Abstract
Thyroid transcription factor 1 (TTF-1) is considered a highly sensitive and specific marker for primary lung adenocarcinoma. However, in recent years retrospective studies of tumor samples have confirmed that, although rare, TTF-1 can also be expressed in colorectal adenocarcinoma. There are a few case reports of patients with TTF-1-positive colon adenocarcinoma in the medical literature but none of TTF-1-positive rectal adenocarcinoma. Here, we present a case of rectal adenocarcinoma with lung metastasis found to be TTF-1 positive on immunohistochemistry. A review and discussion of the available literature is also included.
Keywords: Colorectal cancer, Rectal cancer, Immunohistochemistry, TTF-1, TTF, Thyroid transcription factor, Rectal adenocarcinoma, Colorectal adenocarcinoma
Introduction
Thyroid transcription factor 1 (TTF-1) expression in adenocarcinoma of the lung has been considered a highly sensitive (up to 84% sensitivity) and specific marker (85–100% specificity) for primary lung adenocarcinoma, and it is, therefore, used as a reliable tool in distinguishing primary lung adenocarcinoma from other malignancies [1, 2]. However, in the last decade, a few case reports and retrospective evaluations of tumor samples have confirmed that TTF-1 can also be expressed in colorectal adenocarcinoma [3, 4, 5]. In addition, some variability in detection has been reported depending on the clonal antibody used for testing [1]. TTF-1 expression is rare in colorectal cancer (CRC) and in the literature. The cases available in the medical literature reported patients with TTF-1-positive colon adenocarcinoma; however, to our knowledge, no cases of patients with TTF-1-positive rectal adenocarcinoma have been reported. Here, we present a case of rectal cancer with lung metastasis and TTF-1 positive on immunohistochemistry that highlights the importance of differentiating primary lung adenocarcinoma from TTF-1-positive CRC with lung metastasis by using additional immunohistochemistry markers.
Case Report
A 53-year-old male with no significant medical history was diagnosed with stage IIIC (pT3N2M0) rectal adenocarcinoma in March 2009. He was treated with neoadjuvant 5-fluorouracil plus oxaliplatin (FOLFOX) and chemoradiation, followed by abdominoperineal resection and 8 cycles of adjuvant FOLFOX. In May 2015, he presented to the emergency room with 2 weeks of right upper quadrant abdominal pain and cough productive of yellow sputum. Unfortunately, he had been lost to follow-up for almost 4 years after finishing treatment for his rectal cancer. Additional symptoms included shortness of breath, decreased appetite, and weight loss. A computed tomography of his chest showed postobstructive pneumonia with innumerable bilateral nodules and mass-like consolidations in both lung bases (fig 1). The largest consolidation was located in the right lower lobe and measured 12.6 × 9.9 cm.
Fig. 1.
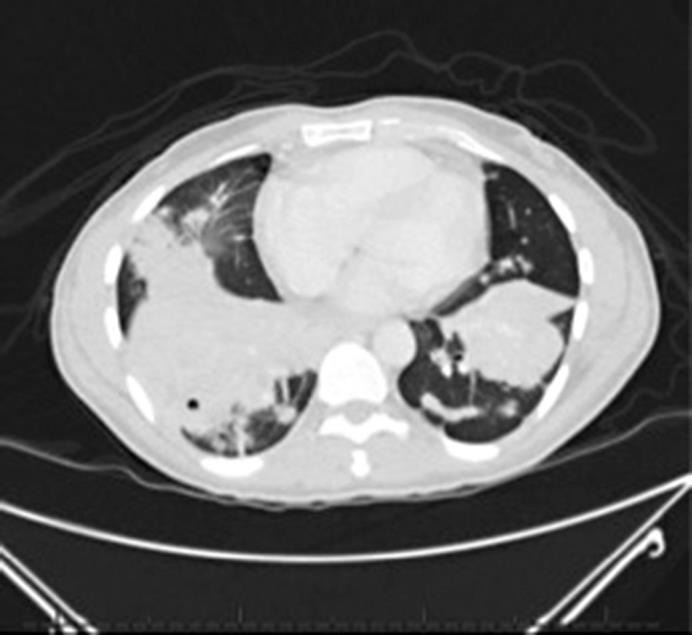
CT scan of the chest at the time of recurrence showing bilateral mass-like consolidations.
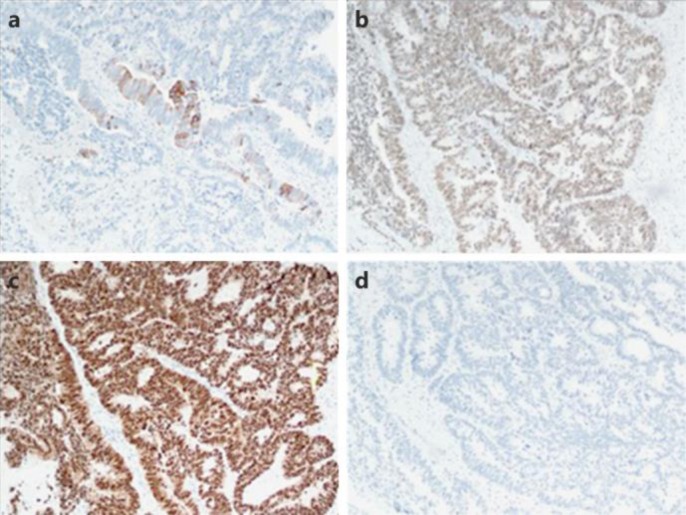
His carcinoembriogenic (CEA) level was 44.2 ng/ml (CEA was 2 at initial diagnosis). He underwent biopsy of the right lower lobe mass that revealed an adenocarcinoma with positivity for caudal type homeobox 2 (CDX2) and scattered positivity for cytokeratin 20 (CK20). TTF-1 was also strongly and diffusely positive (fig 2). The tumor cells were negative for CK7. The positivity for CK20 and CDX2 with negative CK7 supported the diagnosis of metastasis from a colorectal primary. Retrospective review of his previous primary tumor tissue showed similar histologic findings with TTF-1 positivity (fig 3).
Fig. 2.
Histopathology of the metastatic lung lesion. CK20 shows scattered positivity (a); widespread CDX2 expression (b); TTF-1 (SPT24 clone) is strong and diffusely positive (c); CK7 is negative (d).
Fig. 3.
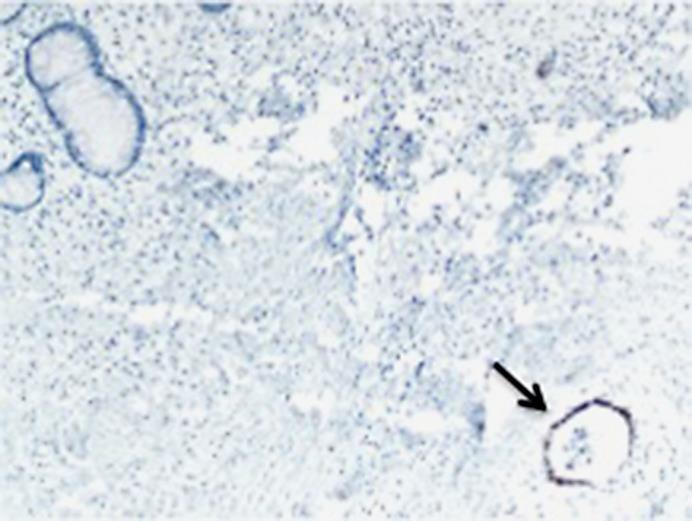
TTF-1 staining of the original primary tumor. Area of normal colon is negative for TTF-1 (left upper corner). Tumor area is positive for TTF-1 (arrow).
As a control, normal colon tissue was stained for TTF-1 using the same antibody and was negative. The patient was treated with FOLFOX and bevacizumab achieving a partial response after 5 cycles of treatment.
Discussion
Metastasis from CRC adenocarcinoma can be found at the time of diagnosis in 20% of patients, and an additional 50–60% will develop metastatic disease at the time of progression [6]. The lung is a common site of CRC metastasis, and TTF-1 is considered a highly sensitive and specific marker to differentiate primary lung adenocarcinoma from metastatic adenocarcinoma [7]. However, there are several case reports in the literature showing that some CRC tumors can express TTF-1, and the sensitivity of the technique used for detection can contribute to our ability to detect these cases [1, 3, 4, 5, 8]. Two clones of antibodies are commonly used to detect TTF-1, namely SPT24 and 8G7G1. The antibody used to detect TTF-1 in our patient's case was SPT24 that has a sensitivity of 84%, whereas 8G7G1 has a reported sensitivity of 65% [1]. This difference in sensitivity was illustrated in a study that compared the performance of both TTF-1 antibody clones in lung-metastatic lesions, primary CRC adenocarcinomas, and primary lung adenocarcinomas [1]. The study proved TTF-1 positivity in 4 out of 41 (10%) cases of lung metastasis from CRC when using SPT24, but no tumors were positive for TTF-1 when using 8G7G1. Out of these 4 cases, TTF-1 was also positive in 3 of the primary tumors with SPT24 and none with 8G7G1 [1]. Another study obtained similar results regarding clonal antibody sensitivity. In this study, 3 out of 6 primary colorectal adenocarcinomas were TTF-1 positive using SPT24 but negative with 8G7G1 [9]. On the other hand, in a study of 120 primary colon cancer tumors, 2.5% were TTF-1 positive, detected by both clones with similar intensity [10].
Overall, it seems that as techniques to detect proteins improve, more cases of positive TTF-1 will be detected in tissues. Therefore, a judicious approach to diagnosis is fundamental. In our case, immunohistochemistry results combined with a previous history of rectal adenocarcinoma pointed to the diagnosis of metastatic disease. However, other clinical presentations may present a diagnostic challenge, including cases of recurrence with a single metastatic lesion or new cases of occult primary malignancies with lung metastasis. We recommend interpreting TTF-1 in the context of additional immunohistochemistry markers including CK20, CDX2, and CK7 and using SPT24 antibody due to its higher sensitivity. Finally, if available, concurrent evaluation of the primary tumor pathology can be of great help in achieving the correct diagnosis and, in consequence, in offering our patients the most appropriate treatment.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Comperat E, Zhang F, Perrotin C, Molina T, Magdeleinat P, Marmey B, et al. Variable sensitivity and specificity of TTF-1 antibodies in lung metastatic adenocarcinoma of colorectal origin. Mod Pathol. 2005;18:1371–1376. doi: 10.1038/modpathol.3800422. [DOI] [PubMed] [Google Scholar]
- 2.Moldvay J, Jackel M, Bogos K, Soltesz I, Agocs L, Kovacs G, et al. The role of TTF-1 in differentiating primary and metastatic lung adenocarcinomas. Pathol Oncol Res. 2004;10:85–88. doi: 10.1007/BF02893461. [DOI] [PubMed] [Google Scholar]
- 3.Remo A, Zanella C, Pancione M, Astati L, Piacentini P, Cingarlini S, et al. Lung metastasis from TTF-1 positive sigmoid adenocarcinoma. Pitfalls and management. Pathologica. 2013;105:69–72. [PubMed] [Google Scholar]
- 4.Reis HG, Metz CH, Baba HA, Bornfeld N, Schmid KW, Metz KA. TTF-1 (8G7G3/1) positive colon adenocarcinoma: diagnostic implications. Pathologe. 2011;32:349–351. doi: 10.1007/s00292-010-1414-y. [DOI] [PubMed] [Google Scholar]
- 5.Xu B, Thong N, Tan D, Khoury T. Expression of thyroid transcription factor-1 in colorectal carcinoma. Appl Immunohistochem Mol Morphol. 2010;18:244–249. doi: 10.1097/PAI.0b013e3181c29407. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Reis-Filho JS, Carrilho C, Valenti C, Leitao D, Ribeiro CA, Ribeiro SG, et al. Is TTF1 a good immunohistochemical marker to distinguish primary from metastatic lung adenocarcinomas? Pathol Res Pract. 2000;196:835–840. doi: 10.1016/S0344-0338(00)80084-9. [DOI] [PubMed] [Google Scholar]
- 8.Dettmer M, Kim TE, Jung CK, Jung ES, Lee KY, Kang CS. Thyroid transcription factor-1 expression in colorectal adenocarcinomas. Pathol Res Pract. 2011;207:686–690. doi: 10.1016/j.prp.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Penman D, Downie I, Roberts F. Positive immunostaining for thyroid transcription factor-1 in primary and metastatic colonic adenocarcinoma: a note of caution. J Clin Pathol. 2006;59:663–664. doi: 10.1136/jcp.2005.030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matoso A, Singh K, Jacob R, Greaves WO, Tavares R, Noble L, et al. Comparison of thyroid transcription factor-1 expression by 2 monoclonal antibodies in pulmonary and nonpulmonary primary tumors. Appl Immunohistochem Mol Morphol. 2010;18:142–149. doi: 10.1097/PAI.0b013e3181bdf4e7. [DOI] [PMC free article] [PubMed] [Google Scholar]