Abstract
Background
Screening with mammography can detect breast cancer early, before clinical symptoms appear. Some cancers, however, are not captured with mammography screening alone. Among women at high risk for breast cancer, magnetic resonance imaging (MRI) has been suggested as a safe adjunct (supplemental) screening tool that can detect breast cancers missed on screening mammography, potentially reducing the number of deaths associated with the disease. However, the use of adjunct screening tests may also increase the number of false-positive test results, which may lead to unnecessary follow-up testing, as well as patient stress and anxiety. We investigated the benefits and harms of MRI as an adjunct to mammography compared with mammography alone for screening women at less than high risk (average or higher than average risk) for breast cancer.
Methods
We searched Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database, from January 2002 to January 2016, for evidence of effectiveness, harms, and diagnostic accuracy. Only studies evaluating the use of screening breast MRI as an adjunct to mammography in the specified populations were included.
Results
No studies in women at less than high risk for breast cancer met our inclusion criteria.
Conclusions
It remains uncertain if the use of adjunct screening breast MRI in women at less than high risk (average or higher than average risk) for breast cancer will reduce breast cancer–related mortality without significant increases in unnecessary follow-up testing and treatment.
BACKGROUND
Clinical Need and Target Population
Breast Cancer
Breast cancer is the most common cancer among Canadian women, with an estimated 1 in 9 women expected to develop the disease during their lifetime.1 In Ontario, an estimated 9,500 women are diagnosed with and 1,950 die from breast cancer annually.1
Most breast cancers are invasive, meaning the cancer invades the surrounding tissue of the breast. Invasive breast cancers can metastasize (spread) to the lymph nodes and other parts of the body, including bone, the lungs, and the brain. Some women will be diagnosed with a noninvasive breast cancer, meaning that abnormal cells have not spread to neighbouring breast tissue. The most common noninvasive breast cancer is ductal carcinoma in situ (abnormal cells in the milk ducts). The natural history of ductal carcinoma in situ is poorly understood, and the significance of these lesions as a precursor to invasive breast cancer is unclear.2,3
Treatment options for breast cancer vary depending on the stage of the disease and the cancer pathology. Treatment often involves a combination of surgery, hormone therapy, chemotherapy, and/or radiation therapy.
Classifying Breast Cancer Risk
The risk of developing breast cancer increases with age: More than 75% of breast cancer cases occur in women over the age of 50 years who have no other specific risk factors for the disease.4 Other factors can also increase the risk of developing breast cancer, including a family history of breast or ovarian cancer, extremely dense breast tissue, endogenous estrogen exposure, and various lifestyle factors. The strongest known risk factor for breast cancer is hereditary, resulting from gene mutations (changes) inherited from a parent. The most common hereditary breast cancers are due to mutations in the BRCA genes.
Numerous models with varying degrees of complexity have been developed to predict the lifetime risk of developing breast cancer; these are based primarily on genetic assessment, age, and family history of breast cancer. Common risk assessment tools include BRCAPRO, the Gail model, the International Breast Cancer Intervention Study (IBIS) Breast Cancer Risk Evaluation Tool, and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA).5–8
Classifications of breast cancer risk are multifaceted and not standardized across countries or in the literature. In Ontario, women are generally considered to be at average risk for breast cancer if they do not have any significant risk factors for the disease relative to the general population.9 Based on population incidence rates for breast cancer, average risk is regularly defined as a less than 15% risk of developing the disease over a lifetime.1,10 Some women have risk factors that place them at higher risk of developing breast cancer than an average woman but do not meet the accepted threshold for high-risk classification. Women are typically considered to be at higher than average, or intermediate, risk for developing the disease when lifetime risk is between 15% and 25%, based on family risk factors such as having two or more first-degree female relatives with breast cancer, having one first-degree relative under the age of 50 years at the time of breast cancer diagnosis, or having a first-degree relative with ovarian cancer.9 Extremely high breast density (defined as greater than 75% fibrous or glandular breast tissue) has also been suggested as a factor increasing the risk of breast cancer four to five times compared to women with less dense breast tissue.11 Additionally, women with a personal history of biopsy-documented high-risk lesions (areas of abnormal tissue), including lobular carcinoma in situ, atypical ductal hyperplasia, and atypical lobular hyperplasia, are at higher than average risk of developing subsequent invasive cancer in either breast. Women with lobular neoplasias are estimated to have a 10% to 25% lifetime risk of developing breast cancer; this risk is independent of breast density but greatly increases when combined with an associated family history of disease.12–14
Women at high risk for breast cancer are commonly defined as having one or more of the following risk factors: being a known mutation carrier, being an untested first-degree relative of a mutation carrier, having a strong family history, or having a 25% or greater lifetime risk of getting breast cancer.9 Women who had radiation therapy to the chest before the age of 30 years and more than 8 years previously are also categorized as high risk.9 Women with BRCA1 or BRCA2 gene mutations are estimated to have a 40% to 80% lifetime risk of developing breast cancer.15,16 It is estimated that less than 1% of the general population are at high risk for breast cancer and that about 5% of all breast cancers are due to inherited genetic mutations.4 Women at high risk for breast cancer often develop the disease at a younger age than those at less than high risk, and their cancers tend to grow faster and be more aggressive.17,18 Women at high risk may choose preventive options to reduce their risk of breast cancer through chemoprevention, prophylactic mastectomy (surgical removal of all or part of the breasts), or oophorectomy (surgical removal of the ovaries).
Breast Cancer Screening
Breast cancer screening is the regular examination of healthy, asymptomatic women. The intent of breast cancer screening programs is to identify breast cancer early so that women can receive timely and effective treatment. Cancers identified and treated at earlier stages tend to have better prognoses than those that have progressed or metastasized.1 The ultimate goal of breast cancer screening is to reduce breast cancer–related deaths, as well as the morbidity associated with advanced stages of the disease.
A successful screening program must also aim to minimize any adverse consequences associated with the screening itself. Screening for breast cancer can pose many challenges. All screening tests have the potential to produce false test results, both false-negative and false-positive. False-negative tests—tests that indicate a person does not have the disease when they actually do—may delay necessary treatment. False-positive tests—tests that indicate a person has the disease when they do not—will lead to additional unnecessary testing to confirm the diagnosis. This process may include diagnostic mammography, ultrasound, and surgical biopsy, each of which poses risks. False-positive tests can also lead to serious distress, anxiety, and uncertainty for patients.19,20 Overdiagnosis and overtreatment are other potential risks of breast cancer screening. Some cancers detected by screening may never cause symptoms or become life-threatening. There is currently no definitive way of determining which screening-detected cancers will progress, meaning that some women may undergo treatment with surgery, radiation therapy, or chemotherapy that is not needed.
The primary method used for breast cancer screening is mammography, which uses low-dose x-rays to image the breast, either on film or digitally. Mammography is currently the only screening tool for breast cancer that has been shown to reduce breast cancer–related deaths through early detection for average-risk women aged 50 to 74 years.21 However, recent reviews have suggested that screening with mammography may not be as effective for this population as originally understood and may result in significant overdiagnosis and overtreatment.22 For younger average-risk women (aged 40–49 years), several reviews have found that mammography is not an effective tool for breast cancer screening.21,23
Mammography is not a perfect test, and several factors, such as breast cancer risk, tumour characteristics, younger age, and increased breast density, can decrease its diagnostic accuracy.24,25 The sensitivity of screening mammography has been shown to be limited in women at high risk for breast cancer due to a strong hereditary risk or known BRCA gene mutations.26,27 A high proportion of dense breast tissue (fibrous and glandular tissue) can make it more difficult to detect cancer on mammography, although there is considerable debate about the potential correlation between breast density and rates of interval cancers (cancers that are diagnosed between screening rounds).28,29 About 40% of all women are estimated to have heterogeneously dense breasts (50–74% dense tissue), and 10% are estimated to have extremely dense breasts (≥ 75% dense tissue).30 Increased breast density is directly related to younger age: Approximately 53% of premenopausal and 23% of postmenopausal women have at least 50% dense breast tissue. A review by Health Quality Ontario found that digital mammography is more sensitive than film mammography among women with heterogeneously or extremely dense breast tissue.26 The accuracy of mammography among women with other specific risk factors for breast cancer has not been extensively researched. One study found that the sensitivity of screening mammography was not significantly different in women with lobular carcinoma in situ or atypical hyperplasia and women without high-risk lesions. However, the specificity of screening was lower and interval cancer rates were higher for women with high-risk lesions.31
Adjunct screening with breast magnetic resonance imaging (MRI) is thought to be an effective approach to improving the sensitivity of screening with mammography alone (i.e., increase the number of true-positive test results), although potentially at the risk of increasing the rate of false-positive findings and unnecessary biopsies and treatments.
Technology
MRI is a tool that uses magnetic fields and radio waves to produce detailed cross-sectional images of tissue structures. Contrast between tissues in the breast depends on the magnetic environment and water content, which contribute to the signal intensity (brightness) of the breast image. Breast MRI generally requires the use of a gadolinium-based contrast agent that is injected intravenously to provide better detail and detection of breast cancers and lesions. Dedicated breast MRI coils are also used to improve image quality. During the procedure, women typically lie prone in the MRI scanner. Scanning takes approximately 30 to 45 minutes, and the MRI images are then interpreted by a radiologist.
Some of the most common clinical indications for breast MRI are for diagnostic purposes such as to evaluate abnormalities detected on mammography or ultrasound, assess the status of breast implants, perform presurgical breast cancer staging, evaluate palpable breast lumps or other breast-related symptoms, guide breast biopsies, and monitor chemotherapy.
In high-risk populations, screening with both MRI and mammography annually improves the sensitivity of screening (sensitivity range 84–94%) but decreases specificity (specificity range 77–95%) relative to screening with mammography alone.26,27 Research in high-risk women has indicated that MRI is limited in its ability to identify noninvasive breast cancers (e.g., ductal carcinoma in situ) and should therefore be used as an adjunct to, rather than a replacement for, breast mammography.26 The benefits and harms of adjunct screening breast MRI among women at less than high risk for breast cancer, however, are unclear.
Regulatory Information
Several MRI systems are approved by Health Canada as Class II medical devices. Most scanners are general systems that can be used on the breast but are not specifically indicated for breast cancer or breast cancer screening. Several licensed MRI systems are also available that include software packages and modules specific to breast imaging, and numerous MRI coils are approved for use specifically in breast imaging in conjunction with a general magnetic resonance scanner.
Context
Breast Cancer Screening in Ontario
Screening for breast cancer can be done either as part of an organized program or opportunistically (when requested by the patient or offered by a health care provider at a routine or unrelated health care visit). The Ontario Breast Screening Program is a province-wide, organized, publicly funded screening program for breast cancer.9 Table 1 summarizes the program's current recommendations for breast cancer screening.9 Average-risk women between the ages of 50 and 74 years are offered screening with mammography every two years, whereas women at higher than average (but not high) risk are offered screening with mammography annually. Screening with both mammography and MRI is currently indicated for women aged 30 to 69 years at high risk for breast cancer; this indication is supported by a 2010 Health Quality Ontario review and Ontario Health Technology Advisory Committee recommendation in addition to several national and international agencies and organizations.26
Table 1:
Summary of Ontario Breast Screening Program Guidelines
| Age, Years | Risk | Screening Tests and Frequency |
|---|---|---|
| Women < 50 | Average | Screening not recommended |
| Women 50–74 | Average | Mammography every 2 years |
| Women 50–74 | Higher than averagea | Annual mammography |
| Women 30–69 | High | Annual mammography and breast MRI, or screening breast ultrasound if MRI is contraindicatedb |
Abbreviation: MRI, magnetic resonance imaging.
Documented pathology of high-risk lesions, including atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ, benign papillary lesion, benign phyllodes tumour, and radial scar; a personal history of ovarian cancer; two or more first-degree female relatives with breast cancer at any age; one first-degree relative with breast cancer under age 50 years; one first-degree relative with ovarian cancer at any age; one first-degree male relative with breast cancer at any age; or breast density greater than 75% as seen on mammogram (reassessed annually by a screening radiologist).
Contraindications include metallic implants (e.g., pacemakers or aneurysm clips), contrast allergies, and claustrophobia. There are also body and weight restrictions to the MRI machines.
Approximately 1.3 million women in Ontario aged 50 to 74 years (65% of those eligible) were screened for breast cancer with mammography between 2013 and 2014. Of these women, more than 77% were screened through the Ontario Breast Screening Program.32
Funding of MRI for Breast Cancer Screening in Ontario
Screening breast MRI is currently funded in Ontario for women at high risk for breast cancer. The Ontario Schedule of Benefits states that breast MRI is not an insured service for the routine screening of an average-risk person.33 No specifications are listed for women who are at higher than average (but not high) risk for breast cancer.
National and International Guidelines for Technology
Several national and international guidelines and health technology assessment organizations have provided recommendations regarding the use of adjunct screening breast MRI in women who are at less than high risk for breast cancer (Table 2). Based on a lack of available evidence, no guidelines recommended the use of MRI in women at average risk for breast cancer. For women with risk factors that place them at above average, but less than high, risk for breast cancer, three sets of guidelines state there is inadequate or insufficient evidence to make a recommendation; one does not recommend the use of MRI; and only one states that MRI may be considered in some of these patients.
Table 2:
Clinical Guideline and Health Technology Assessment Recommendations on the Use of Screening Breast MRI in Women at Less Than High Risk for Breast Cancer
| Guidance, Year | Recommendation |
|---|---|
| U.S. Preventive Services Task Force, 201634,35 |
Average Risk: Current evidence is insufficient to assess the additional benefits and harms of MRI vs. film mammography as a screening modality for breast cancer Dense Breasts: Current evidence is insufficient to assess the balance of benefits and harms of adjunct screening using MRI in women identified as having dense breasts on an otherwise negative screening mammogram |
| International Agency for Research on Cancer, 201536 |
Intermediate Risk: Inadequate evidence that MRI as an adjunct to mammography vs. mammography alone increases the detection rate of breast cancer or the proportion of false-positive screening outcomes in women with LCIS or atypical proliferations |
| Washington State Health Authority, 201537 |
Average Risk: Supplementary screening with MRI is not covered Dense Breasts: Available evidence is not sufficient to support coverage for MRI for supplementary screening following mammography |
| Aetna, 201538 |
Average Risk: Considers breast MRI experimental and investigational…because there is insufficient scientific evidence to support its use |
| National Institute for Health and Care Excellence, 201339 |
Intermediate Risk: Do not offer MRI to women of any age at moderate risk (greater than 17% but less than 30%) of breast cancer |
| Alberta Health Services, 2012,40 and American Cancer Society, 200741 |
Average Risk: Recommend against MRI screening (based on expert consensus opinion)
Intermediate Risk: Insufficient evidence to recommend for or against screening in women with the following:
|
| American College of Radiology, 201242 |
Average Risk: Screening breast MRI is not recommended for the general population of asymptomatic, average-risk women Intermediate Risk: Contrast-enhanced MRI may be indicated in some patients |
| Canadian Task Force on Preventive Health Care, 201121 |
Average Risk:
|
| National Comprehensive Cancer Network, 200943 |
Average Risk: Current evidence does not support the routine use of breast MRI as a screening procedure in average-risk women Intermediate Risk: Consider annual breast MRI for LCIS as an adjunct to mammogram and clinical breast exam |
Abbreviations: ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; LCIS, lobular carcinoma in situ; MRI, magnetic resonance imaging.
Research Question
What are the effectiveness, harms, and diagnostic accuracy of breast MRI as an adjunct to mammography in comparison to mammography alone for breast cancer screening among women at less than high risk (average and higher than average risk) for developing breast cancer?
CLINICAL EVIDENCE REVIEW
Objective
The objective of this clinical evidence review was to assess the effectiveness, harms, and diagnostic accuracy of breast MRI as an adjunct to mammography in comparison to mammography alone for breast cancer screening among women at less than high risk for developing breast cancer.
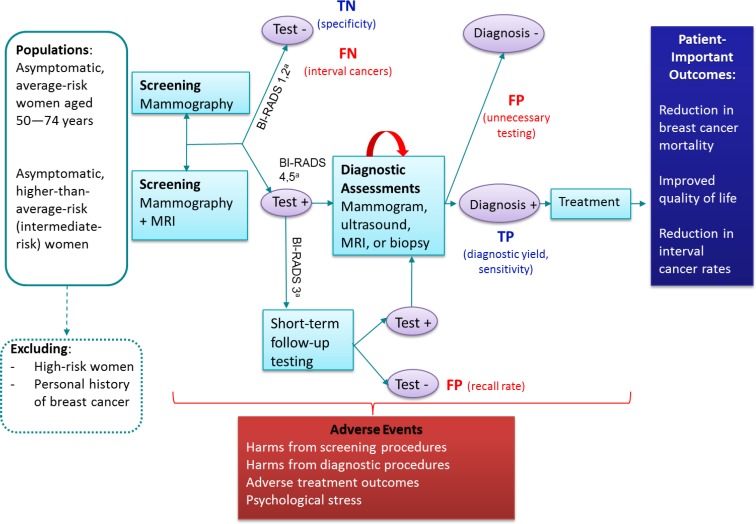
Figure 1 displays the general screening pathway and framework for our research questions. The overarching question was whether adjunct screening with MRI improves patient-important outcomes relative to screening with mammography alone. Improvement in patient-important outcomes may be associated with harms related to screening tests, diagnostic tests, or treatment. False-positive results may lead to unnecessary testing, surgery, or treatment, and false-negative tests may result in more aggressive and difficult-to-treat cancers.
Figure 1: Framework for Screening With MRI as an Adjunct to Mammography.
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; FN, false-negative; FP, false-positive; MRI, magnetic resonance imaging; TN, true-negative; TP, true-positive.
aBI-RADS 1 = negative; BI-RADS 2 = benign; BI-RADS 3 = probably benign; BI-RADS 4 = suspicious abnormality; BI-RADS 5 = highly suggestive of malignancy.
Source: Adapted from Health Quality Ontario.44
Methods
Research questions were developed by Health Quality Ontario in consultation with experts, end users, and/or applicants in the topic area. There is variability in the definitions used to define breast cancer risk; for consistency, the definitions used in this review were based primarily on the definitions used by the Ontario Breast Screening Program.
Sources
We performed a literature search on January 14, 2016, using Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database, for studies published from January 1, 2002, to January 14, 2016. The year 2002 was selected as the start date as previous reviews identified no studies of MRI as an adjunct or comparative test in less-than-high-risk women before this date.26,45
Search strategies were developed by medical librarians using medical subject headings (MESH). See Appendix 1 for full details, including all search terms.
Literature Screening
A single reviewer reviewed the abstracts, and, for those studies meeting the eligibility criteria, we obtained full-text articles. We also examined reference lists for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
-
Studies of asymptomatic women aged 50 years and older at average lifetime risk for breast cancer
-
○
Defined as a less than 15% lifetime risk of breast cancer, or studies excluding intermediate and high-risk women as defined below, or studies of women with dense breasts but no additional high-risk factors
Or studies of asymptomatic women at higher than average, or intermediate, lifetime risk for breast cancer
-
○
Defined as higher-than-average risk but not meeting the threshold of high risk, including a 15% to 25% lifetime risk of breast cancer (based primarily on family history models), or studies of women with a personal history of high-risk lesions (including lobular carcinoma in situ, atypical ductal hyperplasia, atypical lobular hyperplasia, and benign papillary lesions) but no additional high-risk factors
Or studies of asymptomatic women at less than high risk for breast cancer
-
○
Defined as studies excluding high-risk women, where high risk is defined as being a carrier of or having one or more first-degree relatives with a breast cancer gene mutation (e.g., BRCA1, BRCA2), having undergone chest radiation prior to age 30 years, a lifetime risk of breast cancer equal to or greater than 25%, or a high lifetime risk as defined in research articles
-
○
Studies assessing screening breast MRI as an adjunct to screening mammography (provided simultaneously or sequentially with mammography)
Studies using pathology results from biopsy as a reference standard for true-positive tests and a minimum of clinical follow-up for women with negative imaging results
-
Studies reporting on one or more outcomes of interest
-
○
For studies reporting only on diagnostic performance (yield or accuracy) outcomes, sufficient information to construct a two-by-two table (true-positives, true-negatives, false-positives, false-negatives) was required for inclusion
-
○
Exclusion Criteria
Studies among symptomatic women (e.g., clinical symptoms or palpable breast mass prior to enrollment)
Studies of diagnostic MRI (e.g., pre-operative use, to evaluate abnormalities detected on other imaging tests, to assess the status of breast implants, for presurgical staging, to provide image-guided percutaneous biopsies, or to monitor neoadjuvant chemotherapy)
Studies only among women with a personal history of breast cancer
Studies including women at high risk for breast cancer
Studies in which population risk for breast cancer was not specified or results were not stratified by included population risk groups
Studies comparing MRI alone to mammography alone as a primary screening modality
Abstract, editorial, case report, or commentary
Outcomes of Interest
Effectiveness and Harms
Breast cancer mortality
All-cause mortality
Number needed to screen to prevent one additional death
Health-related quality of life
Screening-related harms
Diagnostic Performance (Diagnostic Accuracy and Yield)
-
Incremental diagnostic yield (incremental cancer detection rate)
-
○
Cancer and tumour characteristics: tumour size, invasiveness, lymph node status
-
○
Sensitivity (true-positive rate)
Specificity (true-negative rate)
False-negative rate
False-positive rate
Positive predictive value (the proportion of all positive results that were true-positives) among women who tested positive for disease and among women who received a follow-up biopsy
Biopsy rate and recall rate
Study Designs
-
Only primary research studies were included. We used the following hierarchical approach based on study design:
For effectiveness outcomes
-
1)
Randomized controlled trials and prospective comparative studies
For diagnostic performance outcomes
-
1)
Randomized controlled trials and prospective comparative studies; paired cohort studies were considered the ideal design for observational studies46
-
2)
Prospective noncomparative studies (including studies of MRI among women with negative mammography) and retrospective comparative studies
-
1)
We contacted authors via email where there were missing or incomplete data reported and where clarification was needed regarding study populations or outcome definitions.
Data Extraction
We planned to extract relevant data on study characteristics, risk of bias items, population, interventions, comparators, and outcomes using a standardized data form.
Expert Consultation
In January 2016, expert consultation was solicited on the use of breast MRI as an adjunct to mammography in comparison to mammography alone for breast cancer screening. Members of the consultation included clinicians and researchers in the specialty areas of oncology, radiology, and breast cancer screening. The role of the expert advisors was to review the clinical research plan, contextualize the evidence, and provide advice on screening for breast cancer with MRI. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Literature Search
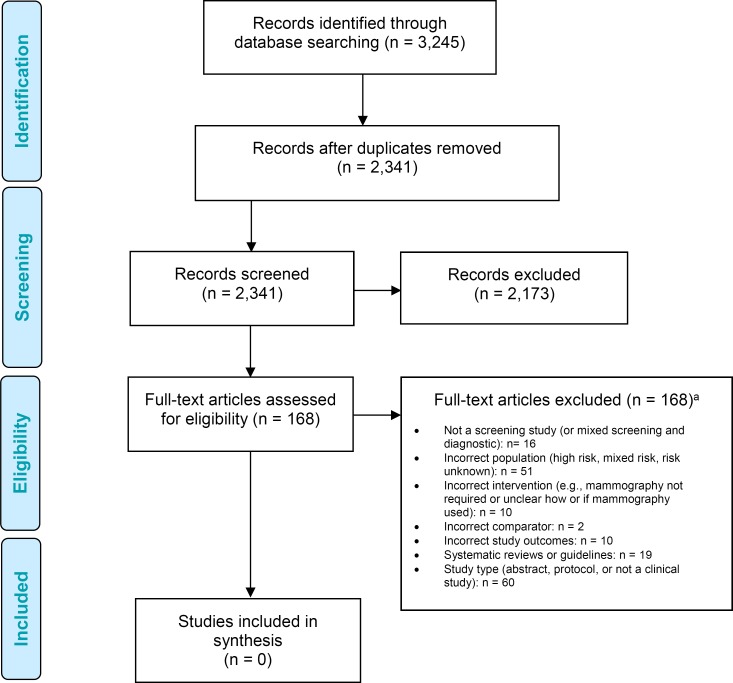
The database search yielded 3,245 citations published between January 1, 2002, and January 14, 2016. After removing duplicates, we reviewed titles and abstracts to identify potentially relevant articles. We obtained the full texts of these articles for further assessment.
Figure 2 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 2: PRISMA Flow Diagram.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
aArticles may have been excluded for more than one of the criteria listed.
Source: Adapted from Moher et al.47
Included Studies
No studies were identified that met the inclusion criteria.
Characteristics of Relevant Excluded Studies
We identified several studies that focused on the use of screening breast MRI in specific groups of women with average or intermediate risk factors for breast cancer. One study evaluated the use of adjunct MRI among a subgroup of women at moderate risk for breast cancer due to familial predisposition, and eight studies evaluated the use of adjunct screening breast MRI among women with a personal history of high-risk lesions or women with dense breasts on mammography.
Given that these excluded studies have formed the evidence base for other reviews, a description of the reasons for exclusion from our review is provided below.
Studies of Women With Moderate Familial or Genetic Predisposition
We identified one primary study that stratified screening results for mammography and adjunct MRI among women at moderate risk for breast cancer (defined as a 15–30% lifetime risk) based on familial or genetic predisposition for the disease.48 This study was excluded because of insufficient outcome reporting. The primary intervention comparison was mammography alone versus MRI alone, with sufficient data to estimate only the additional number of cancers identified (sensitivity) with the use of MRI as an adjunct to mammography.
Studies of Women With High-Risk Lesions
Five studies assessed the use of screening breast MRI in asymptomatic women with a previous biopsy demonstrating lobular carcinoma in situ,49–53 two of which also included women with atypical ductal hyperplasia and atypical lobular hyperplasia.51,52
Two studies were primarily excluded as they did not meet the defined population inclusion criteria for this review. Schwartz et al reported a lifetime risk of breast cancer exceeding 25% (up to 46%) in more than half of the women included,52 and Sung et al did not exclude women with known or suspected BRCA mutations or a strong family history of the disease.53 These studies were further excluded due to insufficient information regarding the use of mammography and MRI (i.e., required for all women vs. a subset; screening vs. diagnostic mammography results; MRI as a simultaneous, sequential, or replacement test).
Three studies did not provide information regarding the overall population risk for breast cancer but were excluded predominately because of inadequate information regarding both the included interventions and reporting of outcome measures.49–51 Friedlander et al excluded women with an abnormality depicted on other imaging modalities but did not indicate if all women received mammography.49 In the studies by King et al and Port et al, details regarding the use of mammography and MRI screening were not described, and insufficient information was provided to determine how and when MRI was performed relative to mammography screening.50,51 All three studies reported on incremental cancer detection rates, but insufficient data were provided to assess the sensitivity and false-positive rates for the two screening groups.
Studies of Women With Dense Breasts
Three primary studies were identified that evaluated the use of adjunct screening breast MRI among women with dense breasts.54–56 However, all were primarily excluded for including women who were known to be at high risk for breast cancer based on a personal or strong family history of breast cancer. No studies were identified that stratified women by breast density as the primary risk factor for breast cancer.
Ongoing Studies
One recently published study protocol was designed to assess the additional value of screening breast MRI among women aged 50 to 75 years with extremely dense breasts and negative screening mammography results.57 This study intends to randomize women either to MRI screening or to a control arm with no MRI screening. The primary study outcome is the difference in the proportion of interval cancers over two years, with secondary outcomes including the number of MRI screening–detected cancers, proportion of false-positive imaging results, positive predictive value, and sensitivity of screening. Among detected cancers, the investigators plan to compare tumour size, stage, and grade distributions. Quality of life and cost-effectiveness will also be assessed. It is unclear if the included study population is limited to women with an otherwise less than high risk for developing breast cancer. The anticipated completion date of the study was not provided in the protocol.57
Discussion
No studies evaluating the use of MRI as an adjunct to mammography in comparison to mammography alone for breast cancer screening met the inclusion criteria of the review.
Our results are consistent with conclusions of other systematic reviews that evaluated the evidence for using adjunct screening breast MRI in women at less than high risk for breast cancer. Several older reviews and international guidelines have reported a lack of published evidence for screening breast MRI as an adjunct test for women at average risk for breast cancer.21,41,45 One review using similar inclusion criteria to ours reported a lack of evidence for the effectiveness of adjunct MRI in either a population with dense breasts or in one defined as intermediate risk (15–20% lifetime risk).58 Other systematic reviews of women with dense breasts used more liberal selection criteria (i.e., they did not exclude high-risk women and did not require comprehensive diagnostic performance outcome reporting). However, these reviews consistently concluded that the effectiveness of adjunct screening MRI among women with dense breasts was uncertain.59 Similarly, a systematic review that evaluated the use of screening breast MRI in women with a personal history of lobular neoplasias or proliferative lesions60 also concluded that the benefits and harms of adjunct MRI in these populations were uncertain.
To date, only studies among women defined as high risk (those with BRCA mutations or a strong hereditary risk of breast cancer) have shown evidence for the improved sensitivity of cancer detection with adjunct screening breast MRI, in addition to the identification of earlier-stage tumours with more favourable prognostic characteristics. However, results from these studies cannot be directly extrapolated to a lower-risk population, as both sensitivity and specificity have been found to differ based on risk status. Additionally, women at high risk for breast cancer exhibit altered tumour histology (microscopic anatomy) and morphology (tissue structure) on mammography and have a higher rate of interval cancers than those at less than high risk. The benefit of screening breast MRI in populations other than high risk may differ among subgroups of women with different risk factor profiles. However, given the lower yearly incidence of breast cancer in the less-than-high-risk population, and the low specificity of MRI in the high-risk population, additional screening breast MRI may result in unnecessary short-term follow-up in a significant proportion of the less-than-high-risk screened population.
Given that no evidence was identified in the clinical evidence review, the economic impact of adjunct screening breast MRI and the lived experience of patients at less than high risk for breast cancer were not assessed.
Study Limitations
This review used a focused definition of breast cancer risk classification that aligns with current Ontario Breast Cancer Screening Program definitions.9 As such, all possible permutations and combinations of breast cancer risk factors could not be thoroughly assessed. Rather, this review provides a focused view of the literature within the context of Ontario.
No research has evaluated the specific use of adjunct screening breast MRI among women at less than high risk for breast cancer, and the vast majority of studies excluded from this literature review focused on women at high risk for breast cancer. While there is general consensus as to which women are considered to be at very high risk for breast cancer (i.e., BRCA mutation, lifetime risk greater than 25%, having undergone chest irradiation prior to age 30 years), the definitions of average, moderate, and intermediate risk have not been standardized. Further, women at less than high risk constitute a much larger and quite heterogeneous group in terms of risk factors. Many high-risk studies that were excluded from our review included women with a risk greater than 15% or women with a personal history of lobular carcinoma in situ or atypical hyperplasia within the studies’ high-risk cohort definitions. Similarly, studies on the evaluation of women with dense breasts or atypical breast lesions also included women with other factors that placed them at high risk for breast cancer. A drawback of a precise focus on women at less than high risk for breast cancer is that only a subset of the overall literature on screening breast MRI could be included in this review. Although this precise focus presented a challenge, including populations with other risk factors may have confounded any benefit found with adjunct screening breast MRI.
In Ontario, the IBIS and BODICEA breast cancer risk evaluation tools are recommended to assess a woman's lifetime risk of breast cancer. The published literature, however, is complicated by the use of a wide variety of other models that assess the lifetime or 10-year risk of developing breast cancer.5 These models were developed with varying populations, and each relies on different aspects of a woman's personal and familial history. While all models include genetic mutations and familial risk factors as input variables, few include factors such as breast density or the presence of high-risk lesions. As such, different models have been shown to result in different lifetime risk classifications.61
Lastly, this review did not look at the evidence for adjunct screening breast MRI among women with a personal history of breast cancer. While a personal history of breast cancer alone can place a women at higher than average risk of developing breast cancer (i.e., a second breast cancer), evaluating this particular risk factor was considered beyond the scope of this review. Screening and surveillance for breast cancer are important for women with a personal history of the disease, however, and require unique considerations of various complexities, such as concurrent treatment, mastectomy, tumour biology, the number and location of tumours, and patient preferences. Currently, women with a personal history of breast cancer are not included in the Ontario Breast Screening Program's average-risk program.
Conclusions
Currently, there is no evidence available to inform us about whether adjunct screening breast MRI improves the detection of breast cancer without significantly increasing the rate of associated false-positives or harms in women at less than high risk for breast cancer. Furthermore, no studies to date have shown a reduction in mortality when comparing mammography plus adjunct MRI to mammography alone for breast cancer screening in any patient population. Ongoing and future studies may provide further insight into the potential benefits and harms of adjunct screening with MRI among women at less than high risk for breast cancer.
Acknowledgments
The medical editor was Kara Stahl. Others involved in the development and production of this report were Chris Pagano, Ana Laing, Claude Soulodre, Andrée Mitchell, Anil Thota, Vivian Ng, Nancy Sikich, and Irfan Dhalla.
We are grateful to our expert advisors: Dr. Anna Chiarelli, senior scientist, Prevention and Cancer Control, provincial scientific lead, Ontario Breast Screening Program, Cancer Care Ontario, and professor, Dalla Lana School of Public Health, University of Toronto; and Dr. Derek Muradali, radiologist in chief, Ontario Breast Screening Program, Cancer Care Ontario.
Glossary
ABBREVIATIONS
- BOADICEA
Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm
- IBIS
International Breast Cancer Intervention Study
- MRI
Magnetic resonance imaging
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
Glossary
GLOSSARY
- BRCA1 and BRCA2
BRCA1 and BRCA2 are genes that help control cell growth. People with abnormalities in these genes are at higher risk of certain cancers, including breast, prostate, and ovarian cancer.
- Diagnostic yield
The diagnostic yield is the proportion of screens in a study who are correctly diagnosed as having the condition tested.
- False-negative
A test result that indicates a person does not have the disease or condition tested for when they actually do have it.
- False-positive
A test result that indicates a person has the disease or condition tested for when they actually do not have it.
- Lesion
Area of abnormal tissue.
- Neoplasia
Abnormal and uncontrolled cell growth.
- Positive predictive value
The proportion of people with a positive test result who actually have the disease or characteristic.
- Sensitivity
The ability of a test to accurately identify persons with the condition tested for (how well it returns positive results in persons who have the condition).
- Specificity
The ability of a test to accurately identify persons who do not have the condition tested for (how well it returns negative results in persons who do not have the condition).
- True-negative
A test result where a person who does not have the disease or condition tested for is correctly identified as not having it.
- True-positive
A test result where a person who has the disease or condition tested for is correctly identified as having it.
APPENDICES
Appendix 1: Literature Search Strategies
Search date: January 14, 2016
Databases searched: Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <December 2015>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to January 08, 2016>, EBM Reviews - Database of Abstracts of Reviews of Effects <2nd Quarter 2015>, EBM Reviews - Health Technology Assessment <4th Quarter 2015>, EBM Reviews - NHS Economic Evaluation Database <2nd Quarter 2015>, Embase <1980 to 2016 Week 02>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
| 1 | exp *Breast Neoplasms/ (467347) |
| 2 | Carcinoma, Lobular/ (23219) |
| 3 | ((breast* or mammar*) adj2 (cancer* or neoplas* or tumo?r* or carcinoma* or adenoma*)).tw. (569839) |
| 4 | or/1–3 (676137) |
| 5 | exp Magnetic Resonance Imaging/ (977720) |
| 6 | (magnetic resonance or MRI or ((MR or NMR) adj2 (imag* or tomograph*))).tw. (835914) |
| 7 | or/5–6 (1212921) |
| 8 | 4 and 7 (20606) |
| 9 | (breast* adj2 MRI*).tw. (3715) |
| 10 | or/8–9 (21218) |
| 11 | exp Mass Screening/ (285163) |
| 12 | “Early Detection of Cancer”/ (63570) |
| 13 | Early Diagnosis/ (98796) |
| 14 | (screen* or (earl* adj (diagnos* or detect*))).tw. (1457364) |
| 15 | or/11–14 (1593702) |
| 16 | 10 and 15 (3997) |
| 17 | limit 16 to (english language and yr=“2002 -Current”) [Limit not valid in CDSR,DARE; records were retained] (3319) |
| 18 | 17 use pmoz,cctr,coch,dare,clhta,cleed (1115) |
| 19 | exp *breast tumor/ (461691) |
| 20 | ((breast* or mammar*) adj2 (cancer* or neoplas* or tumo?r* or carcinoma* or adenoma*)).tw. (569839) |
| 21 | or/19–20 (658680) |
| 22 | exp nuclear magnetic resonance imaging/ (636853) |
| 23 | (magnetic resonance or MRI or ((MR or NMR) adj2 (imag* or tomograph*))).tw. (835914) |
| 24 | or/22–23 (1086320) |
| 25 | 21 and 24 (18989) |
| 26 | (breast* adj2 MRI*).tw. (3715) |
| 27 | or/25–26 (19611) |
| 28 | mass screening/ (137980) |
| 29 | cancer screening/ (67375) |
| 30 | early diagnosis/ (98796) |
| 31 | (screen* or (earl* adj (diagnos* or detect*))).tw. (1457364) |
| 32 | or/28–31 (1551723) |
| 33 | 27 and 32 (3746) |
| 34 | limit 33 to (english language and yr=“2002 -Current”) [Limit not valid in CDSR,DARE; records were retained] (3124) |
| 35 | 34 use emez (2130) |
| 36 | 18 or 35 (3245) |
| 37 | 36 use pmoz (997) |
| 38 | 36 use emez (2130) |
| 39 | 36 use cctr (19) |
| 40 | 36 use coch (30) |
| 41 | 36 use dare (31) |
| 42 | 36 use clhta (20) |
| 43 | 36 use cleed (18) |
| 44 | remove duplicates from 36 (2392)® |
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The lead clinical epidemiologist was Milica Nikitovic-Jokic, the medical librarian was Corinne Holubowich.
KEY MESSAGES
Screening for breast cancer is the process of looking for the disease before symptoms appear so it can be treated early. Women considered at average risk for breast cancer generally have a less than 15% chance of developing the disease over a lifetime, whereas women considered at high risk generally have a genetic mutation or a greater than 25% (1 in 4) risk of developing the disease. Some women have risk factors that place them at higher than average, but less than high, risk of developing breast cancer.
In Ontario, women at less than high risk, both average and higher than average risk, are screened with mammography (x-ray of the breast), but mammography alone may miss breast cancer in some women. Magnetic resonance imaging (MRI) is a potential tool to look for breast cancer that mammography may have missed.
This review intended to look at the impact of adding MRI to mammography to screen for breast cancer in women at less than high risk (average and higher than average risk), to see if screening with both tests catches more invasive breast cancers and saves more lives compared with mammography alone. We also wanted to know if MRI produces more false-positives (test results that show a woman has breast cancer when she does not), as false-positives can lead to unnecessary follow-up testing, treatment, and anxiety.
We found no studies that evaluated using both mammography and MRI to screen average-risk women. No studies of higher-than-average-risk women met the inclusion criteria of the review. Given that no relevant evidence was identified, we conclude that it is uncertain whether screening with both tests will reduce the number of deaths related to breast cancer or if it will lead to more false-positives and potentially unnecessary diagnosis and treatment.
Contributor Information
Health Quality Ontario:
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are.
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do.
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters.
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian cancer statistics 2015 [Internet]. Toronto (ON): Canadian Cancer Society; 2015. [cited 2015 Aug]. Available from: http://www.cancer.ca/statistics [Google Scholar]
- (2).Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006; 97(2):135–44. [DOI] [PubMed] [Google Scholar]
- (3).Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015; 1(7):888–96. [DOI] [PubMed] [Google Scholar]
- (4).Cancer Care Ontario. Ontario Breast Screening Program 2011 report. Toronto (ON): Cancer Care Ontario; 2013. [Google Scholar]
- (5).Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989; 81(24):1879–86. [DOI] [PubMed] [Google Scholar]
- (6).Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004; 23(7):1111–30. [DOI] [PubMed] [Google Scholar]
- (7).Antoniou AC, Pharoah PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004; 91(8):1580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Berry DA, Iversen ES, Jr, Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002; 20(11):2701–12. [DOI] [PubMed] [Google Scholar]
- (9).Cancer Care Ontario. About the Ontario Breast Screening Program [Internet]. Toronto (ON): Cancer Care Ontario; 2014. [cited 2015 May]. Available from: https://www.cancercare.on.ca/pcs/screening/breastscreening/obsp/ [Google Scholar]
- (10).Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975–2013, National Cancer Institute [Internet]. Bethesda (MD): National Cancer Institute; 2016. [cited 2016 Jun]. Available from: http://seer.cancer.gov/csr/1975_2013. [Google Scholar]
- (11).Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005; 6(10):798–808. [DOI] [PubMed] [Google Scholar]
- (12).Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985; 312(3):146–51. [DOI] [PubMed] [Google Scholar]
- (13).Page DL, Kidd TE, Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991; 22(12):1232–9. [DOI] [PubMed] [Google Scholar]
- (14).Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer. 2006; 106(10):2104–12. [DOI] [PubMed] [Google Scholar]
- (15).Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007; 25(11):1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Petrucelli N, Daly MB, Feldman GL. BRCA1 and BRCA2 hereditary breast and ovarian cancer. In: Pagon RA, Adam MP, Ardinger HH, et al, editors. Gene Reviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1998. [cited 2015 Jul]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1247/. [Google Scholar]
- (17).Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005; 365(9473):1769–78. [DOI] [PubMed] [Google Scholar]
- (18).Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Vijver MJ, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998; 90(15):1138–45. [DOI] [PubMed] [Google Scholar]
- (19).Lampic C, Thurfjell E, Bergh J, Sjoden PO. Short- and long-term anxiety and depression in women recalled after breast cancer screening. Eur J Cancer. 2001; 37(4):463–9. [DOI] [PubMed] [Google Scholar]
- (20).Steggles S, Lightfoot N, Sellick SM. Psychological distress associated with organized breast cancer screening. Cancer Prev Control. 1998; 2(5):213–20. [PubMed] [Google Scholar]
- (21).Canadian Task Force on Preventive Health Care, Tonelli M, Connor Gorber S, Joffres M, Dickinson J, Singh H, et al. Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ. 2011; 183(17):1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013; 6:CD001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Health Quality Ontario. Screening mammography for women aged 40 to 49 years at average risk for breast cancer: an evidence-based analysis. Ont Health Technol Assess Ser. 2007; 7(1). [PMC free article] [PubMed] [Google Scholar]
- (24).Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 2003;138:168–75. [DOI] [PubMed] [Google Scholar]
- (25).Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA. 1996; 276(1):33–8. [PubMed] [Google Scholar]
- (26).Health Quality Ontario. Cancer screening with digital mammography for women at average risk for breast cancer, magnetic resonance imaging (MRI) for women at high risk: an evidence-based analysis. Ont Health Technol Assess Ser. 2010; 10(3):1–55. [PMC free article] [PubMed] [Google Scholar]
- (27).Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008; 148(9):671–9. [DOI] [PubMed] [Google Scholar]
- (28).Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. Am J Roentgen. 2015; 204(2):234–40. [DOI] [PubMed] [Google Scholar]
- (29).Kerlikowske S, Zhu W, Tosteson A, Sprague B, Tice J. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Annals Int Med. 2015; 10(162):673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sickles EA, D'Orsi CJ, Bassett LW, Catherine MA, Berg WA, Burnside ES, et al. ACR BI-RADS® Mammography. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al, editors. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston (VA): American College of Radiology; 2013. [Google Scholar]
- (31).Houssami N, Abraham LA, Onega T, Collins LC, Sprague BL, Hill DA, et al. Accuracy of screening mammography in women with a history of lobular carcinoma in situ or atypical hyperplasia of the breast. Breast Cancer Res Treat. 2014; 145(3):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cancer Quality Council of Ontario. Breast cancer screening participation [Internet]. 2013. [cited 2015 July]. Available from: http://www.csqi.on.ca/cms/One.aspx?portalId=289784&pageId=296132 [Google Scholar]
- (33).Ontario Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): The Ministry; 2015. [cited 2016 Jun]. Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/sob_master11062015.pdf. [Google Scholar]
- (34). U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009; 151(10):716–26, W-236. [DOI] [PubMed] [Google Scholar]
- (35).Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016; 164(4):279–96. [DOI] [PubMed] [Google Scholar]
- (36).Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening–viewpoint of the IARC Working Group. N Engl J Med. 2015; 372(24):2353–8. [DOI] [PubMed] [Google Scholar]
- (37).Washington State Health Care Authority. Appropriate imaging for breast cancer screening in special populations - Health Technology Clinical Committee final findings and decision [Internet]. Olympia (WA): The Authority; 2015. [cited 2016 Jan]. Available from: http://www.hca.wa.gov/hta/Documents/breast_imaging_final_findings_decision_033015.pdf [Google Scholar]
- (38).Aetna Inc. Magnetic resonance imaging (MRI) of the breast. Clinical policy bulletin number 0105 [Internet]. Hartford (CT): Aetna Inc.; 2016. [cited 2016 Jun]. Available from: http://www.aetna.com/cpb/medical/data/100_199/0105.html [Google Scholar]
- (39).National Collaborating Centre for Cancer. Familial breast cancer. Classification and care of people at risk of familial breast cancer and management of breast cancer and related risks in people with a family history of breast cancer. London (UK): National Institute for Health and Care Excellence; 2013. [PubMed] [Google Scholar]
- (40).Alberta Provincial Breast Tumour Team. Magnetic resonance imaging for breast cancer screening, preoperative assessment, and follow-up (clinical practice guideline; no. BR-007). Edmonton (AB): Alberta Health Services, Cancer Care; 2012. [Google Scholar]
- (41).Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007; 57(2):75–89. [DOI] [PubMed] [Google Scholar]
- (42).Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, et al. ACR appropriateness criteria breast cancer screening. J Am Coll Radiol. 2013; 10(1):11–4. [DOI] [PubMed] [Google Scholar]
- (43).Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Nat Compr Canc Netw. 2009; 7(10):1060–96. [DOI] [PubMed] [Google Scholar]
- (44).Health Quality Ontario. Ultrasound as an adjunct to mammography for breast cancer screening: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2016. Jul [cited 2016 Jul];16(5):1–71. Available from: http://www.hqontario.ca/Evidence-to-Improve-Care/Journal-Ontario-Health-TechnologyAssessment-Series. [PMC free article] [PubMed] [Google Scholar]
- (45).Irwig L, Houssami N, van Vliet C. New technologies in screening for breast cancer: a systematic review of their accuracy. Br J Cancer. 2004; 90(11):2118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editors. Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 1.0. [Internet]. London: The Cochrane Collaborative; 2010. [cited 2015 Oct]. Available from: http://methods.cochrane.org/sdt/sites/methods.cochrane.org.sdt/files/uploads/Chapter%2010%20-%20Version%201.0.pdf. [Google Scholar]
- (47).Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Rijnsburger AJ, Obdeijn IM, Kaas R, Tilanus-Linthorst MMA, Boetes C, Loo CE, et al. BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC screening study. J Clin Oncol. 2010; 28(36):5265–73. [DOI] [PubMed] [Google Scholar]
- (49).Friedlander LC, Roth SO, Gavenonis SC. Results of MR imaging screening for breast cancer in high-risk patients with lobular carcinoma in situ. Radiology. 2011; 261(2):421–7. [DOI] [PubMed] [Google Scholar]
- (50).King TA, Muhsen S, Patil S, Koslow S, Oskar S, Park A, et al. Is there a role for routine screening MRI in women with LCIS? Breast Can Res Treat. 2013;142(2):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Annals Surg Oncol. 2007; 14(3):1051–7. [DOI] [PubMed] [Google Scholar]
- (52).Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015; 193(2):519–22. [DOI] [PubMed] [Google Scholar]
- (53).Sung JS, Malak SF, Bajaj P, Alis R, Dershaw DD, Morris EA. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology. 2011; 261(2):414–20. [DOI] [PubMed] [Google Scholar]
- (54).Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast Magnetic Resonance Imaging (MRI): First postcontrast subtracted images and maximum-intensity projection - A novel approach to breast cancer screening with MRI. J Clin Oncol. 2014; 32(22):2304–10. [DOI] [PubMed] [Google Scholar]
- (55).Kriege M, Brekelmans CT, Obdeijn IM, Boetes C, Zonderland HM, Muller SH, et al. Factors affecting sensitivity and specificity of screening mammography and MRI in women with an inherited risk for breast cancer. Breast Cancer Res Treat. 2006; 100(1):109–19. [DOI] [PubMed] [Google Scholar]
- (56).Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012; 307(13):1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Emaus MJ, Bakker MF, Peeters PHM, Loo CE, Mann RM, de Jong MDF, et al. MR imaging as an additional screening modality for the detection of breast cancer in women aged 50–75 years with extremely dense breasts: the DENSE trial study design. Radiology. 2015; 277(2):527–37. [DOI] [PubMed] [Google Scholar]
- (58).Olldendorf DA, Loos AM, Tice JA, Pearson SD. Appropriate imaging for breast cancer screening in special populations. Olympia (WA): Washington State Health Care Authority, Institute for Clinical and Economic Review; 2014. [Google Scholar]
- (59).Melnikow J, Fenton JJ, Whitlock EP, Miglioretti DL, Weyrich MS, Thompson JH, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016; 164(4):268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Havrilesky L, Gierish JM, Moorman P, McCrory D, Ghate S, Williams J, et al. Systematic review of cancer screening literature for updating American Cancer Society breast cancer screening guidelines. Durham (NC): Duke Evidence Synthesis Group; 2014. Dec. Contract No.: 13214. Prepared for the American Cancer Society, Inc. [Google Scholar]
- (61).Ozanne EM, Drohan B, Bosinoff P, Semine A, Jellinek M, Cronin C, et al. Which risk model to use? Clinical implications of the ACS MRI screening guidelines. Cancer Epidemiol Biomarkers Prev. 2013; 22(1):146–9. [DOI] [PubMed] [Google Scholar]