Abstract
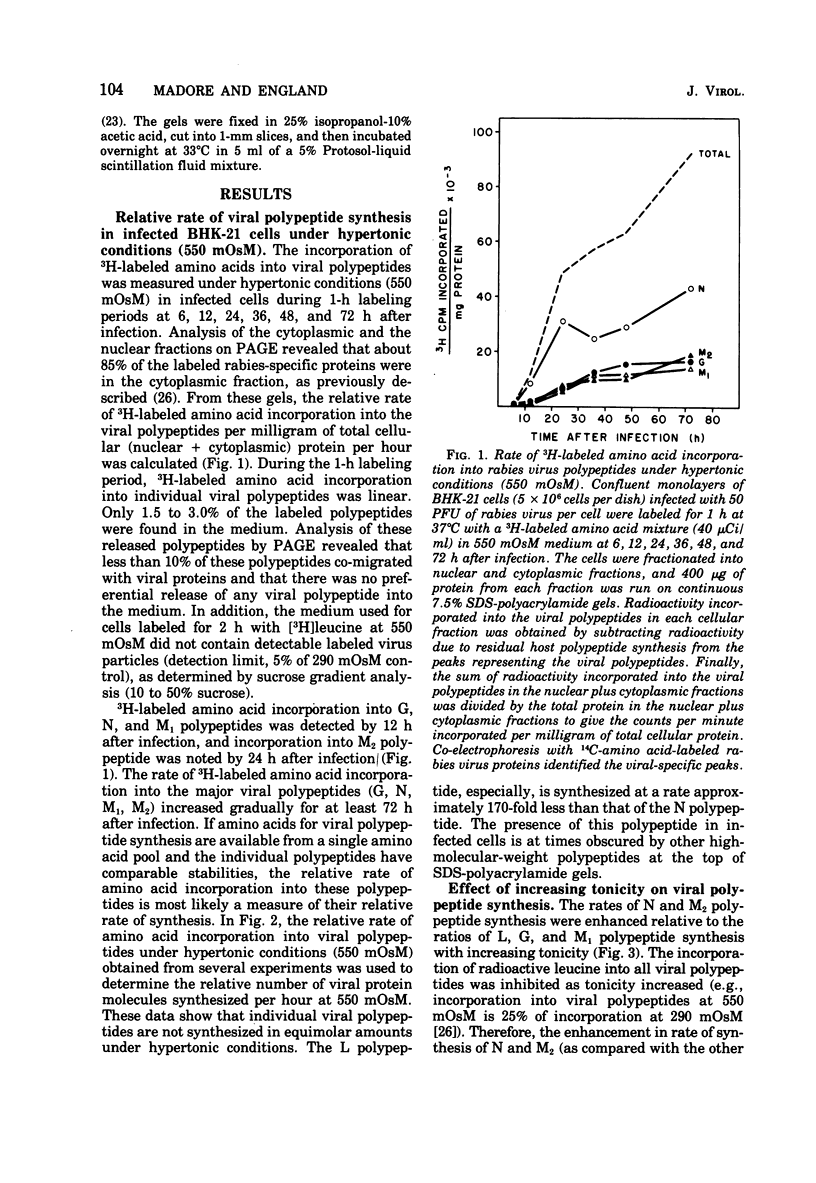
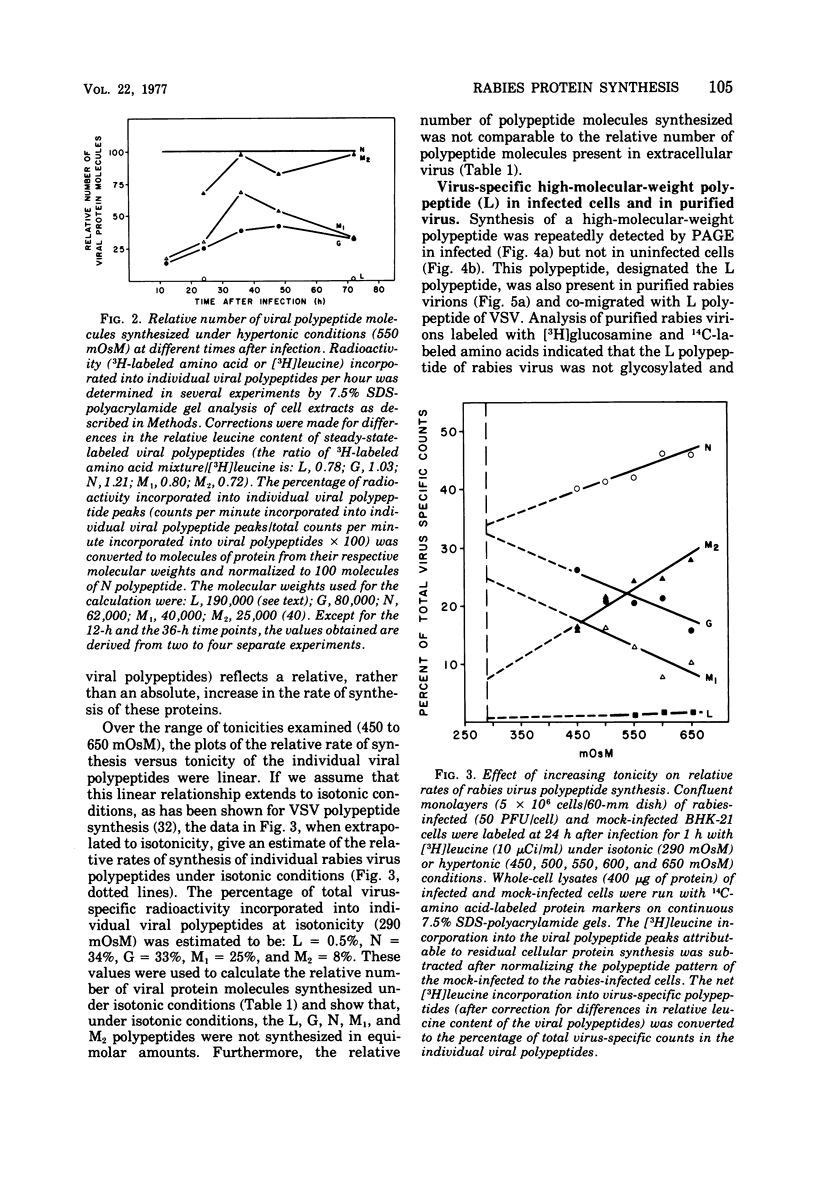
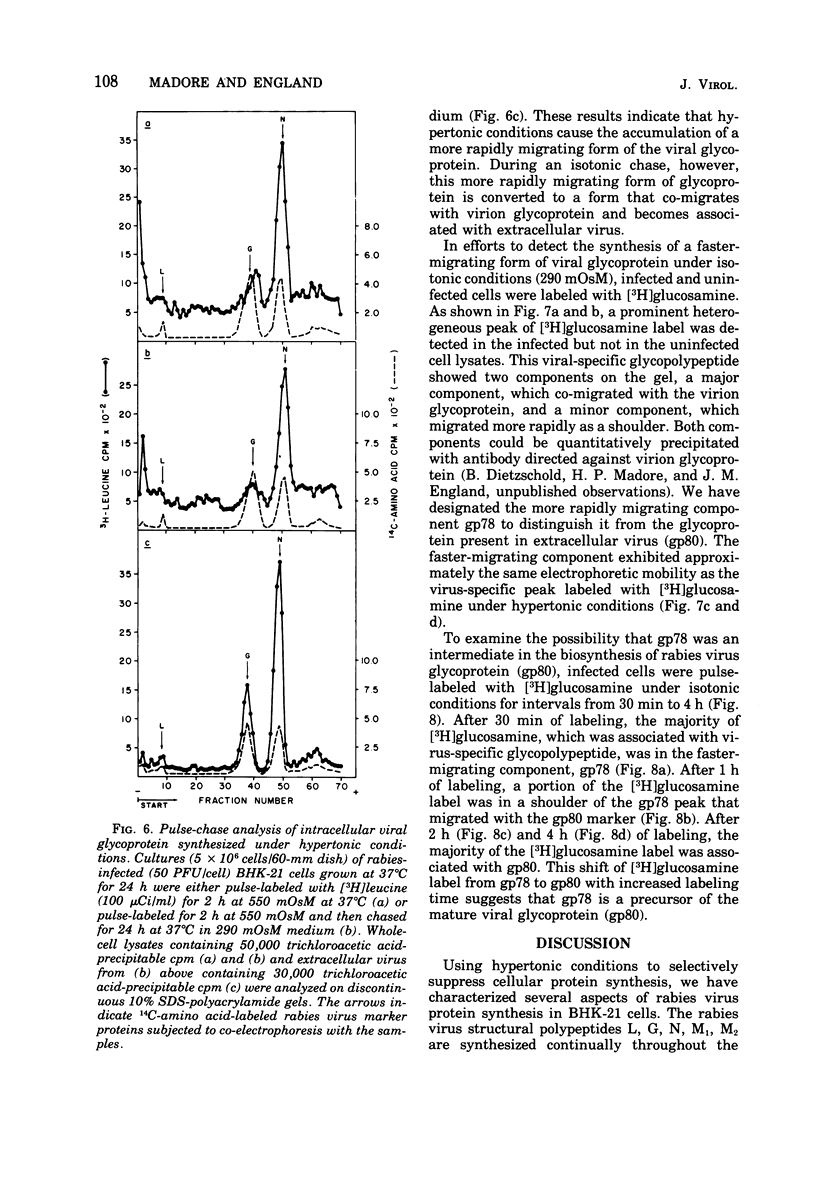
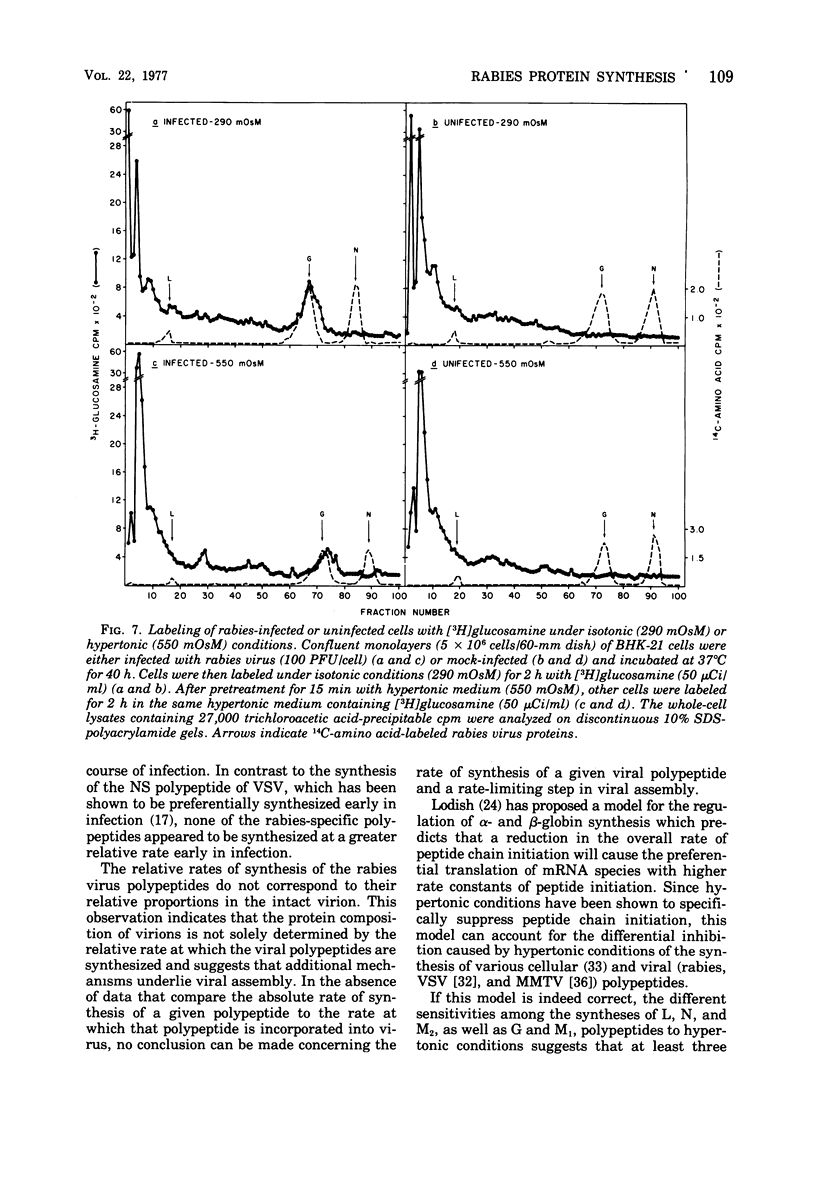
Rabies virus specific polypeptide synthesis was examined under hypertonic conditions, which selectively inhibit cellular protein synthesis. The rabies virus proteins (L, G, N, M1, M2) were synthesized throughout the course of infection, with little change in their relative rates of synthesis. The rates of synthesis of the G and M1 polypeptides were more sensitive to increasing osmolarity than those of the L, N, and M2 polypeptides. Extrapolation to isotonicity of the results obtained under hypertonic conditions indicated that the molar ratios of the polypeptides synthesized under normal conditions were 0.4 (L), 64 (G), 100 (N), 75 (M1) and 35 (M2). A high-molecular-weight polypeptide (190,000), designated polypeptide L, was repeatedly detected both in infected cells and in extracellular virus. The estimated number of L polypeptide molecules per virion was 33. The synthesis of a viral glycoprotein precursor, designated gp78, , preceded the appearance of the mature viral glycoprotein in infected cells labeled with [3H]glucosamine under isotonic conditions. In cells labeled under hypertonic conditions, little or no mature viral glycoprotein was detected, but a virus-specific glycoprotein with an electrophoretic mobility similar to that of gp78 was observed. This glycoprotein could be chased into mature viral glycoprotein when the hypertonic conditions were made isotonic. These results suggest that a reversible block of viral glycoprotein synthesis occurs under hypertonic conditions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Moyer S. A., Summers D. F. Assembly of vesicular stomatitis virus glycoprotein and matrix protein into HeLa cell plasma membranes. J Mol Biol. 1976 Apr 15;102(3):613–631. doi: 10.1016/0022-2836(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N., Ranki M., Morser J., Käriäinen L., Smith A. E. Initiation of translation directed by 42S and 26S RNAs from Semliki Forest virus in vitro. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3059–3063. doi: 10.1073/pnas.73.9.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F., Kuwert E., Koprowski H. Morphology of the nucleoprotein component of rabies virus. J Virol. 1968 Oct;2(10):1191–1199. doi: 10.1128/jvi.2.10.1191-1199.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Emerson S. U., Wagner R. R. RNA- temperature-sensitive mutants of vesicular stomatitis virus: L-protein thermosensitivity accounts for transcriptase restriction of group I mutants. J Virol. 1976 May;18(2):596–603. doi: 10.1128/jvi.18.2.596-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Wagner R. R. Location of the transcription defect in group I temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):28–35. doi: 10.1128/jvi.13.1.28-35.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y., Wiktor T. J., Koprowski H. Early events of rabies virus replicaton in tissue cultures. An electron microscopic study. Lab Invest. 1973 Feb;28(2):142–148. [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G. Early synthesis of Semliki Forest virus-specific proteins in infected chicken cells. J Virol. 1976 Jul;19(1):1–12. doi: 10.1128/jvi.19.1.1-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G. Effect of impaired glycosylation on the biosynthesis of Semliki forest virus glycoproteins. J Virol. 1975 Sep;16(3):602–612. doi: 10.1128/jvi.16.3.602-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Knipe D., Rose J. K., Lodish H. F. Translation of individual species of vesicular stomatitis viral mRNA. J Virol. 1975 Apr;15(4):1004–1011. doi: 10.1128/jvi.15.4.1004-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson D. L. Rhabdoviruses. J Gen Virol. 1973 Jun;20(Suppl):105–130. doi: 10.1099/0022-1317-20-Supplement-105. [DOI] [PubMed] [Google Scholar]
- Lachmi B. E., Käriäinen L. Sequential translation of nonstructural proteins in cells infected with a Semliki Forest virus mutant. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1936–1940. doi: 10.1073/pnas.73.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Madore H. P., England J. M. Selective suppression of cellular protein synthesis in BHK-21 cells infected with rabies virus. J Virol. 1975 Nov;16(5):1351–1354. doi: 10.1128/jvi.16.5.1351-1354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Morrison T., Stampfer M., Baltimore D., Lodish H. F. Translation of vesicular stomatitis messenger RNA by extracts from mammalian and plant cells. J Virol. 1974 Jan;13(1):62–72. doi: 10.1128/jvi.13.1.62-72.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Differential inhibition of vesicular stomatitis virus polypeptide synthesis by hypertonic initiation block. J Virol. 1975 Jan;17(1):283–286. doi: 10.1128/jvi.17.1.283-286.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Variation in the relative synthesis of immunoglobulin G and non-immunoglobulin G proteins in cultured MPC-11 cells with changes in the overall rate of polypeptide chain initiation and elongation. J Mol Biol. 1976 Apr 15;102(3):601–612. doi: 10.1016/0022-2836(76)90337-5. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Schlom J. Independent polypeptide chain initiation sites for the synthesis of different classes of proteins for an RNA tumor virus: mouse mammary tumor virus. Virology. 1976 Sep;73(2):431–441. doi: 10.1016/0042-6822(76)90404-9. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Klenk H. D. Inhibition of glycosylation of the influenza virus hemagglutinin. J Virol. 1974 Nov;14(5):1023–1034. doi: 10.1128/jvi.14.5.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F., Wiktor T. J., McFalls M. L., Bishop D. H., Obijeski J. F. Structural phosphoproteins associated with ten rhabdoviruses. J Gen Virol. 1974 Sep;24(3):433–445. doi: 10.1099/0022-1317-24-3-433. [DOI] [PubMed] [Google Scholar]
- Sokol F. Purification of rabies virus and isolation of its components. Monogr Ser World Health Organ. 1973;(23):165–178. [PubMed] [Google Scholar]
- Sokol F., Schlumberger H. D., Wiktor T. J., Koprowski H. Biochemical and biophysical studies on the nucleocapsid and on the RNA of rabies virus. Virology. 1969 Aug;38(4):651–665. doi: 10.1016/0042-6822(69)90184-6. [DOI] [PubMed] [Google Scholar]
- Sokol F., Stancek D., Koprowski H. Structural proteins of rabies virus. J Virol. 1971 Feb;7(2):241–249. doi: 10.1128/jvi.7.2.241-249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Cell-free synthesis of vesicular stomatitis virus proteins: translation of membrane-bound polyribosomal mRNAs. FEBS Lett. 1975 Feb 15;50(3):369–373. doi: 10.1016/0014-5793(75)80530-8. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Characterization and translation of methylated and unmethylated vesicular stomatitis virus mRNA synthesized in vitro by ribonucleoprotein particles from vesicular stomatitis virus-infected L cells. J Virol. 1976 Feb;17(2):477–491. doi: 10.1128/jvi.17.2.477-491.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang H., Atanasiu P. Réplication du virus rabique fixe en suspension cellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1971 Feb 8;272(6):897–900. [PubMed] [Google Scholar]
- Villarreal L. P., Holland J. J. Transcribing complexes in cells infected by vesicular stomatitis virus and rabies virus. J Virol. 1974 Sep;14(3):441–450. doi: 10.1128/jvi.14.3.441-450.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., György E., Schlumberger D., Sokol F., Koprowski H. Antigenic properties of rabies virus components. J Immunol. 1973 Jan;110(1):269–276. [PubMed] [Google Scholar]
- Wiktor T. J. Laboratoty techniques in rabies: tissue culture methods. Monogr Ser World Health Organ. 1973;(23):101–123. [PubMed] [Google Scholar]



