Abstract
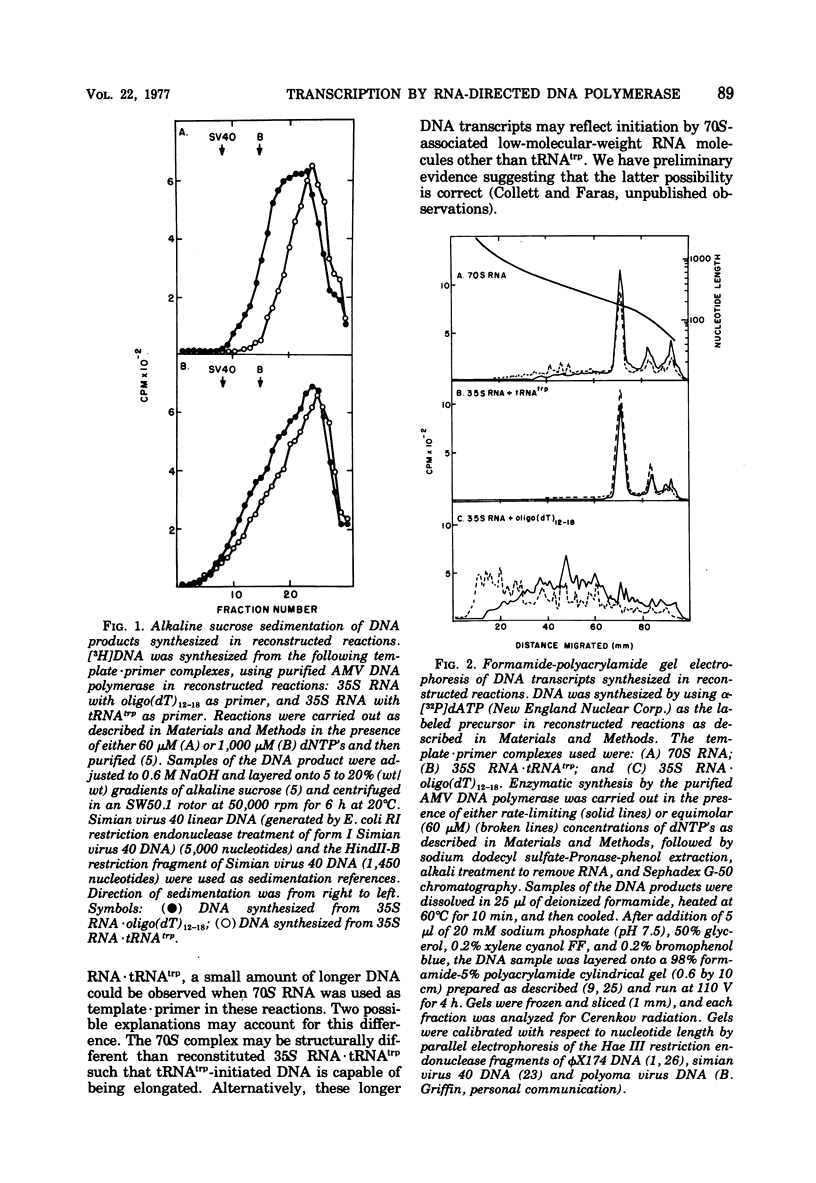
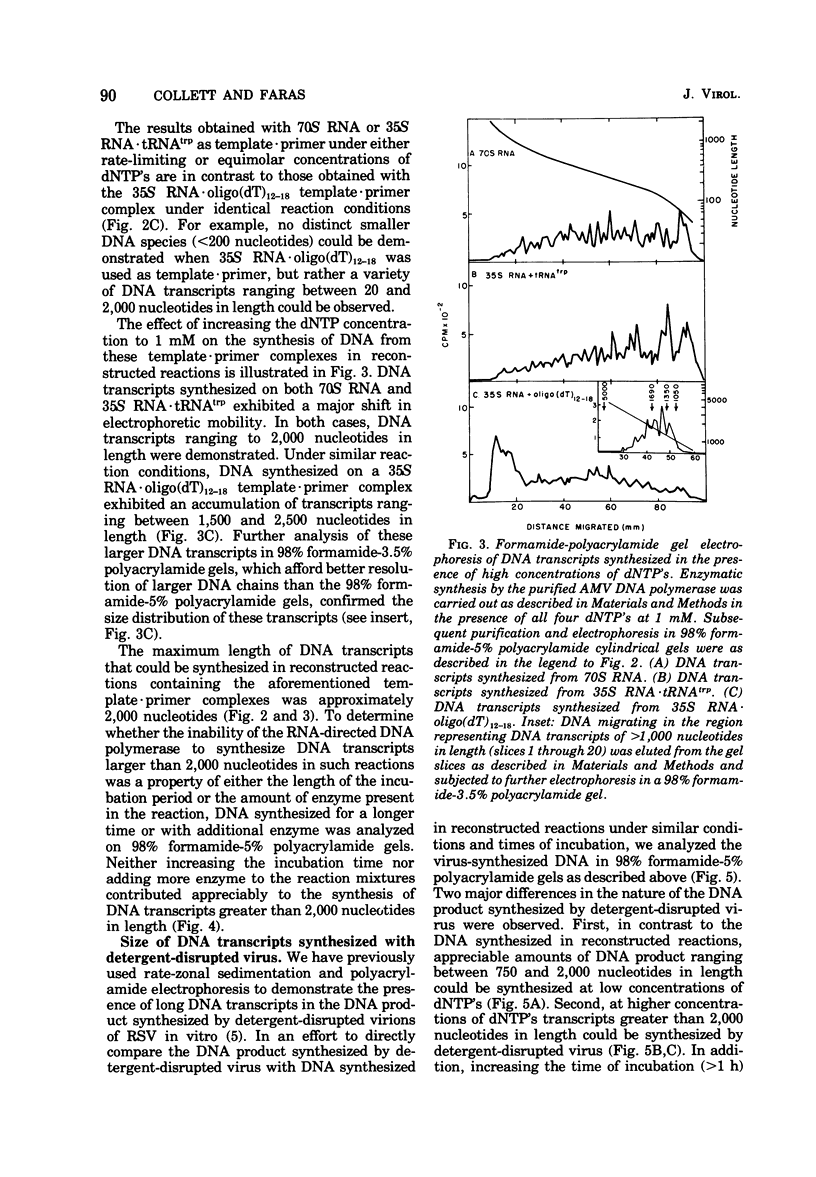
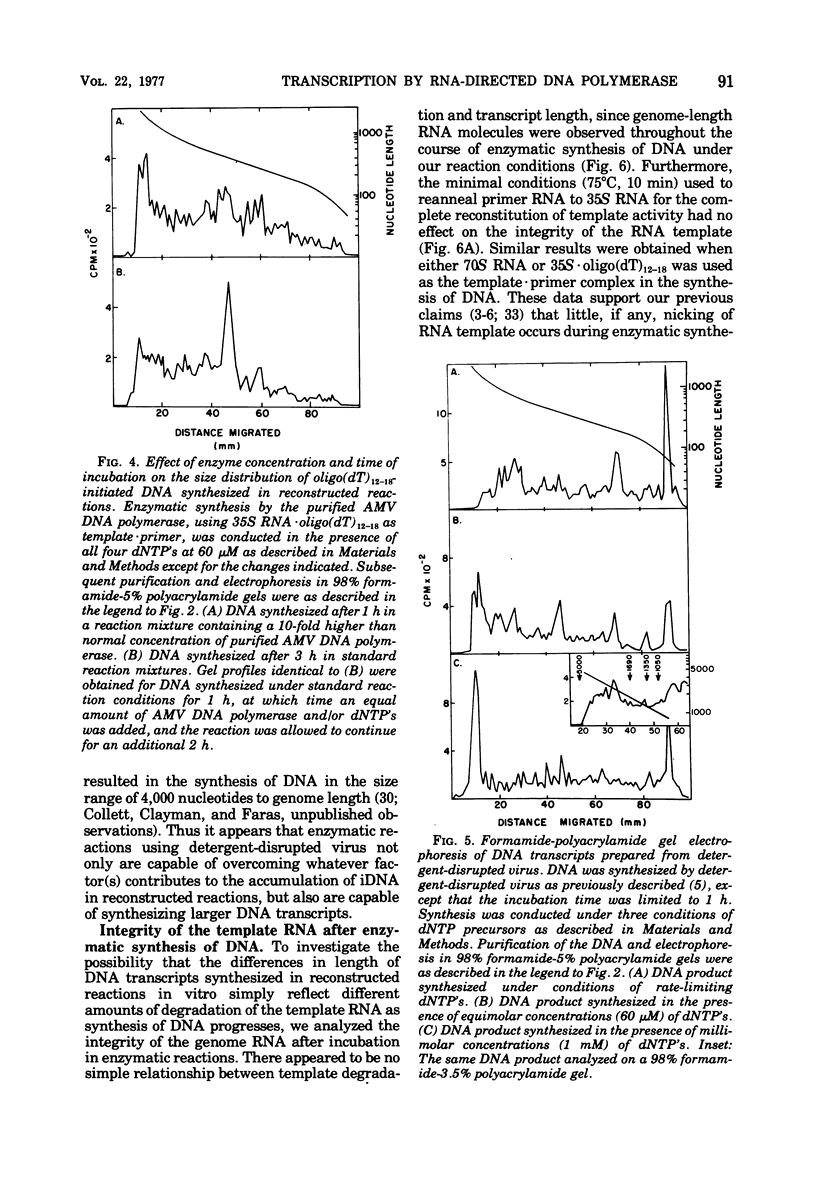
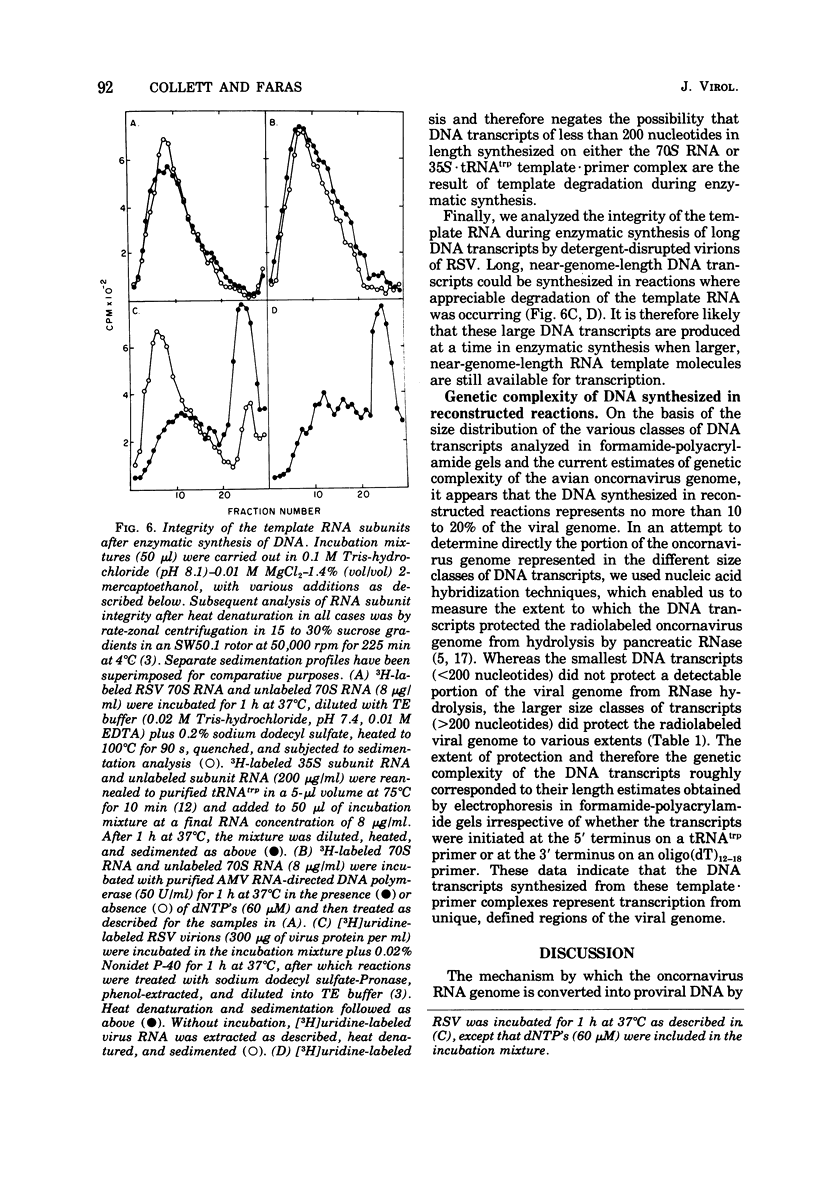
We have analyzed the DNA products synthesized in vitro in reconstructed reactions containing purified avian oncornavirus genome RNA and RNA-directed DNA polymerase. The results of these studies indicate that: (i) the initial DNA product synthesized on either 70S RNA or reconstituted 35S RNA-tRNAtrp template - primer complexes in the presence of low concentrations of deoxynucleoside triphosphates consists of several discrete size classes, none of which exceed 200 nucleotides in length; (ii) large DNA transcripts (about 2,000 nucleotides) can be synthesized on both 70S RNA and the 35S RNA - tRNAtrp complex by increasing the deoxynucleoside triphosphate concentration; and (iii) DNA synthesized by detergent-disrupted virus is considerably longer than DNA synthesized in reconstructed reactions.
Full text
PDF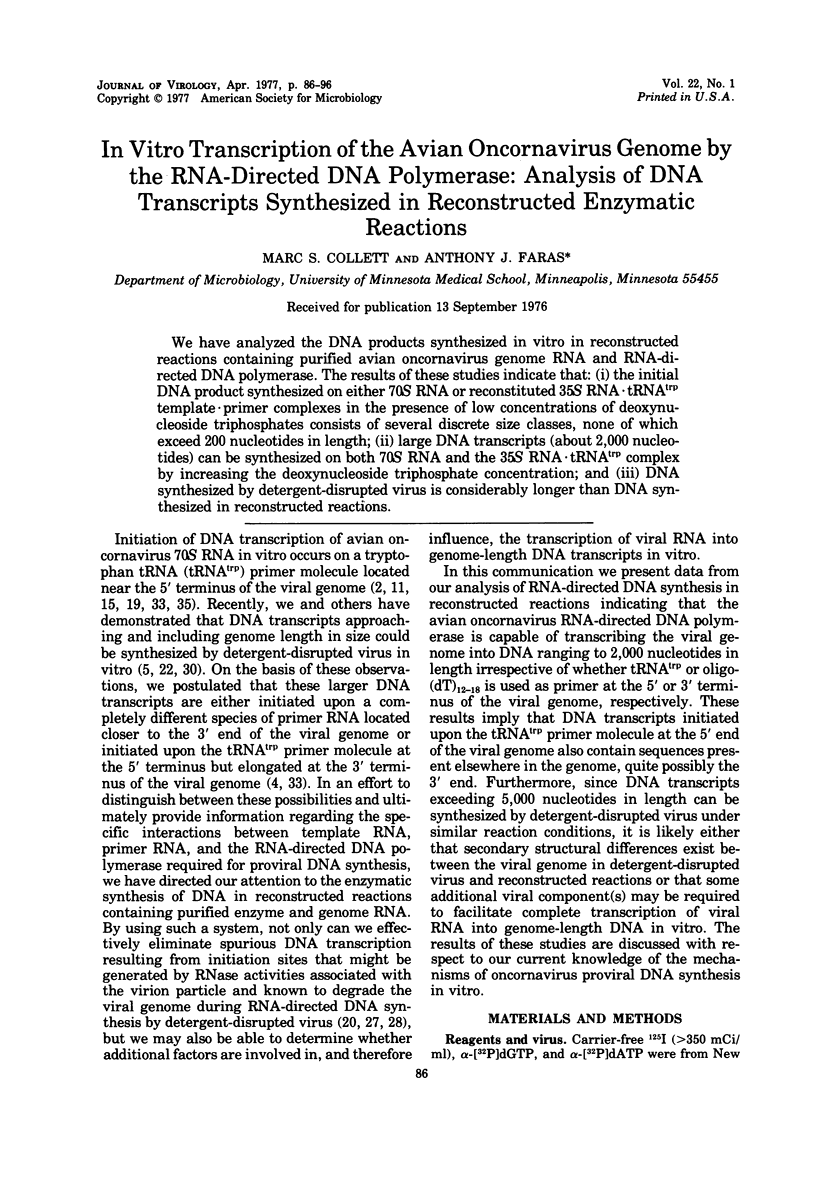






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Cashion L. M., Joho R. H., Planitz M. A., Billeter M. A., Weissmann C. Initiation sites of Rous sarcoma virus RNA-directed DNA synthesis in vitro. Nature. 1976 Jul 15;262(5565):186–190. doi: 10.1038/262186a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Elimination of residual RNase activity from purified preparations of RNA-directed DNA polymerase. Biochem Biophys Res Commun. 1975 Dec 1;67(3):946–955. doi: 10.1016/0006-291x(75)90767-6. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of 70S RNA by the RNA-directed DNA polymerase of Rouse sarcoma virus: lack of influence of RNase H. J Virol. 1975 Jan;17(1):291–295. doi: 10.1128/jvi.17.1.291-295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of DNA from the 70S RNA of Rous sarcoma virus: identification and characterization of various size classes of DNA transcripts. J Virol. 1975 Nov;16(5):1220–1228. doi: 10.1128/jvi.16.5.1220-1228.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Davis J., Scherer M., Tsai W. P., Long C. Low-molecular- weight Rauscher leukemia virus protein with preferential binding for single-stranded RNA and DNA. J Virol. 1976 May;18(2):709–718. doi: 10.1128/jvi.18.2.709-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanshier L., Garapin A. C., McDonnell J., Faras A., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase associated with avian tumor viruses: secondary structure of the deoxyribonucleic acid product. J Virol. 1971 Jan;7(1):77–86. doi: 10.1128/jvi.7.1.77-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Dahlberg J. E., Sawyer R. C., Harada F., Taylor J. M., Levinson W. E., Bishop J. M., Goodman H. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. II. Structure of a 4S RNA primer. J Virol. 1974 May;13(5):1134–1142. doi: 10.1128/jvi.13.5.1134-1142.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Dibble N. A. RNA-directed DNA synthesis by the DNA polymerase of Rous sarcoma virus: structural and functional identification of 4S primer RNA in uninfected cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):859–863. doi: 10.1073/pnas.72.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: tryptophan tRNA as the major species of primer RNA. J Virol. 1976 Mar;17(3):1049–1051. doi: 10.1128/jvi.17.3.1049-1051.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J., Stavnezer E., Bishop J. M. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975 Oct 16;257(5527):618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Lee S. G. Isolation of nucleic acid-binding protein: stimulation of reverse transcriptase-catalysed DNA synthesis. Nature. 1976 Feb 12;259(5543):499–502. doi: 10.1038/259499a0. [DOI] [PubMed] [Google Scholar]
- Hung P. P. Ribonucleases of Rous sarcoma virus. Virology. 1973 Feb;51(2):287–296. doi: 10.1016/0042-6822(73)90429-7. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz P., Siegel W., Sklar J. Hemophilus aegyptius restriction edonuclease cleavage map of the simian virus 40 genome and its colinear relation with the hemophilus influenzae cleavage map of SV40. J Mol Biol. 1974 Sep 5;88(1):105–123. doi: 10.1016/0022-2836(74)90297-6. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Middleton J. H., Edgell M. H., Hutchison C. A., 3rd Specific fragments of phi X174 deoxyribonucleic acid produced by a restriction enzyme from Haemophilus aegyptius, endonuclease Z. J Virol. 1972 Jul;10(1):42–50. doi: 10.1128/jvi.10.1.42-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M., Kodama M., Wells R. T. DNA ligase and exonuclease activities in virions of rous sarcoma virus. Nat New Biol. 1971 Apr 21;230(16):232–235. doi: 10.1038/newbio230232a0. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Fanshier L., Evans B., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase(s) of Rous sarcoma virus: effects of virion-associated endonuclease on the enzymatic product. J Virol. 1971 Jul;8(1):17–27. doi: 10.1128/jvi.8.1.17-27.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Abrell J. W., Trainor C. D., Gallo R. C. Precipitation of nucleic acids with cetyltrimethylammonium bromide: a method for preparing viral and cellular DNA polymerase products for cesium sulfate density gradient analysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):30–38. doi: 10.1016/0006-291x(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. Specificity of in vitro binding of primate Type C viral RNA and the homologous viral p12 core protein. Science. 1976 Jul 23;193(4250):326–328. doi: 10.1126/science.180601. [DOI] [PubMed] [Google Scholar]
- Staskus K. A., Collett M. S., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: structural and functional localization of the major species of primer RNA on the oncornavirus genome. Virology. 1976 May;71(1):162–168. doi: 10.1016/0042-6822(76)90102-1. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Faras A. J., Varmus H. E., Goodman H. M., Levinson W. E., Bishop J. M. Transcription of ribonucleic acid by the ribonucleic acid directed deoxyribonucleic acid polymerase of Rous sarcoma virus and deoxyribonucleic acid polymerase I of Escherichia coli. Biochemistry. 1973 Jan 30;12(3):460–467. doi: 10.1021/bi00727a016. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



