Abstract
Background:
One of the new methods of scaffold fabrication is a nano-micro hybrid structure in which the properties of the scaffold are improved by introducing nanometer and micrometer structures. This method could be suitable for scaffold designing if some features improve.
Materials and Methods:
In this study, electrospun nanofibers of 9% weight solution of poly (3-hydroxybutyrate) (P3HB) and a 15% weight of chitosan by trifluoroacetic acid were coated on both the surface of a silk knitted substrate in the optimum condition to improve the mechanical properties of scaffolds for cartilage tissue engineering application. These hybrid nano-micro fibrous scaffolds were characterized by structural and mechanical evaluation methods.
Results:
Scanning electron microscopy values and porosity analysis showed that average diameter of nanofibers was 584.94 nm in electrospinning part and general porosity was more than 80%. Fourier transform infrared spectroscopy results indicated the presence of all elements without pollution. The tensile test also stated that by electrospinning, as well as adding chitosan, both maximum strength and maximum elongation increased to 187 N and 10 mm. It means that the microfibrous part of scaffold could affect mechanical properties of nano part of the hybrid scaffold, significantly.
Conclusions:
It could be concluded that P3HB-chitosan/silk hybrid scaffolds can be a good candidate for cartilage tissue engineering.
Keywords: Cartilage tissue engineering, chitosan, hybrid scaffold, poly (3-hydroxybutyrate), silk
INTRODUCTION
The articular cartilage task is providing movements without friction. Cartilage tissue can be damaged by diseases such as arthritis or trauma resulting from injuries. Extracellular matrix has limited the ability to repair the extracellular matrix damage because of the complex structure and lack of blood or lymphatic vessels, as well as low-density.[1] Because of the cost of expensive treatment and due to the drawbacks of current treatments for cartilage injuries and diseases, tissue engineering is a new solution by the interaction of biomaterials as scaffold, growth factors, and cells to regenerate tissue function.[2,3] The three-dimensional scaffold provides mechanical support while gradually scaffold material destruct. Scaffolds can be derived from natural or synthetic ingredients in various physical forms such as foam, viscose fluids, hydrogels, and porous matrices.[3] The basic requirements of cartilage scaffold as target tissue can be the lack of immune response and inflammation, the attachment of chondrocytes, the maintenance of chondrocyte phenotype, and mechanical stability inherent in the injury.[4] Many materials in a wide range of chemical compositions, mechanical strength, structural topography, and biochemical properties have been developed for tissue engineering purposes.[3] One of the most interesting polymers to fabricate cartilage tissue engineering scaffolds is poly (3-hydroxybutyrate) (P3HB).
P3HB known as a thermoplastic, biodegradable, and biocompatible polymer that can be made by some species of microorganisms. It should be noted that P3HB compared to other polymers have higher mechanical properties. However, besides the benefits, this polymer shows disadvantages such as low hydrophilicity, slow degradation rate, and weak brittleness.[5,6,7] These drawbacks could be improved by using natural polymers beside P3HB. For instance, chitosan is of these natural polymers, which can modify brittleness and hydrophilicity.
Chitosan material is a biocompatible and hydrophilic natural polymer, which increases cell adhesion, proliferation, and differentiation. In addition, chitosan has antibacterial activity and shows minimal tissue reaction to the host. However, chitosan is mechanically weak and unstable. To increase and improve the mechanical and biological properties, chitosan is developed as a biologically active material.[8]
Considering the listed material properties, these materials together can provide a good choice for cartilage tissue engineering scaffolds. Sadeghi et al. were investigated in electrospun nanofibers as an alloy of P3HB and chitosan in different weight percentages. According to results, optimum percentages of chitosan were obtained by 15% and 20%. Adhesion and infiltration of chondrocyte cells on mentioned scaffolds were improved. However, poor mechanical properties can be a disadvantage of this alloyed scaffold.[9] Electrospinning is a method to fabricate nanofiber in different applications like designing scaffolds in tissue engineering. This method shows advantages such as high specific surface area which leads to increasing adhesion, migration, and cell growth in nanoscale. Moreover, it is some disadvantages like three-dimensional applications according to their pore size that is smaller than the diameter of the cell and may not allow the migration of cells within the structure.[10,11] Considering these problems, using another fabrication method or material would seem useful to adjust. One of the promising methods to produce three-dimensional tissue engineering scaffolds with high porosity is a combination of fibers at the nanoscale and microscale.[11]
The first step in fabricating of these hybrid scaffolds is a proper method for providing microscale base to provide proper structural environment.[11] Textile-based structures such as those braided, knitted, or woven in the microscale could be a good option for applications as tissue engineering materials. Knitting is based on multiple loops of yarn which is applicable to match mechanical properties by using biocompatible and biodegradable yarns. Rib texture is one of the weft knitted fabrics.[12] Studies based on knitted textured scaffolds have shown that three-dimensional silk scaffolds support, attach, and proliferate of stem cells.[13] The second step is fabricating the nanoscale part of the scaffold to supply a compatible surface for attachment and growth of cells and microfibers.[12] One of the production methods of nano-micro fibrous hybrid structures is a direct coating on textile structures. Electrospinning method as a flexible method for coating nanofibers on textiles is considered.[14] The benefits of both these techniques are the orientation of the fibers to improve the mechanical properties in weft knitting and the effect of electrospun nanofiber to improve cell behavior in electrospinning in the final scaffold.
Based on Sahoo et al. investigation, a hybrid biodegradable polymer scaffold was made of electrospun poly (lactic-glycolic acid) (PLGA) nanofibers coated on an annular tissue PLGA micro-fiber was provided. Hybrid scaffolds have shown good mechanical strength and integrity due to the presence of both microfiber knitted structure and high specific surface area made of nanofibers dispersed on the surface and between microfibers. The final hybrid scaffold is accompanied by increasing the surface area and pore size reduction of scaffolds, better adhesion of cells, and the formation of new tissue and extracellular matrix.[15] Growth and cell proliferation on knitted scaffolds in comparison with woven scaffolds also have significantly increased due to dense structure and low porosity of woven scaffolds. Terms of mechanical properties of woven scaffolds showed much better rigidity and strength than knitted substrate.[16] In another study, three types of hybrid scaffolds also include coverage of thin polycaprolactone (PCL) film, PLGA nanofibers, and thin collagen film on the knitted texture were produced and studied. In general, hybrid scaffolds illustrate better behavior in mechanical properties and cellular growth. The type of coating including nanofibers or collagen film also helped cell proliferation and growth. Coating nanofibers on PLGA annular texture have shown more cell growth.[16] Naghashzargar et al. modeled and designed a hybrid nano-micro fibrous scaffold for tendon-ligament applications using silk twisted yarn as microscale part and electrospun P3HB fibers among the twisted yarn as nanoscale part. The results demonstrated an increasing in mechanical properties and no cytotoxic effects and a good level of cytocompatibility.[17] Based on these studies, silk could be an appropriate option to fabricate microscale part of a nano-micro hybrid structure.
Silk is a natural polymer which is clinically used as sutures for centuries. Silk fibroin in various forms (film, fiber, network, mesh, membrane, yarn, and sponges) support stem cell adhesion, proliferation, and differentiation in vitro and promote tissue repair in vivo.[4] Because of good biocompatibility, blood compatibility, and the permeability of water and oxygen, silk can be used as a scaffold. Using of silk-based biomaterials as a scaffold is suitable for a wide range of skeletal tissue engineering such as bones, ligaments, cartilage, as well as the connective tissue, such as skin. Because of disadvantages of silk such as hydrophobicity and long degradation rate, it is usually used as a scaffold with other polymers.[4] Therefore, silk, P3HB, and chitosan are the three biomaterials that can be useful in a hybrid nano-micro scaffold for cartilage tissue engineering.
In this study, we aim to create a new structure of nano-micro substrate using the silk weft knitted and electrospun nanofibers of P3HB-chitosan hybrid to take advantage of any of the mentioned properties, methods, and materials to improve cartilage tissue engineering scaffolds.
MATERIALS AND METHODS
Materials
P3HB (Lot Number: ST_BB9669V, Cas Number: 29435-48-1, Mw = 300000 g/mol) and chitosan (Lot Number: STBF3507V, Cas Number: 9012-76-4 Mw = 1526.454 g/mol) were purchased from Sigma-Aldrich Company (United States of America). Trifluoroacetic acid (Lot Number: 607-091-00-1 Cas Number: 76-05-1 Mw = 169.87 g/mol) as a solvent was purchased from Merck Company (Germany).
Construction of the microscale silk knitted structure
Silk yarn was provided by the commercial vendor. First of all to degumming and removing sericin due to prevent the negative effects on chondrocytes, silk was treated at 25% weight sodium carbonate solutions for 30 min at a temperature of 90°C.[17] Knitted structure was fabricated in Passap Duomatic machine by 5 and 10 gauge needles. Rib 1 × 1 structure with low density was selected as the basic knitted substrate. In following, knitted fabricated structure was put in distilled water for 1 day and then for 2 h at a temperature of 120°C in the oven because of setting the structure and making it release of any tension.
Construction of the nano-micro hybrid scaffold
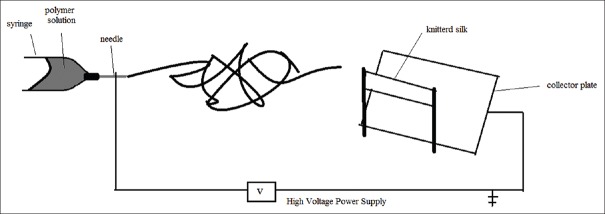
According to the previous studies,[9] the concentration of the solution, applied voltage, and the ratio of solvent were chosen to construct a nanofiber web by electrospinning method. A polymer solution P3HB-chitosan using trifluoroacetic acid as a solvent at 40°C temperature was prepared with 9% weight P3HB and 15% weight chitosan in 45 min. Then, the prepared polymer solution at a rate of feeding 10 ml per hour was set. The distance between needle and collector and voltage was adjusted 15 cm and 210 kV, respectively. Both sides of knitted sample were coated for 2 h, separately. To prepare the nano-micro hybrid structure, silk knitted fabric was adjusted in front of the needle of electrospinning close to the collector as shown in Figure 1. Different samples that comprised silk knitted substrate and P3HB-chitosan nanofibers were listed in Table 1.
Figure 1.
Schematic of electrospinning for coating nanofibers on the silk knitted structure
Table 1.
Fabricated samples made of silk knitted substrate, poly (3-hydroxybutyrate) and chitosan

Scanning electron microscope
Evaluating the morphology of polymeric scaffolds in terms of pore size, characterizing fiber diameter, and being interconnected was performed by scanning electron microscopy (SEM, with Philips XI30).
To measure porosity, SEM images as inputs in image processing were used in MATLAB 7.11.0.584 software (developed by MathWorks, Natick, Massachusetts, United States of America, since 1984).[9] The MATLAB program was set to measure porosity. The diameters of micro and nanofibers were measured by ImageJ software on the SEM images. For each sample to measure fiber diameter, thirty nanofibers of fabricated web were selected and analyzed.
Fourier transforms infrared spectroscopy
To examine functional groups, all materials including silk, pure P3HB, pure chitosan in addition hybrid types including P3HB-silk, P3HB-chitosan, and final structure made of P3HB-chitosan/silk scaffold were analyzed by Fourier transforms infrared spectroscopy (FTIR) instrument (Model JASCO6300 with 400–4000 cm−1). Due to the thickness of the samples, attenuated total reflectance-FTIR was used and finally the results were compared with each other.
Tensile test
To evaluate the mechanical properties, the longitudinal tensile test was performed on pure silk knitted structure, silk texture with electrospun P3HB, and silk texture with electrospun P3HB and 15% weight chitosan according to Table 1. Each test was repeated twice by Zwick tensile tester. Test speed was considered 50 mm per minute and sample length was set 6 inches, based on Naghashzargar et al. studies.[18] For more accurate comparison, the tensile test was performed on the pure P3HB and P3HB-20% weight chitosan scaffolds.
RESULTS
Scanning electron microscope
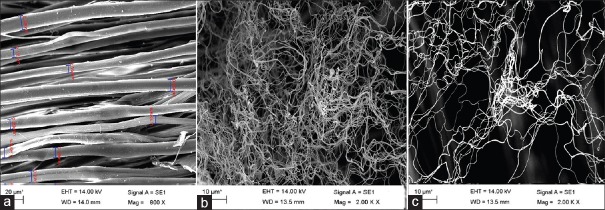
Fiber diameter and porosity of the nanofibrous layer are the two main parameters that can analyze the morphological structure of nanofibers.[19] To obtain optimal conditions, three types of scaffolds made of silk knitted structure, P3HB-silk hybrid scaffold, and P3HB-chitosan/silk hybrid scaffold were fabricated. Figure 2 shows SEM images which obtained from prepared samples. Average porosity was calculated in the first layer by MATLAB software as surface porosity. The porosity of various surface layers of the scaffold was measured using image analysis of SEM images of nanofibers mat by Ghasemi-Mobarakeh et al.[20] Table 2 shows the results of fiber diameters and surface porosity of scaffolds. Based on the table, fiber diameters range is decreasing by adding chitosan from 985.27 to 584.94. In addition, the porosity of all samples was measured more than 80%. One-way analysis of variance (ANOVA) was carried out for statistical analysis. A P < 0.05 was considered statistically significant at 0.95 level.
Figure 2.
Scanning electron microscopy images of (a) silk knitted substrate, (b) hybrid poly (3-hydroxybutyrate)/silk scaffold, (c) hybrid poly (3-hydroxybutyrate)-chitosan/silk scaffolds
Table 2.
Fiber diameter and surface porosity of fabricated samples mentioned in Table 1

Fourier transforms infrared spectroscopy
Figure 3 shows the results of FTIR of different type of scaffolds. As seen in Figure 3a, the most important peaks appear in the spectrum are the absorption peak in 3282 cm−1 wavelength as a result of the A amide group bond or NH bond in silk. There are a variety of groups, type I amide related to the stretching CH, type II amide group is related to stretching to the extended CH and bending NH, III, and IV type amide group of silk sample at 1658, 1512, 1226, and 1064 cm−1 wavelengths are clear and obvious.[21,22]
Figure 3.
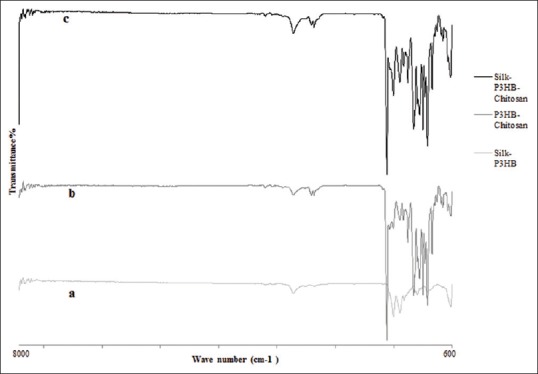
The results of Fourier transforms infrared spectroscopy (a) hybrid poly (3-hydroxybutyrate)-chitosan/silk scaffold, (b) hybrid poly (3-hydroxybutyrate)/silk scaffold (c) poly (3-hydroxybutyrate)-chitosan electrospun sample
In Figure 3a and b, the most important wavelengths of the P3HB electrospun fibers are mentioned. One of the wavelengths of the most important wavelength of the polymer is 1725 cm−1 relating to the stretching crystalline C=O bond. The absorption in 1188 cm−1 is related to asymmetric stretching vibration C_O_C, and the symmetric stretching vibration 1284 cm−1 relating to this bond and also peak at 1455 cm−1 was assigned to the asymmetric methyl group. Infrared spectroscopy of chitosan [Figure 3b] shows a strong absorption band in 3454 cm−1 due to the presence of OH and symmetric stretching vibration because of N_H of amine. A peak observed in 2923 cm−1 due to the CH2 stretching vibration is related to the pyranose ring. A peak in 1156 cm−1 is related to the saccharides structure. A sharp peak is in 1384 cm−1 is related to CH3 in the amide group. Broad peaks in 1021 cm−1 and 1098 cm−1 represent the stretching vibration C_O in chitosan, and peaks in 1628 cm−1 and 1540 cm−1 are due to the tension - C = O (amide type I) and stretching N_H (amide type II). The absorption bands in 1151 cm−1 related to nonsymmetric stretching C_O_C bridge and 1021 cm−1 and 1098 cm−1 is related to the vibrations of stretching C_O.[23]
Tensile test
In Table 3, the average of maximum force and maximum elongation at the mentioned force with their standard deviation were calculated and summarized. Figure 4 shows the force-elongation graph of mentioned scaffolds according to Table 1. Based on Table 3, the maximum force range is increasing from 171.66 to 187.485 N and maximum elongation improves from 8.32 to 10.165 mm.
Table 3.
Maximum force and maximum elongation of fabricated samples mentioned in Table 1
Figure 4.
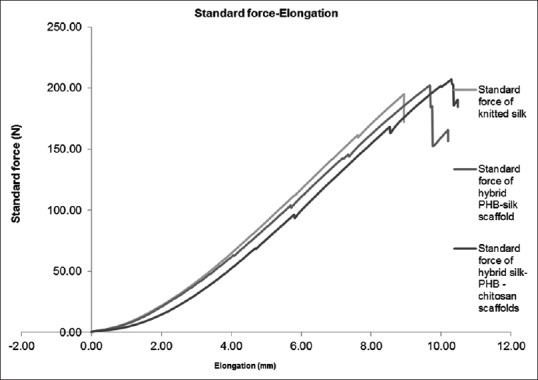
Diagram of force-elongation silk knitted substrate, hybrid poly (3-hydroxybutyrate)/silk scaffold, and hybrid poly (3-hydroxybutyrate)-chitosan/silk scaffolds
DISCUSSION
Scanning electron microscope
The value of average surface porosity for silk knitted structure, P3HB-silk hybrid scaffold, and P3HB-chitosan/silk hybrid scaffolds was measured 83.5%, 84.5%, and 86.5%, respectively. Porosity measurements illustrate that the percentage of porosity is more than 80% even by coating electrospun nanofibers on the surface of microscale silk fibers. In addition, average electrospun fiber diameter was measured 985.27 and 584.94 nm for P3HB-silk hybrid scaffold and P3HB-chitosan/silk hybrid scaffolds which is 198.9 µm for silk knitted substrate. Nano-fibers have diameters between 528 and 1314 nm for P3HB-silk hybrid scaffold and 477–735 nm for P3HB-chitosan/silk hybrid scaffold, while the diameter of micron fibers is between 150 and 280 µm. According to ANOVA, there is a significant difference between the average diameter values and porosity of silk knitted structure, P3HB-silk hybrid scaffold and P3HB-chitosan/silk hybrid scaffolds because of P < 0.5.
The results show that the values of fiber diameter by adding chitosan in electrospinning solution are decreasing significantly. Fiber diameter, which is reported in Sadeghi et al., is 1.811 µm for the optimized sample contained 15% weight chitosan and 9% weight P3HB.[9] Tehrani et al.[24] were obtained smooth P3HB fibrous scaffolds without beads with diameters of approximately 700 nm to 4 µ. Moreover, Masaeli et al.[25] could fabricate electrospinning of P3HB nanofibers with an average diameter of 993 nm and porosity 84.31. Masaeli et al.[26] in another study on P3HB and poly (3-hydroxy butyrate-co-3-hydroxyvalerate) (PHBV) blend reported the fiber diameter changed from 1208 ± 149 to 451 ± 65 nm.
In another study, Correia et al.[27] prepared 7% and 10% weight of P3HB solution using a combination solvent dimethylformamide/chloroform (7/3 ratio) and electrospun. The most fiber diameter was obtained about 400–600 nm. Garrigues et al.[28] fabricated electrospun single and multilayer PCL scaffolds for cartilage tissue engineering with an average fiber diameter of 0.56 ± 0.01 μm. Ricotti et al.[29] also were obtained the average fiber diameter of 200–400 nm for random and oriented P3HB electrospinning scaffolds.
By comparison the obtained results with previous investigations, P3HB-silk hybrid or P3HB-chitosan/silk hybrid structures are acceptable scaffolds in the final application in cartilage tissue engineering.
Fourier transforms infrared spectroscopy
By examining the FTIR spectrum of hybrid silk scaffold and P3HB, according to Figure 3a-c, it is clear that all the wavelengths related to the bonds of mentioned polymers are visible in hybrids scaffold. Based on Figure 3a, no new or changed chemical reaction between the silk and P3HB during the electrospinning process is created which is matched to the results of Naghashzargar et al.[18]
With regard to the spectrum of the sample containing chitosan and P3HB, shown in Figure 3b, peaks move in higher wavenumbers. Shifting toward in Figure 3b, higher wave numbers is due to the disturbance in crystalline phase of P3HB because of the addition of chitosan. The presence of these peaks and weakening them with increasing chitosan suggested a P3HB crystalline phase but more amorphous structures. This phenomenon comes from the formation of intramolecular hydrogen bonds between amino side groups in the chitosan and carbonyl groups of P3HB, which in turn reduces the crystallinity of the P3HB fibers. This type of interaction acts as a bridge between the polymers and facilitates the miscibility of two polymers in the alloy. This result confirms FTIR results of Sadeghi et al.[9]
As seen in Figure 3c and according to other figures, the presence of all materials is evident in the hybrid scaffold. As seen in Figure 3c, new bond or reaction between the materials has not been maintained.
Tensile test
The mechanical properties of cartilage vary according to location, type of duty, age, weight, and other conditions, but an important cartilage-like knee cartilage or a scaffold to replacement should bear a significant part of body weight.[30] However, one of the disadvantages of electrospun scaffolds is poor mechanical properties.[31] Hence, the combination of this fabricating method could improve some mechanical properties.
Based on the tension results, electrospinning generally increases both maximum tensile strength and maximum elongation. This trend has continued with increasing chitosan to P3HB and silk in nano-micro hybrid scaffold and as a result, mechanical properties have increased. However, there is no statistically significant difference between P3HB-silk and P3HB-chitosan/silk hybrid structures because of P > 0.05. It should be considered that an electrospun P3HB scaffold maximum tensile strength and maximum elongation shows a significant difference rather than all hybrid scaffolds. In this kind of hybrid structure, major suppliers of mechanical properties are the duty of the microfiber in silk knitted substrate, but according to the research of Sahoo et al.,[16] the impact of nano-mechanical properties cannot be ignored. At the same research in cartilage tissue engineering by Dai et al.,[32] hybrid structures of the three-dimensional scaffolds that combined type I collagen and PLGA knitted mesh was developed because of the mechanically strong PLGA knitted mesh and histologically facilitated cell seeding of collagen.
Kweon et al. in a study on silk fibroin/chitosan film blend obtained 4.5 MPa as the tensile strength of silk fibroin film, while that chitosan film was 30 MPa. This result confirms the probable effect of increasing chitosan in the improvement of mechanical properties.[33]
Steele et al. in a similar study on zonal articular cartilage engineering using PCL, hexafluoroisopropanol, and dichloromethane, fabricated combinatorial scaffold morphologies applying salt leaching and electrospinning method. Tensile stress at failure reported at this study was 0.4 ± 0.2 for this bilayered scaffold.[34] Ricotti et al. in their study were reported 6.3 ± 1.5 N as the ultimate tensile strength of random nanofibers P3HB scaffolds.[29] In a study by Ramier et al.,[35] tensile strength at break was reported 16.16 ± 0.86 MPa for electrospun P3HB-nano hydroxyapatite scaffolds. In a research has been done by Karbasi et al. at the same condition, the maximum force of electrospun P3HB scaffolds was measured 3.28 N, while the maximum elongation was 1.71 mm.[36] It is obvious that all these mentioned scaffolds and their mechanical properties are not suitable for cartilage tissue engineering, but the mechanical properties of hybrid scaffolds are significantly better than the electrospun scaffold.
A simple comparison between these two mentioned results illustrates a significant improvement of maximum force and elongation.
CONCLUSIONS
The aim of this study was to compare and interaction the three biomaterials including silk, P3HB, and chitosan in cartilage tissue engineering. Three main samples comprised silk knitted substrate, P3HB-silk, and P3HB-chitosan/silk were prepared by coating nanofibers on the surface of silk knitted substrate. According to the results, the porosity of samples was measured more than 80% with 477 nm as minimum fiber diameter and 735 nm as the maximum value of fiber diameter for P3HB-chitosan/silk sample. FTIR results showed the presence of all the materials without pollution and tensile tests were displayed a significant increasing in strength and elongation rather than electrospun P3HB-chitosan scaffolds without any negative effect on porosity or fibers diameter. Base on mechanical properties and comparison with other electrospinning scaffolds, these materials and fabrication method are a good choice to improve mechanical compliance for hard tissues such as cartilage. As a result, nano-micro hybrid scaffold comprised P3HB-chitosan/silk can be a promising substrate in cartilage tissue engineering.
Financial support and sponsorship
Isfahan University of Medical Sciences
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McDevitt CA. Biochemistry of articular cartilage. Nature of proteoglycans and collagen of articular cartilage and their role in ageing and in osteoarthrosis. Ann Rheum Dis. 1973;32:364–78. doi: 10.1136/ard.32.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinatier C, Bouffi C, Merceron C, Gordeladze J, Brondello JM, Jorgensen C, et al. Cartilage tissue engineering: Towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther. 2009;4:318–29. doi: 10.2174/157488809789649205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelttari K, Wixmerten A, Martin I. Do we really need cartilage tissue engineering? Swiss Med Wkly. 2009;139:602–9. doi: 10.4414/smw.2009.12742. [DOI] [PubMed] [Google Scholar]
- 4.Cheung HY, Lau KT, Lu TP, Hui D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos Part B Eng. 2007;38:291–300. [Google Scholar]
- 5.Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7:2249–58. doi: 10.1021/bm060317c. [DOI] [PubMed] [Google Scholar]
- 6.Luklinska ZB, Schluckwerder H. In vivo response to HA-polyhydroxybutyrate/polyhydroxyvalerate composite. J Microsc. 2003;211(Pt 2):121–9. doi: 10.1046/j.1365-2818.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 7.Doyle C, Tanner ET, Bonfield W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials. 1991;12:841–7. doi: 10.1016/0142-9612(91)90072-i. [DOI] [PubMed] [Google Scholar]
- 8.Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49:780–92. [Google Scholar]
- 9.Sadeghi D, Karbasi S, Bonakdar S, Razavi S. Evaluation of the structural properties and cellular behavior of electrospun poly (hydroxybutyrate)/chitosan blend scaffolds for cartilage tissue engineering. J Isfahan Med Sch. 2015;33:1441–58. [Google Scholar]
- 10.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006;12:1197–211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Jang DH, Park WH, Min BM. Fabrication and characterization of 3-dimensional PLGA nanofiber/microfiber composite scaffolds. Polymer. 2010;51:1320–7. [Google Scholar]
- 12.Anbumani N. Knitting Fundamentals, Machines, Structures and Developments, India. New Age International. 2007 [Google Scholar]
- 13.Almqvist KF, Wang L, Wang J, Baeten D, Cornelissen M, Verdonk R, et al. Culture of chondrocytes in alginate surrounded by fibrin gel: Characteristics of the cells over a period of eight weeks. Ann Rheum Dis. 2001;60:781–90. doi: 10.1136/ard.60.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou FL, Gong RH, Porat I. Nanocoating on filaments by electrospinning. Surf Coat Technol. 2009;204:621–8. [Google Scholar]
- 15.Sahoo S, Ouyang H, Goh JC, Tay TE, Toh SL. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12:91–9. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 16.Sahoo S, Cho-Hong JG, Siew-Lok T. Development of hybrid polymer scaffolds for potential applications in ligament and tendon tissue engineering. Biomed Mater. 2007;2:169–73. doi: 10.1088/1748-6041/2/3/001. [DOI] [PubMed] [Google Scholar]
- 17.Naghashzargar E, Semnani D, Karbasi S, Nekoee H. Application of intelligent neural network method for prediction of mechanical behavior of wire-rope scaffold in tissue engineering. J Text Inst. 2014;105:264–74. [Google Scholar]
- 18.Naghashzargar E, Farè S, Catto V, Bertoldi S, Semnani D, Karbasi S, et al. Nano/micro hybrid scaffold of PCL or P3HB nanofibers combined with silk fibroin for tendon and ligament tissue engineering. J Appl Biomater Funct Mater. 2015;13:e156–68. doi: 10.5301/jabfm.5000216. [DOI] [PubMed] [Google Scholar]
- 19.Širc J, Hobzová R, Kostina N, Munzarová M, Juklícková M, Lhotka M, et al. Morphological characterization of nanofibers: Methods and application in practice. J Nanomate 2012. 2012:121. [Google Scholar]
- 20.Ghasemi-Mobarakeh L, Semnani D, Morshed M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J Appl Polym Sci. 2007;106:2536–42. [Google Scholar]
- 21.Lu Q, Hu X, Wang X, Kluge JA, Lu S, Cebe P, et al. Water-insoluble silk films with silk I structure. Acta Biomater. 2010;6:1380–7. doi: 10.1016/j.actbio.2009.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Um IC, Kweon HY, Park YH, Hudson S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int J Biol Macromol. 2001;29:91–7. doi: 10.1016/s0141-8130(01)00159-3. [DOI] [PubMed] [Google Scholar]
- 23.Padermshoke A, Katsumoto Y, Sato H, Ekgasit S, Noda I, Ozaki Y. Melting behavior of poly (3-hydroxybutyrate) investigated by two-dimensional infrared correlation spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2005;61:541–50. doi: 10.1016/j.saa.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Tehrani AH, Zadhoush A, Karbasi S, Khorasani SN. Experimental investigation of the governing parameters in the electrospinning of poly (3-hydroxybutyrate) scaffolds: Structural characteristics of the pores. J Appl Polym Sci. 2010;118:2682–9. [Google Scholar]
- 25.Masaeli E, Morshed M, Rasekhian P, Karbasi S, Karbalaie K, Karamali F, et al. Does the tissue engineering architecture of poly (3-hydroxybutyrate) scaffold affects cell-material interactions? J Biomed Mater Res Part A. 2012;100:1907–18. doi: 10.1002/jbm.a.34131. [DOI] [PubMed] [Google Scholar]
- 26.Masaeli E, Morshed M, Nasr-Esfahani MH, Sadri S, Hilderink J, van Apeldoorn A, et al. Fabrication, characterization and cellular compatibility of poly (hydroxy alkanoate) composite nanofibrous scaffolds for nerve tissue engineering. PLoS One. 2013;8:e57157. doi: 10.1371/journal.pone.0057157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correia DM, Ribeiro C, Ferreira JC, Botelho G, Ribelles JL, Lanceros-Méndez S, Sencadas V. Influence of electrospinning parameters on poly (hydroxybutyrate) electrospun membranes fiber size and distribution. Polymer Engineering and Science. 2014;54(7):1608–17. [Google Scholar]
- 28.Garrigues NW, Little D, Sanchez-Adams J, Ruch DS, Guilak F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J Biomed Mater Res A. 2014;102:3998–4008. doi: 10.1002/jbm.a.35068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricotti L, Polini A, Genchi GG, Ciofani G, Iandolo D, Vazão H, et al. Proliferation and skeletal myotube formation capability of C2C12 and H9c2 cells on isotropic and anisotropic electrospun nanofibrous PHB scaffolds. Biomed Mater. 2012;7:035010. doi: 10.1088/1748-6041/7/3/035010. [DOI] [PubMed] [Google Scholar]
- 30.Kempson GE, Freeman MA, Swanson SA. Tensile properties of articular cartilage, nature. 1968;220:1127–1128. doi: 10.1038/2201127b0. [DOI] [PubMed] [Google Scholar]
- 31.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 32.Dai W, Kawazoe N, Lin X, Dong J, Chen G. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials. 2010;31:2141–52. doi: 10.1016/j.biomaterials.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 33.Kweon H, Ha HC, Um IC, Park YH. Physical properties of silk fibroin/chitosan blend films. J Appl Polym Sci. 2001;80:928–34. [Google Scholar]
- 34.Steele JA, McCullen SD, Callanan A, Autefage H, Accardi MA, Dini D, et al. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater. 2014;10:2065–75. doi: 10.1016/j.actbio.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramier J, Bouderlique T, Stoilova O, Manolova N, Rashkov I, Langlois V, et al. Biocomposite scaffolds based on electrospun poly (3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2014;38:161–9. doi: 10.1016/j.msec.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 36.Karbasi S, Zarei M, Foroughi MR. Effects of multi-wall carbon nano-tubes (MWNTs) on structural and mechanical properties of poly (3-hydroxybutyrate) electrospun scaffolds for tissue engineering applications. Sci Iran. 2016 accepted-in press. In press. [Google Scholar]