Abstract
Background:
Lactoperoxidase (LPO) is related to mammalian peroxidase family which contains a wide spectrum of biological activities. Despite the wide studies on the LPO, there is little study has been performed to simplify and shorten the procedure of enzyme purification. The aim of this project was to purify the enzyme through a simple method, and investigating enzyme kinetic parameters.
Materials and Methods:
LPO was purified from bovine whey through modified method of Yoshida (1990) using two steps of ammonium sulfate precipitation and ion-exchange chromatography. The purity of isolated enzyme was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Results:
The enzyme was purified 59.13-fold with a recovery of 10.26 having a specific activity of 5.78 U/mg protein and an Rz value of 0.8. The enzyme activity was measured using guaiacol as a chromogenic substrate in phosphate buffer pH 6. SDS-PAGE showed a single bond with molecular weight of 78 kDa. The purified enzyme displayed optimum activity at pH 6 in 30 mM phosphate buffer and at a temperature of 50°C, with a Km value of 178 mM and Vmax 0.63 U/ml.min for guaiacol.
Conclusion:
Using only one step ion-exchange chromatography, LPO was isolated from bovine whey in high purity.
Keywords: Bovine milk, enzyme, kinetic parameters, lactoperoxidase, purification, whey
INTRODUCTION
Lactoperoxidase (LPO) (EC.1.11.1.7), a peroxidase enzyme, is present in exocrine secretion liquids including milk, saliva, and tears.[1] This enzyme catalyzes the reaction of inorganic and organic substrates in the presence of hydrogen peroxide (H2O2). Thiocyanate (SCN−) is one of the most important substrates of the enzyme due to the generation of the hypothiocyanite (OSCN−) through the enzymatic reaction catalyzed by LPO.[2] OSCN− is a potent antibacterial agent due to its propensity to oxidase sulfhydryl groups of microbial enzymes. LPO together with SCN−, OSCN−, and H2O2 is known as LPO system.[3] Concentration of LPO in bovine milk is around 30 mg/L depending on season, diet, calving, and breeding season.[4] Hence, bovine milk LPO is the favorite enzyme in vitro or in vivo application.[5] It is to be noted that whey may be better than milk for purification LPO because it has not casein contamination. Bovine milk LPO is a calcium and iron-containing glycoprotein consisting of a single polypeptide chain of 612 amino acid residues with a molecular weight of about 78 kDa and carbohydrate content about 10%. A calcium ion plays a significant role in the stability of the enzyme. Bovine milk LPO is a cationic protein with an isoelectric pH of 9.6.[6] A heme prosthetic group which consist of a protoporphyrin IX derivative, located at the active site of the enzyme. Since protohemin, LPO has maximum absorbance at 412 nm. Calculating the ratio of absorbance 412 nm (indicating heme group) over absorbance 280 nm (indicating protein concentration) is an index for evaluating of the purity of isolated peroxidase enzyme from various sources. This ratio is commonly referred to as the Rz value which translate to number of purity.[7] Purification of the enzyme using different purification methods has been focused by many researcher groups. For example, Ozdemir et al. purified the enzyme using Amberlit CG50H+ resin, CM-sephadex C-50 ion-exchange chromatography; and sephadex G-100 gel filtration.[8] Mecitoğlu and Yemenicioğlu using toyopearl-sp Cation-exchange chromatography purified the enzyme.[9] In addition, affinity chromatography was used for the purification of LPO.[10] All of the research on the LPO purification have indicated that purification of the LPO can be performed using very-time consuming and complicated methods.
Carboxymethyl cellulose (CM-cellulose), as a chromatographic resin, has a carboxymethyl functional group which is negatively charged and in pH 5–9 acts as a cation exchanger. The buffer pH of CM-cellulose chromatography should be one unit below the PI (Isoelectric pH) of the LPO (pH 9.6) to give a net positive charge and ensure adequate binding. Thus, the buffer pH in the chromatography step was chosen to be 8.6. As above-mentioned, at the chosen conditions, CM-cellulose is a suitable resin for purification of the LPO from bovine milk. Ye et al. also used 0.05 M Tris-HCl buffer (pH 8.5) for isolating LPO from bovine rennet whey using ion exchange chromatography.[11] The aim of this project was to design a simple and short-time consuming method for purification of LPO from bovine whey and determine kinetic parameters of the purified enzyme. Removing of globulins by 50% ammonium sulfate saturation is a simple step for decreasing cost and yielding high pure LPO.
MATERIALS AND METHODS
Fresh unpasteurized bovine milk was purchased from the local dairy in Isfahan. Guaiacol and CM-cellulose resin was obtained from Sigma–Aldrich (USA). Chemicals for electrophoresis and preparation of buffers were obtained from Merck (Germany). Bradford reagent was purchased from BioRad (USA). All chemicals were of analytical grade.
Preparation of skim milk
Skim milk was prepared by centrifugation of fresh bovine milk at 2500 rpm for 15 min at 4°C. Then, the upper layer which is a lipid layer was separated by pipetting and was discarded.[10]
Preparation of whey
The skim milk was warmed to 37°C and then 20 mg/L rennet was added to it, and kept it for 1 h at the same temperature. The rennet whey initially separated by filtration using Whatman filter paper number 1 (W and R Balston, England).[12]
Precipitation of whey globulins with 50% saturation ammonium sulfate
To precipitate whey globulins, 314 g/L ammonium sulfate (50% ammonium sulfate saturation) was added slowly to the whey in three steps and allowed to shake for 3 h at 4°C.[13] Precipitated globulins were removed by centrifugation at 15,000 rpm for 30 min (using Sigma 3K30 Centrifuge) and discarded.
Precipitation of remaining proteins from the last supernatant with 70% saturation of ammonium sulfate
Remaining proteins in the resulting supernatant from the previous step was precipitated by adding 135 g/L ammonium sulfate (70% ammonium sulfate saturation) in the same manner as mentioned above. The obtained pellet was diluted by 2 ml of 50 mM Tris-HCl buffer pH 8.6 and was dialyzed against the 2 l of the same buffer, with repeated changes of the buffer tree times at least.
Preparation of carboxymethyl-cellulose and column chromatography
CM-cellulose, with a capacity of 1.05–1.45 g/g was used as a cation exchanger resin in the ion-exchange chromatography. 2 g of dry resin was slowly suspended to distilled water and was allowed to swell for 1 h. Then the resin was stirred in 50 mM Tris-HCl buffer (pH 8.6) and packed in a 1.2 cm × 30 cm column and equilibrated with the same buffer.[14] The dialyzed sample with using a pipette was applied to the column.
The column-bound enzyme was washed with 50 ml of 50 mM Tris-HCl buffer pH 8.6 at a flow rate of 0.7 ml/min. Elution of bovine LPO was performed with a linear gradient of 0.1–0.5 M NaCl in the same buffer. For each NaCl gradient step 25 ml of related buffer was used and the collected fraction size was about 2.5 ml. The absorbance of each fraction was monitored at 280 nm and 412 nm, and Rz value of each fraction was calculated. Fractions having Rz values of 0.7 or higher were collected. To decrease the volume of enzyme solution, an amount of 657 g/L ammonium sulfate (90% ammonium sulfate saturation) was added to 25 ml of enzyme solution as mentioned above and was shaking for 3 h in 4°C. Then the solution was centrifuged at 15,000 rpm for 30 min at 4°C. The pellet was dissolved in 1 ml of phosphate buffer pH 6 and then dialyzed overnight against 500 ml of the same buffer. The enzyme solution was checked for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Electrophoresis
To check the purity of obtained LPO, SDS-PAGE was performed according to the procedure of Laemmli.[15] Briefly, electrophoresis was run with a 5% staking gel and a 12% separating gel, with a constant current of 100 mA.
Protein determination
Determination of protein was carried out spectrophotometrically at 595 nm using the method of Bradford.[16] Bovine serum albumin was used for the preparation of standard curve. Seven Concentration of BSA (2, 1.5, 1, 0.75, 0.5, 0.25, and 0.125 mg/mL) was prepared.
Enzyme assay
LPO activity was measured according to the method of Maehly and Chance[17] with some modification. The reaction mixture contained 775 µl of 30 mM sodium phosphate buffer (PH 6), 25 µL of the enzyme sample, 100 µl of 25 mM guaiacol, and 100 µl of 25 mM H2O2, in a final volume of 1 ml. reaction was started by adding of H2O2. This method is based on the oxidation of guaiacol by H2O2, which results in tetra-guaiacol, a product which is absorbent at 470 nm. The absorbance measured at 470 nm, every 15 s for 3 min, using a Beckman model ultraviolet-visible spectrophotometer (Molar extinction coefficient of tetra-guaiacol is 26.6/mM/cm). One unit of enzyme activity was defined as the amount of enzyme catalyzing the conversion of 1 µmol guaiacol to tetra-guaiacol per min.
Determination of Km and Vmax
To determine Km and Vmax values of LPO for guaiacol, activity of the enzyme using four different concentration of guaiacol (100, 200, 400, and 800 mM) determined. Km and Vmax of the LPO were determined by the Lineweaver-burk graph.[18]
Determination of optimum pH
Optimum pH for the purified LPO was determined by performing enzyme assay using two wide ranges of pH. Buffers were 30 mM sodium citrate (pH 3–5.5) and 30 mM sodium phosphate buffer (pH 6–8).[19]
Determination of optimum temperature and thermal stability
Optimum temperature for LPO was determined by assaying at an increasing temperature range, 20–80°C using a multitemperature water bath. For evaluation thermal stability of the enzyme, LPO were incubated at 75°C in water bath. At definite time intervals, LPO was withdrawn, chilled immediately, and its activity was determined.[19]
Statistical analysis
The Statistical Package for the Social Sciences (version 20 SPSS, SPSS Inc., Chicago, Illinois, USA) program was used for the data analysis. Data were expressed as mean ± standard deviation. Comparisons were tested using an analysis of variance test. A difference was considered to be significant at P < 0.05.
RESULTS
LPO from bovine whey was isolated and purified through two steps of ammonium sulfate precipitation and CM-cellulose cation-exchange chromatography with a Rz value of 0.8. The fraction obtained in 50% ammonium sulfate saturation showed no peroxidase activity while high level of enzyme activity was found in the fraction obtained in 70% ammonium sulfate saturation. After loading the sample with peroxidase activity on a CM-cellulose column and used a linear gradient of 0.1–0.5 NaCl in the washing buffer, the enzyme was eluted with a 0.4 M NaCl gradient. As the result of the purification procedure in this study, the enzyme was purified 59.13-fold with a yield of 10.26, the result of the LPO purification from rennet whey has been shown in Table 1. As shown in Figure 1, the obtained enzyme exhibited single bound on SDS-PAGE. The molecular weight of LPO was estimated to be 78 kDa according to the molecular weight of markers on SDS-PAGE [Figure 1]. Km and Vmax values of the enzyme for guaiacol were 178 mM and 0.63 U/ml, respectively [Figure 2]. The optimum pH and optimum temperature were pH 6 and 50°C, respectively [Figures 3 and 4]. Thermal stability studies showed that incubation of the enzyme in 75°C for 120 s results in losing the enzyme activity [Figure 5].
Table 1.
Steps of purification of lactoperoxidase from bovine whey
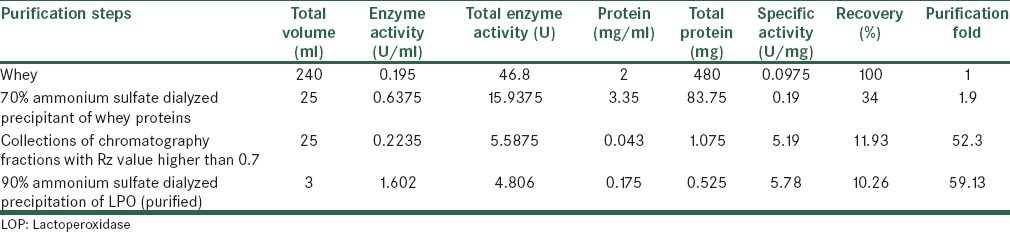
Figure 1.

Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of bovine lactoperoxidase: (Lane 1) standards proteins; aldolase (158 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), ribonoclease A (13.7 kDa), insulin (6.6 kDa) (Lane 2). The purified Lactoperosxidas from bovine milk
Figure 2.

Lineweaver-Burk reciprocal plot of lactoperoxidase for four different concentrations of guaiacol (100, 200, 400 and 800 mM). The concentration of H2O2 was constant (25 mM). Data are shown as a mean of the activity ± standard deviation of three independent experiments
Figure 3.

Optimum pH of lactoperoxidase activity. Assay was performed using two wide range of pH. 30 mM citrate-sodium (pH: 3.0–5.5) and 30 mM sodium phosphate buffer (pH 6–8). Data are shown as a mean of the activity ± standard deviation of three independent experiments
Figure 4.

Optimum temperature of lactoperoxidase activity. Assay was performed in temperature ranges of (20–80°C). Data are shown as a mean of the activity ± standard deviation of three independent experiments
Figure 5.

Thermal stability of purified lactoperoxidase from bovine milk. The enzyme was incubated at the 75°C in water bath for different times and it was chilled immediately and its activity was calculated. Data are shown as a mean of the activity ± standard deviation of three independent experiments
DISCUSSION
LPO, one of the main enzymes in whey, exhibit both bactericidal and bacteriostatic effects.[20,21,22] These features cause LPO considered as an important natural conservative which can be used in various industries including dairy, cosmetics, ophthalmic solutions, dental and wound treatment, as an antitumor and antiviral agent.[4,23,24] This study was designed to develop a simple isolation protocol of LPO with high purity and concentration from bovine whey. Meanwhile, we tried to establish simple and short time-consuming method. Initial efforts to purify LPO from bovine milk back to 1925 using ammonium sulfate precipitation.[25] We used 50% of ammonium sulfate saturation for removing of globulins from bovine whey. Then, the supernatant has been used for LPO and remaining proteins precipitation by 70% ammonium sulfate saturation. Bovine whey enriched with globulins which cause low purity of extracted LPO.[26] After dialysis of 70% precipitation, CM-cellulose ion-exchange chromatography has been employed as a cation exchanger resin for LPO purification. As seen in Figure 1, using 50% ammonium sulfate for precipitation of globulins cause very pure LPO with high yields as comparable with Ozdemir et al.[8]
Ozdemir et al.,[8] Nandini and Rastogi[27] and Mecitoğlu and Yemenicioğlu[9] used different successive methodes for separation LPO from bovine milk. However, by simply removing step of globulins, we use only CM-cellulose ion-exchange chromatography which is cost effective and short time-consuming. Otherwise, using time-consuming chromatographic procedures may lose enzyme activity during purification process.
In this study, we were able to purify LPO from bovine whey using simple ammonium sulfate precipitation and single CM-cellulose cation exchange chromatography 59.13-fold with a yield of 10.26 and Rz value 0.8. Purified LPO in our study showed more purity than the several studies. The purified LPO by Ozdemir et al. showed 11.52-fold with a yields of 27% and a Rz value of 0.7,[8] which is comparable with our results. These researchers used three different resins, Amberlite CG50H+ resin, CM-Sephadex C-50 ion exchange chromatography, and Sephadex G-100 gel filtration chromatography.
Furthermore, Nandini and Rastogi purified LPO from bovine whey using ultrafiltration method (a complicated method), 3.53-fold with a recovery of 149.85%.[27]
The optimum pH of purified enzyme was found to be 6.0 which are the same of previously purified LPO.[28] As shown in Figure 3, activity of LPO on acidic conditions is very low, it is due to the realized of calcium ion from enzyme under acidic pH.[4]
At this optimum pH, the Km and Vmax values of LPO for guaiacol, as substrate, was found to be 178 mM and 0.63 U/ml/min, respectively. These Km and Vmax values are comparable with purified peroxidase from rosemary (Rosmarinus officinalis L.) leaves, Km: 28.8 mM and Vmax: 0.312 (U/ml/min),[19] and cauliflower (Brassica oleracea L.), Km: 141.64 mM and Vmax: 7500 (U/ml/min),[29] using guaiacol. The reason of these differences in Km and Vmax of mentioned enzymes for the same substrate may relate to their different source.
Optimum temperature of our purified LPO has been evaluated 50°C. According to J. Tenovuo and Kurkijärvi LPO seems to have optimum temperature between 45°C and 50°C.[30] Thermal stability studies on LPO showed that heat denaturation of LPO starts at temperature up to 70°C.[4]
As shown in Figure 5, this enzyme is unable to tolerant high temperatures. Incubation of the enzyme at 75°C for about 120 s resulted in a significant reduction of enzyme activity. The purified LPO by Jafary et al. showed same temperature tolerance.[28] Thermal instability of LPO may be related to the heme prosthetic group, which Buried deep in the molecule, and is crucial for enzyme structure, and mechanism of activity.[31]
CONCLUSION
According to our results, using this method can be cost effective and short time-consuming for high yields and pure LPO. Yielding high pure LPO may be useful for industrial applications.
Financial support and sponsorship
This study was supported by Research Council of Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by Research Council of Isfahan University of Medical Sciences.
REFERENCES
- 1.Sharma S, Singh AK, Kaushik S, Sinha M, Singh RP, Sharma P, et al. Lactoperoxidase: Structural insights into the function, ligand binding and inhibition. Int J Biochem Mol Biol. 2013;4:108–28. [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel A, Parker H, Turner R, Dickerhof N, Khalilova IS, Wilbanks SM, et al. Uric acid and thiocyanate as competing substrates of lactoperoxidase. J Biol Chem. 2014;289:21937–49. doi: 10.1074/jbc.M113.544957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Baarri AN, Ogawa M, Hayakawa S. Application of lactoperoxidase system using bovine whey and the effect of storage condition on lactoperoxidase activity. Int J Dairy Sci. 2011;6:72–8. [Google Scholar]
- 4.Kussendrager KD, van Hooijdonk AC. Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and applications. Br J Nutr. 2000;84(Suppl 1):S19–25. doi: 10.1017/s0007114500002208. [DOI] [PubMed] [Google Scholar]
- 5.Boots JW, Floris R. Lactoperoxidase: From catalytic mechanism to practical applications. Int Dairy J. 2006;16:1272–6. [Google Scholar]
- 6.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 7.Andersson LA, Bylkas SA, Wilson AE. Spectral analysis of lactoperoxidase. Evidence for a common heme in mammalian peroxidases. J Biol Chem. 1996;271:3406–12. doi: 10.1074/jbc.271.7.3406. [DOI] [PubMed] [Google Scholar]
- 8.Ozdemir H, Aygul I, Küfrevioglu OI. Purification of lactoperoxidase from bovine milk and investigation of the kinetic properties. Prep Biochem Biotechnol. 2001;31:125–34. doi: 10.1081/PB-100103378. [DOI] [PubMed] [Google Scholar]
- 9.Mecitoğlu Ç, Yemenicioğlu A. Partial purification and preparation of bovine lactoperoxidase and characterization of kinetic properties of its immobilized form incorporated into cross-linked alginate films. Food Chem. 2007;104:726–33. [Google Scholar]
- 10.Atasever A, Ozdemir H, Gulcin I, Irfan Kufrevioglu O. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem. 2013;136:864–70. doi: 10.1016/j.foodchem.2012.08.072. [DOI] [PubMed] [Google Scholar]
- 11.Ye X, Yoshida S, Ng TB. Isolation of lactoperoxidase, lactoferrin, alpha-lactalbumin, beta-lactoglobulin B and beta-lactoglobulin A from bovine rennet whey using ion exchange chromatography. Int J Biochem Cell Biol. 2000;32:1143–50. doi: 10.1016/s1357-2725(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 12.Defaie M, Sharieat ZS, Divsalar A. Kinetic investigation on lactoperoxidase upon interaction with lead ion. Int J Environ Sci Dev. 2010;1:97. [Google Scholar]
- 13.Neyestani TR, Djalali M, Pezeshki M. Isolation of alpha-lactalbumin, beta-lactoglobulin, and bovine serum albumin from cow's milk using gel filtration and anion-exchange chromatography including evaluation of their antigenicity. Protein Expr Purif. 2003;29:202–8. doi: 10.1016/s1046-5928(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 14.Scopes R. Protein Purification: Principles and Practice. New York: Springer-Verlag; 1982. Ion exchangers-principles, properties and uses; pp. 75–101. [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Maehly A, Chance B. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–75. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- 18.Koksal E. Peroxidase from leaves of spinach (Spinacia oleracea): Partial purification and some biochemical properties. Int J Pharmacol. 2011;7:135–9. [Google Scholar]
- 19.Aghelan Z, Shariat SZ. Partial purification and biochemical characterization of peroxidase from rosemary (Rosmarinus officinalis L.) leaves. Adv Biomed Res. 2015;4:159. doi: 10.4103/2277-9175.161586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aune TM, Thomas EL. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977;80:209–14. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- 21.Dajanta K, Chukeatirote E, Apichartsrangkoon A. Effect of lactoperoxidase system on keeping quality of raw cow's milk in Thailand. Int Dairy Sci. 2008;3:112–6. [Google Scholar]
- 22.Tayefi-Nasrabadi H, Asadpour R. Effect of heat treatment on buffalo (Bubalus bubalis) lactoperoxidase activity in raw milk. J Biol Sci. 2008;8:1310–5. [Google Scholar]
- 23.Atamer M, Kocak C, Cimer A, Odabasi S, Tamucay B, Yamaner N. Some quality characteristics of kasar cheese manufactured from milk preserved by activation of lactoperoxidase/thiocyanate/hydrogen peroxide (LP) system. Milchwissenschaft. 1999;54:553–6. [Google Scholar]
- 24.Pourtois M, Binet C, Van Tieghem N, Courtois PR, Vandenabbeele A, Thirty L. Saliva can contribute in quick inhibition of HIV infectivity. AIDS. 1991;5:598–600. [PubMed] [Google Scholar]
- 25.Dumontet C, Rousset B. Identification, purification, and characterization of a non-heme lactoperoxidase in bovine milk. J Biol Chem. 1983;258:14166–72. [PubMed] [Google Scholar]
- 26.Hahn R, Schulz PM, Schaupp C, Jungbauer A. Bovine whey fractionation based on cation-exchange chromatography. J Chromatogr A. 1998;795:277–87. doi: 10.1016/s0021-9673(97)01030-3. [DOI] [PubMed] [Google Scholar]
- 27.Nandini KE, Rastogi NK. Integrated downstream processing of lactoperoxidase from milk whey involving aqueous two-phase extraction and ultrasound-assisted ultrafiltration. Appl Biochem Biotechnol. 2011;163:173–85. doi: 10.1007/s12010-010-9026-9. [DOI] [PubMed] [Google Scholar]
- 28.Jafary F, Kashanian S, Sharieat ZS, Jafary F, Omidfar K, Paknejad M. Stability improvement of immobilized lactoperoxidase using polyaniline polymer. Mol Biol Rep. 2012;39:10407–12. doi: 10.1007/s11033-012-1919-y. [DOI] [PubMed] [Google Scholar]
- 29.Köksal E, Gülçin I. Purification and characterization of peroxidase from cauliflower (Brassica oleracea L. var. botrytis) buds. Protein Pept Lett. 2008;15:320–6. doi: 10.2174/092986608784246506. [DOI] [PubMed] [Google Scholar]
- 30.Tenovuo J, Kurkijärvi K. Immobilized lactoperoxidase as a biologically active and stable form of an antimicrobial enzyme. Arch Oral Biol. 1981;26:309–14. doi: 10.1016/0003-9969(81)90052-2. [DOI] [PubMed] [Google Scholar]
- 31.Boscolo B, Leal SS, Ghibaudi EM, Gomes CM. Lactoperoxidase folding and catalysis relies on the stabilization of the α-helix rich core domain: A thermal unfolding study. Biochim Biophys Acta Proteins Proteomics. 2007;1774:1164–72. doi: 10.1016/j.bbapap.2007.07.003. [DOI] [PubMed] [Google Scholar]


