprot web server[115] and are shown as orange sticks and labeled according to the order of their bound asparagine residues in the E2 sequence (N1-N11; N5 not visible). All antibodies shown are human, except for those denoted by asterisks, which are mouse-derived (HCV1 is from human antibody transgenic mice).
Not all broadly neutralizing antibodies are created equally. In virus co-culture studies, some antigenic domain B antibodies are associated with viral escape (with and without compromising viral fitness), whereas others are not associated with escape. At a critical antibody concentration of HC-1, a domain B HMAb, a complete elimination of infectious virions will occur.[52] These studies point to two discontinuous regions that participate in domain B epitopes, aa 425-443 and aa 529-535. The variable region aa 425-443 is responsible for escape and the conserved region aa 529-525 is responsible for virus interaction with CD81. Antibodies to antigenic domain E (aa 412-423) are also associated with different patterns of viral escape. Escape from virus neutralization with AP33 and HCV1 nAbs occurs when there is an N-glycan shift from Asn417 to Asn415 or residue substitutions at Asn415.[53–55] However, glycan shift-mediated escape is not associated with other antigenic domain E HMAbs, such as HC33.1.[56] A particular concern for antigenic domain E in vaccine design is that this region is not highly immunogenic in humans. The rate of HCV infected subjects with antibodies to this region ranges from 2–15%[57, 58], while antibodies to antigenic domain B are frequently observed. Escape from antigenic domain D HMAbs (a subset of the antigenic domain B-D/AR3 supersite in Fig 1A) has not been observed, despite some variability at critical binding residue Phe442, as noted above. Structural studies provided an explanation for the lack of viral escape in virus co-culture studies.[51] Three E2 residues, located at 441-443, form a hydrophobic protrusion that serves as the core binding site for domain D HMAbs. When Phe442Ile or Phe442Leu mutations are present, the interaction with the paratope formed by the heavy chain CDRs leads to a decrease in binding energy of the complex that can be compensated by increasing the antibody concentrations.
In summary, vaccine development should focus on specific epitopes within antigenic domains B and D that are not associated with viral escape and less on domain E that is of poor immunogenicity and possibly elicits neutralizing antibodies that are associated with viral escape without compromised viral fitness. Moreover, these studies illustrate the importance of functional and biochemical characterization of broadly reactive nAbs to create a high-resolution, functional map of neutralizing and non-neutralizing epitopes that can be employed for rational design of a B cell vaccine.
3. Viral evasion from neutralizing antibodies
The ability of HCV to adapt to its host is a major limitation for the development of a prophylactic vaccine. In patients, HCV circulates as a pool of quasispecies, viral variants which are genetically distinct and continually evolving due to a high viral mutation rate.[59] HCV variants reinfecting the liver graft are characterized by both enhanced entry in hepatocytes and escape from nAbs.[60] HCV has developed numerous strategies to avoid recognition by host humoral responses. HCV envelope glycoproteins (GPs) are the principal HCV proteins exposed during viral infection and the first target of humoral responses.[61] One study of over 26 years of HCV GP E1E2 sequences in a chronically infected patient demonstrated that humoral immunity applies a selective pressure to HCV, resulting in the continuous generation of variants with E1E2 mutations that are not neutralized by circulating nAbs.[34] Similarly, Dowd et al. observed that during the acute phase of HCV infection, the evolution of HCV GP sequences is driven by nAbs.[62] Nevertheless, the exact determinants of HCV genetic evolution and diversity are only partially understood and it is likely that although host selective pressure is a major determinant of viral quasispecies evolution, it is not the only one.
Many mutations occur in specific regions called hypervariable regions (HVR) of HCV GPs. Farci et al. demonstrated a correlation between HCV diversity and viral clearance. Patients infected with a relatively stable HCV population are more susceptible to viral clearance, whereas chronicity correlates with genetic variability and evolution, especially in HVR1 of HCV GP E2.[63] The importance of this region for HCV persistence has been further confirmed by several studies demonstrating that HVR1 deletion increases the exposure of conserved epitopes facilitating virus neutralization by monoclonal antibodies and patient sera in vitro and in vivo.[35, 36, 64] In addition to a direct shielding role of HVR1, it has recently been proposed that nAbs binding poorly to HVR1 may interfere with the binding of broadly nAbs to other antigenic domains, due to steric hindrance which ultimately favors viral persistence.[37]
Although HCV variability already represents a strong obstacle for the design of a B cell vaccine, HCV has also developed other mechanisms to avoid nAbs recognition. The N-glycosylation of HCV E2 has been described as an important mechanism for viral escape from nAbs, by masking epitopes to which these antibodies bind.[65–67] More precisely, five E2 glycans have been associated with a decreased sensitivity of HCV to nAbs, with four of them shielding CD81 binding sites that are highly targeted by nAbs.[66] Furthermore, under selection pressure, a shift in the glycan attachment site within the CD81 binding epitope on E2 can also occur, which decreases the efficacy of nAbs.[54] The capacity of HCV to disseminate using cell-to cell transmission has also been described as an efficient strategy to avoid the extracellular compartments where nAbs circulate.[68, 69] Interestingly, host targeting agents such as anti-receptor antibodies are able to prevent HCV cell-to-cell transmission, as well as DAA resistance related to this process.[70–73] Other studies also demonstrated that mutations in HCV GP E2 may alter the use of host-receptors CD81 and scavenger receptor B1, thus conferring resistance to nAbs.[74, 75] In patients, circulating HCV is associated with lipoproteins and forms hybrid lipo-viral particles (LVP). These associations have been shown to reduce the access of nAbs to their epitopes, facilitating HCV persistence.[75, 76] A recent study further demonstrated that host apolipoprotein E present at the surface of the LVP mediates evasion from nAbs.[77]
4. Impact of neutralizing antibodies for control of HCV infection: lessons from clinical cohorts
Since the discovery of HCV in 1989, many studies have focused on host immune responses following viral infection to elucidate the contributions of both cellular and humoral immunity for control and protection against HCV infection. The development and improvement of in vitro models for HCV has provided essential tools to elucidate the exact roles played by nAbs during the early phase of HCV infection.[78] Pseudotyped retroviral particles expressing HCV E1E2 glycoproteins (HCVpp) have been extensively used to evaluate the neutralization capacities of HCV-infected patient sera. Pestka et al. used sera from a cohort of pregnant women accidentally infected with a single HCV strain and followed for over 17 years.[79] Generation of HCVpp bearing the GPs of this specific strain, followed by neutralization experiments, revealed that viral clearance is associated with early appearance of nAbs during the early phase of HCV infection. The titer of nAbs then diminished following viral clearance. In contrast, women who developed chronic hepatitis only presented an absent or low titer of nAbs during the initial phase of HCV infection, and the titer increased only during the late phase of infection.[79] Similarly, Osburn et al. evaluated the breadth of nAbs produced during the acute phase of HCV infection and observed that spontaneous control of viral infection is associated with a rapid production of broad nAbs. In contrast, patients who developed persistent infection had a delayed appearance of broad nAbs.[80] Importantly, the study of a patient who spontaneously recovered from HCV chronic infection highlighted that the appearance of nAbs at week 48 was followed by viral clearance at 65 weeks post-infection, confirming their key role for the control of HCV infection.[81]
The importance of nAbs for protection against HCV recurrence has been further demonstrated in a retrospective study of patients with advanced hepatitis B virus (HBV) or HCV infection undergoing LT and who were treated with HBV immunoglobulins (HBIG). These HBIG were potentially contaminated with anti-HCV nAbs, due to the absence of HCV serological screening before 1990.[82] Interestingly, patients infected with HCV before LT and treated with HBIG had a lower incidence of HCV viremia after transplantation, compared to patients who did not receive HBIG or received HBIG after March 1990.[82] Moreover, patients who were HCV-negative before LT and received HBIG were less susceptible to acquire HCV infection than non-treated patients.[82]
Because they are frequently exposed to HCV, people who inject drugs have been included in many cohorts to study HCV recurrence. Osburn et al. observed that while only 25% of HCV-infected people are able to spontaneously clear a primary infection, a clearance rate of 83% is observed after HCV re-exposure.[83] Additionally, patients clearing the secondary infection generate broad humoral immune responses, whereas these cross-nAbs are rarely detected in patients developing chronic infection.[83] These studies clearly demonstrate the crucial role played by nAbs to protect against HCV infection. It is important to note that neutralizing antibodies are considered to exert their function in concert with T cell responses which have been shown to play a key role for HCV control and clearance.[81, 84–88] Furthermore, recent data from clinical cohorts with acute HCV infection demonstrate an important role for T cell responses in the induction of B cell responses.[89] Indeed, in HCV-infected patients, the presence of HCV-specific CD4+ T cells expressing markers of follicular T-helper (Tfh) cells and secreting interleukin 21 following viral exposure were observed.[89] Furthermore, expression of inducible T-cell co-stimulator was associated with induction of HCV-specific antibodies in patients with acute HCV infection.[89]
5. B cell vaccine candidates in preclinical and clinical development
One of the first approaches in vaccine development was the concept of a subunit vaccine based on recombinant envelope glycoproteins. Early studies of the Houghton group at Chiron showed that immunization of seven chimpanzees with recombinant E1/E2 glycoproteins derived from genotype 1a induced nAbs that appeared to protected five of the seven animals from homologous HCV challenge.[90, 91] Furthermore, the vaccine reduced rates of chronic infection following homologous and heterologous HCV challenge,[92] an important finding given that most HCV-associated disease occurs during chronic infection.
The recombinant E1E2 vaccine combined with MF59 adjuvant was then evaluated in a phase I clinical trial with 60 healthy volunteers.[93] The vaccine induced strong humoral and CD4+ T cell responses, and was well tolerated.[93] Studies with HCVpp demonstrated cross-neutralizing activity of the volunteer antisera against heterologous 1a, 1b and 2a HCV genotypes.[94] Using chimeric cell culture-derived HCV assays, Law et al. demonstrated that the recombinant E1E2 vaccine elicited broadly cross-nAbs in the volunteers, suggesting the presence of epitopes conserved among all major genotypes of HCV.[95] Indeed, peptide mapping and competition studies demonstrated that immunization with recombinant E1E2 elicits antibodies targeting multiple cross-neutralizing epitopes.[96]
The recombinant E1E2-MF59 vaccine was further evaluated in a phase 1b study, either alone or in combination with pegylated-interferon (Peg-IFN) alpha2a and ribavirin for the retreatment of 78 treatment-experienced genotype 1a/1b patients.[97] The vaccine was safe and elicited E1E2 nAbs, which positively correlated with better treatment response. Although the vaccine alone did not induce significant changes in viral load, it was associated with a higher sustained virological response rate when used in combination with Peg-IFN/ribavirin (RBV) (17% combined compared to 8% Peg-IFN/ribavirin alone).[97] This study suggests a potential for using HCV vaccines in combination with DAAs for difficult to treat patients or multiresistance.
The immunogenicity of an E1 candidate vaccine with aluminum hydroxide adjuvant was evaluated in a phase I study in 2004.[98] The individuals who received this vaccine developed humoral responses against E1, although the neutralization capacity of the antibodies was not evaluated.[98] Interestingly, when chimpanzees were vaccinated with either genotype 1b E1 or E2 recombinant protein with alum adjuvant, only antibodies against E1 were shown to neutralize HCVpp and protect the chimpanzees from persistent hepatitis following homologous challenge.[99] These findings highlight the importance of E1 for candidate HCV vaccines. Further insight into the structure and function of the E1/E2 complex will likely improve our understanding of nAbs targets and contribute to further vaccine development.
HCV-like particles (LPs) derived from recombinant HCV structural proteins (core, E1 and E2) are morphologically similar to authentic HCV virions.[100] These HCV-LPs induce strong humoral and cellular immune responses in mice[101] and baboons,[102] but only limited B cell responses were observed in chimpanzees, despite strong induction of cellular immune responses.[103] Since HCV-LPs were produced in insect cells, they were likely not fully representative of authentic HCV virions, which may have limited their immunogenicity. Indeed, glycosylation of envelope glycoproteins is slightly different in insect cells,[100] which may explain the limited induction of nAbs. Pseudotyped virus-like particles expressing E1 and/or E2 envelope glycoproteins that were produced in mammalian cells induced cross-nAbs in macaques.[104] Inactivated cell culture-derived HCV virions induce cross-nAbs that confer protection from HCV infection in human liver chimeric mice.[105] These findings suggest that virus-like particles produced in mammalian cells may better reflect the immunogenicity of authentic HCV virions with superior induction of nAbs.
Vector-based immunization is also capable of inducing humoral immune responses in chimpanzees. A DNA vaccine encoding E2 prevented progression to chronicity in vaccinated chimpanzees, although did not induce sterilizing immunity.[106] In another chimpanzee study, vaccination with recombinant DNA (prime) and adenovirus (boost) vaccines expressing HCV core, E1E2 and NS3-5 genes induced long-lasting E2-specific antibody responses.[107] Although one vaccinated chimpanzee had sterilizing immunity against heterologous virus challenge, other vaccines with lower levels of E2-specific antibody developed chronic infections.[107] A therapeutic DNA vaccine expressing HCV structural proteins and NS3 was evaluated in a Phase I clinical trial of chronically infected patients who did not respond to previous IFN/RBV treatment.[108] All patients generated antibodies against core, and the majority (86.6%) of patients developed antibodies against E1. Following vaccination, 6 of the 15 patients previously lacking nAbs responses became positive for nAbs. Furthermore, over 40% of vaccinated individuals demonstrated improved liver histology (i.e. reduction in fibrosis), although this was attributed to cellular immune responses as opposed to nAbs.[108]
Interestingly, chimeric HBV/HCV vaccines have also been explored. These vaccines are based on the fact that the hepatitis B envelope protein (S) self-assembles into subviral particles, which constitutes the HBV vaccine in current use. Foreign epitopes, such as HCV envelope E1 and E2 protein sequences, can be inserted into the S protein to generate chimeric subviral particles.[109] One such vaccine replaced the N-terminal transmembrane domain of the HBV surface antigen with the transmembrane domain of genotype 1a HCV E1 and E2.[110] Vaccination of rabbits induced a strong humoral response to both HBV and HCV envelope proteins. These antibodies demonstrated cross-neutralizing activity against heterologous genotype 1a, 1b, 2a and 3 HCVpp, while HBV responses showed no loss of activity.[110] Further evaluation in other animal models and humans is warranted.
Collectively, these studies from the past decades have indicated the potential for a protective B cell-based vaccine against HCV. Although humoral responses generated by vaccines will likely be able to play a key role for prevention of infection, induction of cellular immune responses will likely be required for efficient protection against chronic infection.[2–7, 9] Interestingly, for several of the above reviewed approaches, concomitant induction of T cell responses has been demonstrated.[93, 104, 111] Ultimately, immunogens inducing both humoral and cell-mediated immune responses may be the optimal approach to provide full protection against HCV infection and liver disease.
6. Expert commentary
Despite hopes raised following the approval of new highly effective therapies able to cure the majority of patients, in the absence of a vaccine, it is probable that treatment-induced HCV eradication will remain a dream. Indeed, limitations of these treatments, especially in term of costs and access, restrain their use in countries with limited resources, where HCV incidence is high. Furthermore, an important proportion of patients are unaware of being infected with HCV and may further transmit the disease, thus favouring HCV spread and persistence in the population. Finally, re-infection following treatment-induced cure limits this approach in particular in injection drug users. Thus, an effective vaccine may be needed to control and eliminate HCV infection on a global level.
The development of B cell-based vaccines has been hampered by the high viral heterogeneity and efficient escape to host immune responses. The recent understanding of the molecular mechanisms of viral neutralization including the elucidation of the crystal structure of HCV envelope glycoprotein E2 has contributed to the identification of epitopes for cross-neutralizing antibodies.[27, 28] Detailed studies in patient cohorts have shown that neutralizing antibody responses are associated with control of infection, and B cell-based vaccine candidates show promise in preclinical animal models and clinical trials. Induction of T cell responses may further enhance the efficacy of vaccine candidates.[2, 4] Future preclinical and clinical studies will provide further insights to bring a protective vaccine closer to reality.
7. Five-year view
Vaccine development is a long and complex process characterized by decades between preclinical studies and licensure and large-scale production. Current HCV vaccine candidates in development are still at the beginning of this process and the full impact of various mechanisms of viral escape to host immune responses still remains to be seen. Recent clinical trials studies have shown promise to induce cross-neutralizing antibodies although their clinical proof-of-concept for protection against infection remains to be shown. The recent advances in understanding the structure of the viral envelope combined with novel animal models assessing protective immune responses[112] will accelerate the development of improved vaccine candidates which ultimately may protect against HCV infection. A continuing effort is needed to reach that goal which appears to be possible to achieve within the next decade.
8. Key issues.
Hepatitis C virus (HCV) infection is a major global health concern, with approximately 170 million people infected worldwide at risk for life-threatening liver disease and cancer.
While recently licensed DAAs cure the majority of HCV-infected patients, high costs and limited access are major challenges for global control and eradication.
Key challenges for the development of B cell vaccines are viral heterogeneity and evasion
Studies in clinical cohorts demonstrated that neutralizing antibodies play a crucial role in viral clearance among individuals who spontaneously resolve HCV infection as well as protection against HCV re-infection.
B cell vaccine candidates have been shown to induce cross-neutralizing antibodies in clinical trials, however proof-of-concept for protection against HCV infection remains to be shown.
Ultimately, applying immunogens inducing both humoral and cell-mediated immune responses may offer the best approach for full protection against HCV infection and virus-induced liver disease.
The recent advances in understanding the structure of the viral envelope combined with novel animal models assessing protective immune responses will accelerate the development of improved vaccine candidates
A continuing effort is needed to develop a protective vaccine – a goal which appears to be possible to achieve within the next decade.
Abbreviations
- DAA
direct acting antivirals
- GP
envelope glycoprotein
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HBIG
hepatitis B immunoglobulins
- HCV
hepatitis C virus
- HCVpp
hepatitis C virus pseudoparticles
- HIV
human immunodeficiency virus
- HVR
hypervariable region
- pegIFN/RBV
pegylated interferon/ribavirin
- LT
liver transplantation
- LVP
lipoviroparticle
- nAbs
neutralizing antibodies
Footnotes
Declaration of Interests
T Baumert acknowledges grant support from the European Union (ERC-2008-AdG-HEPCENT, ERC-AdG-2014-HEPCIR, FP7 HepaMab, EU H2020 HEPCAR, and Interreg IV FEDERHepato-Regio-Net 2012), the Agence Nationale de Recherche sur le SIDA (ANRS), and the Direction Générale de l’Offre de Soins (A12027MS). CC Colpitts is supported by a fellowship from the Canadian Institutes of Health Research (201411MFE-338606-245517). This work has been published under the framework of the LABEX ANR-10-LABX-0028_HEPSYS and benefits from funding from the state managed by the French National Research Agency as part of the Investments for the Future Programme. Research reported in this publication was also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U19AI123862. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ. Current progress in development of hepatitis C virus vaccines. Nat Med. 2013;19(7):869–878. doi: 10.1038/nm.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Holz L, Rehermann B. T cell responses in hepatitis C virus infection: historical overview and goals for future research. Antiviral Res. 2015;114:96–105. doi: 10.1016/j.antiviral.2014.11.009. informative review dissecting the role of T cell responses in HCV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumert TF, Fauvelle C, Chen DY, et al. A prophylactic hepatitis C virus vaccine: a distant peak still worth climbing. J Hepatol. 2014;61(1 Suppl):S34–44. doi: 10.1016/j.jhep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Lauer GM. Immune responses to hepatitis C virus (HCV) infection and the prospects for an effective HCV vaccine or immunotherapies. J Infect Dis. 2013;207(Suppl 1):S7–S12. doi: 10.1093/infdis/jis762. [DOI] [PubMed] [Google Scholar]
- 6.Feinstone SM, Hu DJ, Major ME. Prospects for prophylactic and therapeutic vaccines against hepatitis C virus. Clin Infect Dis. 2012;55(Suppl 1):S25–32. doi: 10.1093/cid/cis362. [DOI] [PubMed] [Google Scholar]
- 7.Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert Rev Vaccines. 2011;10(5):659–672. doi: 10.1586/erv.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung RT, Baumert TF. Curing chronic hepatitis C--the arc of a medical triumph. N Engl J Med. 2014;370(17):1576–1578. doi: 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 9.Cox AL. MEDICINE. Global control of hepatitis C virus. Science. 2015;349(6250):790–791. doi: 10.1126/science.aad1302. [DOI] [PubMed] [Google Scholar]
- 10.Callaway E. Hepatitis C drugs not reaching poor. Nature. 2014;508(7496):295–296. doi: 10.1038/508295a. [DOI] [PubMed] [Google Scholar]
- 11.Liang TJ, Ghany MG. Therapy of hepatitis C--back to the future. N Engl J Med. 2014;370(21):2043–2047. doi: 10.1056/NEJMe1403619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manns M, Samuel D, Gane EJ, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00052-9. published online 18 February 2016. [DOI] [PubMed] [Google Scholar]
- 13.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 14.Midgard H, Bjoro B, Maeland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020–1026. doi: 10.1016/j.jhep.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Hahn JA, Wylie D, Dill J, et al. Potential impact of vaccination on the hepatitis C virus epidemic in injection drug users. Epidemics. 2009;1(1):47–57. doi: 10.1016/j.epidem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Scott N, McBryde E, Vickerman P, et al. The role of a hepatitis C virus vaccine: modelling the benefits alongside direct-acting antiviral treatments. BMC Med. 2015;13:198. doi: 10.1186/s12916-015-0440-2. Modeling approach suggesting that both an effective antiviral therapy as well as an efficient vaccine will be required to fully control HCV spread. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krahn MD, John-Baptiste A, Yi Q, et al. Potential cost-effectiveness of a preventive hepatitis C vaccine in high risk and average risk populations in Canada. Vaccine. 2005;23(13):1549–1558. doi: 10.1016/j.vaccine.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Honegger JR, Zhou Y, Walker CM. Will there be a vaccine to prevent HCV infection? Semin Liver Dis. 2014;34(1):79–88. doi: 10.1055/s-0034-1371081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Damme P, Ward J, Shouval D, et al. Hepatitis B vaccines. In Vaccine. 2013:205–234. [Google Scholar]
- 20.Vidor E, Plotkin SA. Poliovirus vaccine—inactivated. In Vaccines. 2013:573–597. [Google Scholar]
- 21.Schiller JT, Lowy DR, Markowitz LE. Human papillomavirus vaccines. In Vaccines. 2013:235–256. [Google Scholar]
- 22.Ball JK, Tarr AW, McKeating JA. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res. 2014;105:100–111. doi: 10.1016/j.antiviral.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeisel MB, Felmlee DJ, Baumert TF. Hepatitis C virus entry. Curr Top Microbiol Immunol. 2013;369:87–112. doi: 10.1007/978-3-642-27340-7_4. [DOI] [PubMed] [Google Scholar]
- 24.Nabel GJ. Designing tomorrow’s vaccines. N Engl J Med. 2013;368(6):551–560. doi: 10.1056/NEJMra1204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ndongo N, Berthillon P, Pradat P, et al. Association of anti-E1E2 antibodies with spontaneous recovery or sustained viral response to therapy in patients infected with hepatitis C virus. Hepatology. 2010;52(5):1531–1542. doi: 10.1002/hep.23862. [DOI] [PubMed] [Google Scholar]
- 26.Petit MA, Jolivet-Reynaud C, Peronnet E, et al. Mapping of a conformational epitope shared between E1 and E2 on the serum-derived human hepatitis C virus envelope. J Biol Chem. 2003;278(45):44385–44392. doi: 10.1074/jbc.M304047200. [DOI] [PubMed] [Google Scholar]
- 27*.Kong L, Giang E, Nieusma T, et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science. 2013;342(6162):1090–1094. doi: 10.1126/science.1243876. elucidation of the crystal structure of HCV envelope glycoprotein E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Khan AG, Whidby J, Miller MT, et al. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature. 2014;509(7500):381–4. doi: 10.1038/nature13117. elucidation of the crystal structure of HCV envelope glycoprotein E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarselli E, Ansuini H, Cerino R, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21(19):5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pileri P, Uematsu Y, Campagnoli S, et al. Binding of hepatitis C virus to CD81. Science. 1998;282(5390):938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 31.Douam F, Dao Thi VL, Maurin G, et al. Critical interaction between E1 and E2 glycoproteins determines binding and fusion properties of hepatitis C virus during cell entry. Hepatology. 2014;59(3):776–788. doi: 10.1002/hep.26733. [DOI] [PubMed] [Google Scholar]
- 32.Farci P, Alter HJ, Wong DC, et al. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci U S A. 1994;91(16):7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu YK, Hijikata M, Iwamoto A, et al. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68(3):1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132(2):667–678. doi: 10.1053/j.gastro.2006.12.008. key study of continuous escape of HCV from nAbs and T-cell responses during chronic infection in vivo. [DOI] [PubMed] [Google Scholar]
- 35.Bankwitz D, Steinmann E, Bitzegeio J, et al. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol. 2010;84(11):5751–5763. doi: 10.1128/JVI.02200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prentoe J, Jensen TB, Meuleman P, et al. Hypervariable region 1 differentially impacts viability of hepatitis C virus strains of genotypes 1 to 6 and impairs virus neutralization. J Virol. 2011;85(5):2224–2234. doi: 10.1128/JVI.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keck ZY, Girard-Blanc C, Wang W, et al. Antibody response to the hypervariable region-1 interferes with broadly neutralizing antibodies to hepatitis C virus. J Virol. 2016;90(6):3112–22. doi: 10.1128/JVI.02458-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giang E, Dorner M, Prentoe JC, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A. 2012;109(16):6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keck ZY, Op De Beeck A, Hadlock KG, et al. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J Virol. 2004;78(17):9224–9232. doi: 10.1128/JVI.78.17.9224-9232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keck ZY, Li TK, Xia J, et al. Analysis of a highly flexible conformational immunogenic domain a in hepatitis C virus E2. J Virol. 2005;79(21):13199–13208. doi: 10.1128/JVI.79.21.13199-13208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keck ZY, Xia J, Wang Y, et al. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog. 2012;8(4):e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Law M, Maruyama T, Lewis J, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14(1):25–27. doi: 10.1038/nm1698. in vivo evidence of broadly neutralizing antibodies protecting against HCV infection. [DOI] [PubMed] [Google Scholar]
- 43.Flint M, Maidens C, Loomis-Price LD, et al. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73(8):6235–6244. doi: 10.1128/jvi.73.8.6235-6244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabo MC, Luca VC, Prentoe J, et al. Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step. J Virol. 2011;85(14):7005–7019. doi: 10.1128/JVI.00586-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owsianka A, Tarr AW, Juttla VS, et al. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol. 2005;79(17):11095–11104. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broering TJ, Garrity KA, Boatright NK, et al. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J Virol. 2009;83(23):12473–12482. doi: 10.1128/JVI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancini N, Diotti RA, Perotti M, et al. Hepatitis C virus (HCV) infection may elicit neutralizing antibodies targeting epitopes conserved in all viral genotypes. PLoS One. 2009;4(12):e8254. doi: 10.1371/journal.pone.0008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alhammad Y, Gu J, Boo I, et al. Monoclonal Antibodies Directed toward the Hepatitis C Virus Glycoprotein E2 Detect Antigenic Differences Modulated by the N-Terminal Hypervariable Region 1 (HVR1), HVR2, and Intergenotypic Variable Region. J Virol. 2015;89(24):12245–12261. doi: 10.1128/JVI.02070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keck ZY, Li TK, Xia J, et al. Definition of a conserved immunodominant domain on hepatitis C virus E2 glycoprotein by neutralizing human monoclonal antibodies. J Virol. 2008;82(12):6061–6066. doi: 10.1128/JVI.02475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owsianka AM, Timms JM, Tarr AW, et al. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J Virol. 2006;80(17):8695–8704. doi: 10.1128/JVI.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krey T, Meola A, Keck ZY, et al. Structural basis of HCV neutralization by human monoclonal antibodies resistant to viral neutralization escape. PLoS Pathog. 2013;9(5):e1003364. doi: 10.1371/journal.ppat.1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keck ZY, Li SH, Xia J, et al. Mutations in hepatitis C virus E2 located outside the CD81 binding sites lead to escape from broadly neutralizing antibodies but compromise virus infectivity. J Virol. 2009;83(12):6149–6160. doi: 10.1128/JVI.00248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung RT, Gordon FD, Curry MP, et al. Human monoclonal antibody MBL-HCV1 delays HCV viral rebound following liver transplantation: a randomized controlled study. Am J Transplant. 2013;13(4):1047–1054. doi: 10.1111/ajt.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pantua H, Diao J, Ultsch M, et al. Glycan shifting on hepatitis C virus (HCV) E2 glycoprotein is a mechanism for escape from broadly neutralizing antibodies. J Mol Biol. 2013;425(11):1899–1914. doi: 10.1016/j.jmb.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 55.Dhillon S, Witteveldt J, Gatherer D, et al. Mutations within a conserved region of the hepatitis C virus E2 glycoprotein that influence virus-receptor interactions and sensitivity to neutralizing antibodies. J Virol. 2010;84(11):5494–5507. doi: 10.1128/JVI.02153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keck ZY, Angus AG, Wang W, et al. Non-random escape pathways from a broadly neutralizing human monoclonal antibody map to a highly conserved region on the hepatitis C virus E2 glycoprotein encompassing amino acids 412–423. PLoS Pathog. 2014;10(8):e1004297. doi: 10.1371/journal.ppat.1004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarr AW, Owsianka AM, Jayaraj D, et al. Determination of the human antibody response to the epitope defined by the hepatitis C virus-neutralizing monoclonal antibody AP33. J Gen Virol. 2007;88(Pt 11):2991–3001. doi: 10.1099/vir.0.83065-0. [DOI] [PubMed] [Google Scholar]
- 58.Keck Z, Wang W, Wang Y, et al. Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. J Virol. 2013;87(1):37–51. doi: 10.1128/JVI.01941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarr AW, Khera T, Hueging K, et al. Genetic Diversity Underlying the Envelope Glycoproteins of Hepatitis C Virus: Structural and Functional Consequences and the Implications for Vaccine Design. Viruses. 2015;7(7):3995–4046. doi: 10.3390/v7072809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fafi-Kremer S, Fofana I, Soulier E, et al. Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation. J Exp Med. 2010;207(9):2019–2031. doi: 10.1084/jem.20090766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fauvelle C, Felmlee DJ, Baumert TF. Unraveling hepatitis C virus structure. Cell Res. 2014;24(4):385–386. doi: 10.1038/cr.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dowd KA, Netski DM, Wang XH, et al. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136(7):2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farci P, Shimoda A, Coiana A, et al. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288(5464):339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 64*.Prentoe J, Verhoye L, Velazquez Moctezuma R, et al. HVR1-mediated antibody evasion of highly infectious in vivo adapted HCV in humanised mice. Gut. 2015 doi: 10.1136/gutjnl-2015-310300. published online 20 November 2015in vivo evidence that HVR1 protects cross-genotype conserved HCV neutralisation epitopes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helle F, Goffard A, Morel V, et al. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81(15):8101–8111. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helle F, Vieyres G, Elkrief L, et al. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J Virol. 2010;84(22):11905–11915. doi: 10.1128/JVI.01548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Falkowska E, Kajumo F, Garcia E, et al. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. J Virol. 2007;81(15):8072–8079. doi: 10.1128/JVI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brimacombe CL, Grove J, Meredith LW, et al. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol. 2011;85(1):596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Timpe JM, Stamataki Z, Jennings A, et al. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47(1):17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- 70.Xiao F, Fofana I, Heydmann L, et al. Hepatitis C virus cell-cell transmission and resistance to direct-acting antiviral agents. PLoS Pathog. 2014;10(5):e1004128. doi: 10.1371/journal.ppat.1004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fofana I, Xiao F, Thumann C, et al. A novel monoclonal anti-CD81 antibody produced by genetic immunization efficiently inhibits Hepatitis C virus cell-cell transmission. PLoS One. 2013;8(5):e64221. doi: 10.1371/journal.pone.0064221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mailly L, Xiao F, Lupberger J, et al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33(5):549–554. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meuleman P, Catanese MT, Verhoye L, et al. A human monoclonal antibody targeting scavenger receptor class B type I precludes hepatitis C virus infection and viral spread in vitro and in vivo. Hepatology. 2012;55(2):364–372. doi: 10.1002/hep.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fofana I, Fafi-Kremer S, Carolla P, et al. Mutations that alter use of hepatitis C virus cell entry factors mediate escape from neutralizing antibodies. Gastroenterology. 2012;143(1):223–233. doi: 10.1053/j.gastro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grove J, Nielsen S, Zhong J, et al. Identification of a residue in hepatitis C virus E2 glycoprotein that determines scavenger receptor BI and CD81 receptor dependency and sensitivity to neutralizing antibodies. J Virol. 2008;82(24):12020–12029. doi: 10.1128/JVI.01569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andre P, Komurian-Pradel F, Deforges S, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76(14):6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fauvelle C, Felmlee DJ, Crouchet E, et al. Apolipoprotein E Mediates Evasion From Hepatitis C Virus Neutralizing Antibodies. Gastroenterology. 2016;150(1):206–217. doi: 10.1053/j.gastro.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 78.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1–E2 envelope protein complexes. J Exp Med. 2003;197(5):633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Pestka JM, Zeisel MB, Blaser E, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A. 2007;104(14):6025–6030. doi: 10.1073/pnas.0607026104. key study correlating rapid induction of virus-nAbs and viral clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osburn WO, Snider AE, Wells BL, et al. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology. 2014;59(6):2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raghuraman S, Park H, Osburn WO, et al. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis. 2012;205(5):763–771. doi: 10.1093/infdis/jir835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feray C, Gigou M, Samuel D, et al. Incidence of hepatitis C in patients receiving different preparations of hepatitis B immunoglobulins after liver transplantation. Ann Intern Med. 1998;128(10):810–816. doi: 10.7326/0003-4819-128-10-199805150-00003. [DOI] [PubMed] [Google Scholar]
- 83.Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138(1):315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park SH, Shin EC, Capone S, et al. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology. 2012;143(4):1048–1060. doi: 10.1053/j.gastro.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119(7):1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thimme R, Oldach D, Chang KM, et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194(10):1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thimme R, Bukh J, Spangenberg HC, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99(24):15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urbani S, Amadei B, Fisicaro P, et al. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44(1):126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 89*.Raziorrouh B, Sacher K, Tawar RG, et al. Virus-Specific CD4+ T Cells Have Functional and Phenotypic Characteristics of Follicular T-Helper Cells in Patients With Acute and Chronic HCV Infections. Gastroenterology. 2016;150(3):696–706. doi: 10.1053/j.gastro.2015.11.005. study of the role of T-follicular helper cells in spontaneous clearance of HCV. [DOI] [PubMed] [Google Scholar]
- 90*.Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci U S A. 1994;91(4):1294–1298. doi: 10.1073/pnas.91.4.1294. key study demonstrating that vaccination of chimpanzees with HCV envelope glycoproteins can protect against HCV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meunier JC, Gottwein JM, Houghton M, et al. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J Infect Dis. 2011;204(8):1186–1190. doi: 10.1093/infdis/jir511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Houghton M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol Rev. 2011;239(1):99–108. doi: 10.1111/j.1600-065X.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 93.Frey SE, Houghton M, Coates S, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28(38):6367–6373. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stamataki Z, Coates S, Abrignani S, et al. Immunization of human volunteers with hepatitis C virus envelope glycoproteins elicits antibodies that cross-neutralize heterologous virus strains. J Infect Dis. 2011;204(5):811–813. doi: 10.1093/infdis/jir399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Law JL, Chen C, Wong J, et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS One. 2013;8(3):e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong JA, Bhat R, Hockman D, et al. Recombinant hepatitis C virus envelope glycoprotein vaccine elicits antibodies targeting multiple epitopes on the envelope glycoproteins associated with broad cross-neutralization. J Virol. 2014;88(24):14278–14288. doi: 10.1128/JVI.01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colombatto P, Brunetto MR, Maina AM, et al. HCV E1E2-MF59 vaccine in chronic hepatitis C patients treated with PEG-IFNalpha2a and Ribavirin: a randomized controlled trial. J Viral Hepat. 2014;21(7):458–465. doi: 10.1111/jvh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leroux-Roels G, Depla E, Hulstaert F, et al. A candidate vaccine based on the hepatitis C E1 protein: tolerability and immunogenicity in healthy volunteers. Vaccine. 2004;22(23–24):3080–3086. doi: 10.1016/j.vaccine.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 99.Verstrepen BE, Depla E, Rollier CS, et al. Clearance of genotype 1b hepatitis C virus in chimpanzees in the presence of vaccine-induced E1-neutralizing antibodies. J Infect Dis. 2011;204(6):837–844. doi: 10.1093/infdis/jir423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baumert TF, Ito S, Wong DT, et al. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol. 1998;72(5):3827–3836. doi: 10.1128/jvi.72.5.3827-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lechmann M, Murata K, Satoi J, et al. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34(2):417–423. doi: 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 102.Jeong SH, Qiao M, Nascimbeni M, et al. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J Virol. 2004;78(13):6995–7003. doi: 10.1128/JVI.78.13.6995-7003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Awady MK, Tabll AA, Yousif H, et al. Murine neutralizing antibody response and toxicity to synthetic peptides derived from E1 and E2 proteins of hepatitis C virus. Vaccine. 2010;28(52):8338–8344. doi: 10.1016/j.vaccine.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 104.Garrone P, Fluckiger AC, Mangeot PE, et al. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med. 2011;3(94):94ra71. doi: 10.1126/scitranslmed.3002330. [DOI] [PubMed] [Google Scholar]
- 105.Akazawa D, Moriyama M, Yokokawa H, et al. Neutralizing antibodies induced by cell culture-derived hepatitis C virus protect against infection in mice. Gastroenterology. 2013;145(2):447–455. doi: 10.1053/j.gastro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 106.Forns X, Payette PJ, Ma X, et al. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32(3):618–625. doi: 10.1053/jhep.2000.9877. [DOI] [PubMed] [Google Scholar]
- 107.Youn JW, Park SH, Lavillette D, et al. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology. 2005;42(6):1429–1436. doi: 10.1002/hep.20934. [DOI] [PubMed] [Google Scholar]
- 108.Alvarez-Lajonchere L, Shoukry NH, Gra B, et al. Immunogenicity of CIGB-230, a therapeutic DNA vaccine preparation, in HCV-chronically infected individuals in a Phase I clinical trial. J Viral Hepat. 2009;16(3):156–167. doi: 10.1111/j.1365-2893.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 109.Patient R, Hourioux C, Vaudin P, et al. Chimeric hepatitis B and C viruses envelope proteins can form subviral particles: implications for the design of new vaccine strategies. N Biotechnol. 2009;25(4):226–234. doi: 10.1016/j.nbt.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Beaumont E, Patient R, Hourioux C, et al. Chimeric hepatitis B virus/hepatitis C virus envelope proteins elicit broadly neutralizing antibodies and constitute a potential bivalent prophylactic vaccine. Hepatology. 2013;57(4):1303–1313. doi: 10.1002/hep.26132. [DOI] [PubMed] [Google Scholar]
- 111.Elmowalid GA, Qiao M, Jeong SH, et al. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci U S A. 2007;104(20):8427–8432. doi: 10.1073/pnas.0702162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scull MA, Shi C, de Jong YP, et al. Hepatitis C virus infects rhesus macaque hepatocytes and simianized mice. Hepatology. 2015;62(1):57–67. doi: 10.1002/hep.27773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaufmann KW, Lemmon GH, Deluca SL, et al. Practically useful: what the Rosetta protein modeling suite can do for you. Biochemistry. 2010;49(14):2987–2998. doi: 10.1021/bi902153g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kong L, Giang E, Robbins JB, et al. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc Natl Acad Sci U S A. 2012;109(24):9499–9504. doi: 10.1073/pnas.1202924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bohne-Lang A, von der Lieth CW. GlyProt: in silico glycosylation of proteins. Nucleic Acids Res. 2005;33:W214–219. doi: 10.1093/nar/gki385. [DOI] [PMC free article] [PubMed] [Google Scholar]