Abstract
Background:
In this paper, we have compared the cytomorphologic characteristics of liquid-based preparation (LBP) [SurePath (SP)] cytology and conventional smear (CP) preparations on fine-needle aspiration (FNAC) material by a semi-quantitative scoring system for cases of lymphadenopathy.
Materials and Methods:
In this prospective study, a total of 52 consecutive cases of FNAC of lymphadenopathy were included. The first pass was used for CP followed by LBP with the help of SP technique. The smears were independently compared and assessed by two observers (PS and PD).
Results:
The semiquantitative grading was compared in two groups by Wilcoxon signed-rank test. The background information, cell architecture, pleomorphism, nuclear and cytoplasmic details, and three-dimensional structures were significantly different in LBP and CP smears.
Conclusions:
Liquid-based cytology (LBC) is a relatively simple technique, which exhibits good nuclear and cytoplasmic details with the absence of obscuring background material. Even the number of slides and area per slide to be screened were less than the conventional preparation but caution must be applied to interpret the slides and secure a diagnosis, especially if LBC is the first and only method applied for diagnosis.
Keywords: Fine-needle aspiration cytology (FNAC), liquid-based cytology (LBC), SurePath (SP)
Introduction
Fine-needle aspiration cytology (FNAC) is a first-line investigative technique in the management of patients with lymphadenopathy, which is highly cost-effective and accurate. It is safe, simple, rapid, and relatively pain-free, and has become an acceptable and widely practiced minimally invasive technique.[1,2] The main limitation in FNAC of the lymph node is the high rate of false negative diagnosis in cases of low-grade non-Hodgkin lymphoma and specimen adequacy.[3,4] Liquid-based cytology (LBC) is being extensively used for both gynecological and nongynecological specimens. This technique enables cells to be suspended in liquid medium and spread in monolayer and thus, permitting better morphological assessment.[5,6] Two widely used technologies of LBC are ThinPrep (TP) (Hologic, Marlborough, MA, USA) and BD SurePath (BD Diagnostics - TriPath, Burlington, NC, USA).[5] The cytological criteria used for conventional preparation are also applicable to liquid-based preparations (LBPs); however, changes due to the effect of fixatives and processing techniques in the background, and architectural and morphological alterations or artifacts of LBP need to be recognized.[6]
Only a few papers have compared the conventional preparation and LBP for nongynecologic samples in the past.[7,8,9,10] However, the role of LBC in FNAC of the lymph node using TriPath Prep system (BD Diagnostics-TriPath, Burlington, NC, USA) has been described in very few studies.[11] The present study aims to compare the cytomorphologic characteristics of LBC [SurePath (SP)] and conventional smear (CP) preparations on FNAC material by a semi-quantitative scoring system for cases of lymphadenopathy.
Materials and Methods
We routinely perform FNAC of superficial lesions with the informed consent of the patient. Oral informed consent was taken in each patient before doing the routine FNAC of lymph node. In this study, we did not perform any special technique on the patient. SP preparation is also routinely used by us for diagnosis; therefore, no extra ethical clearance was taken from the ethics committee. So no ethical violation was done and the study was ethically justified by ethical rules. In this prospective study, a total of 52 consecutive cases of FNAC of lymphadenopathy were included. In each case, FNAC was performed by an experienced cytologist using a 23-gauge needle, 10-mL syringe, and a pistol handle. In each case, two passes were performed. The first pass produced a CP and direct smear that was fixed immediately in 95% alcohol for Papanicolaou or hematoxylin and eosin (H&E) stain. For SP, the material was collected in CytoRich fluid (BD Diagnostics-TriPath, Burlington, NC, USA) and mixed to homogenize. The sample was then transferred to a 50 mL centrifuge tube (BD Diagnostics-TriPath, Burlington, NC, USA) and centrifuged for 10 min in 600 g and the supernatant was decanted. Then 10 mL Tris buffer was added and centrifugation was done for 5 min in 600 g. The sample was decanted and vortexed to homogenize. Then the centrifuge tubes were loaded on PrepStain for processing. In each case of LBP preparation, one smear was stained with Papanicolaou stain. The LBP and CP slides were studied independently by two observers on different occasions. The representative CP and LBP slides were compared by a semi-quantitative scoring system [Table 1]. The presence of three-dimensionsal (3D) clusters was defined as cell clusters difficult to examine at high magnification, 20x, due to overlapping and tight arrangement if present on more than 5% of the entire slide.
Table 1.
Semi-quantitative grading of the cytological features on conventional and liquid-based preparations

Statistical analysis was performed by the Wilcoxon signed-rank test on the Statistical Package for the Social Sciences (SPSS) program (IBM, USA).
Results
Table 2 shows the number of cases and the various diagnoses made in the patients with lymphadenopathy on FNAC. Comparison of the various cytologic features between LBP and CP is shown in Table 3 (Wilcoxon signed-rank test). No cell was seen in eight cases in LBPs compared to six cases in CP; however, the overall difference in cellularity was not statistically significant (P =.082). In one case of tuberculosis, no cellularity was present in both the preparations but CP showed necrosis and on Ziehl-Neelsen (ZN) staining acid-fast bacilli (AFB) were identified. In another case of malignant small round blue cell tumor, CP showed predominantly blood while cellularity was adequate in case of LBP. This difference could be attributed to the different passes of aspirations taken. No statistically significant difference was seen in quality and clarity of both the preparations; however, there was a significant difference in the absence of blood and debris in LBP as compared to CP (P < 0.001). The background was clear in LBPs, apart from the presence of occasional red blood cells and cell debris in a few cases. It was easier and faster to screen and interpret LBP because the cells were in small areas and with a clear background. As far as informative background was concerned, it was well-preserved and recognized even in LBPs. The presence of 3D clusters was observed more in LBPs and was statistically significant (P = .035). However, the presence of cells as cohesive aggregates [Figures 1a and b] was noted more frequently in CP while there was no statistically significant difference in the presence of monolayer cells. Regarding the cell architecture, nuclear detail, and cytoplasmic detail, all were appreciated better in LBP (P = 0.02,.01, and 0.008, respectively). In case of Hodgkin and non-Hodgkin lymphomas, cytoplasmic detail was poorly preserved and Reed-Sternberg (RS) cells were not well-appreciated in the LBPs although the background blood was absent. In these cases, LBPs displayed more disruption of the cytoplasm and an increased number of naked nuclei. The distinction between various lymphoid cells is also difficult in LBPs. Many lymphoid cells show eccentric cytoplasmic projections in LBP [Figures 2a and b]. Lymphoglandular bodies were not seen in the background of LBPs. Although nuclear details were well-visualized in LBPs, no significant difference was seen in the presence of chromatin pattern and prominence nucleoli in the two preparations. There were occasional exceptions as in the case of Langerhans cell histiocytosis, in which characteristic nuclear grooving of the histiocytes was better appreciated in CP [Figures 2c and d]. In cases of malignant neoplasms, LBPs show less degree of pleomorphism as compared to CPs.
Table 2.
Distribution of cases and the various diagnoses made in the patients with lymphadenopathy

Table 3.
Comparison of the various cytologic features between LBP and CP (Wilcoxon signed-rank test)
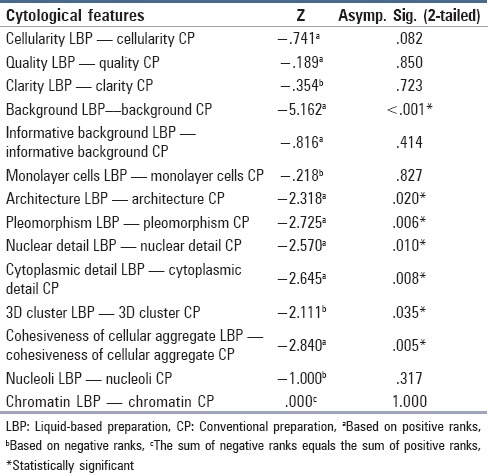
Figure 1.

(a) Conventional smear of metastatic squamous cell carcinoma in the cervical lymph node showing cells in clusters (H&E stain, ×440) (b) LBP of the same case showing predominantly singly scattered cells ((Pap stain, ×440)
Figure 2.

(a) Conventional smear showing scattered atypical cells in a case of Lymphnode showing infiltration by Mycosis Fungoides. (H&E stain, ×1200) (b) LBP of the same showing scattered atypical cells and reactive lymphoid cells with eccentric cytoplasmic projections. (Pap stain, ×1200) (c) Conventional smear of Langerhans cell histiocytosis showing characteristic nuclear grooving (H&E stain, ×1200) (d) LBP of the same showing less defined nuclear details. (Pap stain, ×1200)
In the five cases of granulomatous/tubercular lymphadenitis, epithelioid cell granuloma was noted in four cases in CP and three cases in LBPs [Figures 3a and b]. In one case, only necrosis was seen in CP; however, neither necrosis nor cellularity was present in LBP smear. The granulomas were not well-defined in LBPs as compared to those seen in CP.
Figure 3.

(a) Conventional preparation smear showing granuloma in case of tubercular lymphadenitis. [(H&E stain, ×440) (b) LBP smear of the same (Pap stain, ×440)
Discussion
LBP is generally favored over CP for evaluation of gynecological cytology specimens. However, studies comparing the diagnostic accuracy and morphology of these preparations in the evaluation of nongynecological cytology specimens have drawn variable conclusions.[7] Lee et al. have described several differences between the two preparation techniques and emphasized the importance of experience with LBPs for a correct interpretation.[12]
LBP produces a single slide with representative cells and less obscuring material; however, this feature could be a hindrance because background material such as tumor diathesis and necrosis may be helpful in establishing a diagnosis.[13] In the present study, no statistically significant difference was seen between LBP and CP regarding informative background, monolayer sheets, and cellularity. Garbar et al.[11] found more inadequate samples for LBP than for CS; however, it was not statistically significant as seen in our study. There was absence of blood in the background and better nuclear and cytological details was seen in LBPs in the present study. In a study comparing TP and CP in head and neck FNAC, the authors found no statistical difference between the two groups with regard to the presence of monolayer cells, cell architecture, nuclear details, and cytoplasmic details (P > 0.05). Cellularity, informative background, and cytoplasmic details were statistically more significant in the CP group (P < 0.05).[10] Dey et al. noted that TPs were superior to CP with regard to clear background, monolayer cells, and cell preservation.[9] In the study by Ford et al., TP was found to be equal to CP in terms of the degree of monolayer detail and cellular yield.[14] Ryu et al.[8] reported that when compared with CP, SP produced prominent 3D configurations for epithelial clusters that occasionally caused difficulty in recognizing nuclear characteristics. Similarly, in our study more 3D clusters were present more frequently in LBC preparations. Previous studies have shown that LBPs show more hyperchromatic nuclei and prominent nucleoli; however, no statistical significant difference was seen in the present study although the nuclear details were better visualized.
Garbar et al.[11] stated that the Reed–Sternberg cells were more difficult to observe, probably because of retraction or lysis of their cytoplasm by the alcohol fixation and hemolytic propriety of CytoRich Red or mechanical process. This finding was also noted in the present study where Reed-Sternberg cells were not easily discernible. The size difference among centroblasts, small lymphocytes, and centrocytes is not obvious. Centrocytes are more difficult to detect because their nuclei form is more rounded and less indented than those observed in conventional preparation (CP).[11] Kim et al.[13] documented that lymphoma was difficult to evaluate using LBC because lymphoid cells clumped together and appeared smaller. Ford et al.[14] also highlighted that lymphoid cells have a tendency to aggregate so they might be erroneously considered as epithelial cells; however, similar aggregations were not observed in other studies[10] and even in the present study.
Dey et al.[9] described that in granulomatous lymphadenitis, granulomas have a rounded contour and consist of epithelioid histiocytes with interspersed lymphoid cells in the case of LBPs. Granulomas seen on LBC in our study were ill-formed architecturally as compared to those seen in CS. In our study, granuloma was not identified in one case in LBP. Kim et al.[13] identified three benign lesions with granuloma on CP but none on LBC in their study. Wildi et al.[15] suggested that endoscopic ultrasound-guided FNAC (EUS-FNA) using CS was a good diagnostic tool for granulomatous lesions.
Ford et al.[14] stated that accuracy is the ultimate diagnostic goal of FNAC with secondary goals to achieve as much safety, speed, and cost-effectiveness as possible. The cytopreparatory technique used to prepare the specimen is integral to obtaining diagnostic accuracy. Other important considerations for choosing between CP technique and the SP technique are efficiency, level of skill required, safety, and cost. SP technique can be a viable alternative for clinicians who infrequently perform FNAC. From the pathologist's view, diagnosis obtained from using SP is more efficient because only one slide is needed for the technique. However, the technique becomes inefficient if a poor specimen is obtained and necessitates repeated aspiration.[12] Moreover, the cytology laboratory should obtain experience with and verify the features produced on LBPs when compared to CPs on a wide variety of lesions before implementing LBP methodology to FNAC material.[7]
The key advantages of the LBP technique are efficiency, minimal skill requirement, and safety while diagnostic accuracy and cost favor the use of the conventional (smear) technique.[14] Most of the studies have suggested that because of diagnostic problems and unfamiliarity, application of the LBC method on FNAC cytology is limited. So currently, the LBC method can supplement CP but cannot replace it on FNAC cytology.[7,16]
Conclusion
LBC is a relatively simple technique, which exhibits good nuclear and cytoplasmic details with the absence of obscuring background material. Even the number of slides and area per slide to be screened are less than the conventional preparation but caution must be applied to interpret the slides and secure a diagnosis, especially if LBC is the first and only method applied as adequate experience is required to familiarize with various lesions on LBC. Other aspects to be considered are the amount of material yield by FNA and cost efficacy.
Financial support and sponsorship
No grant was taken for this project.
Conflicts of interest
There is no conflict of interest in this paper.
References
- 1.Steel BL, Schwart MR, Ramzy I. Fine needle aspiration biopsy in the diagnosis of lymphadenopathy in 1,103 patients. Role, limitations and analysis of diagnostic pitfalls. Acta Cytol. 1995;39:76–81. [PubMed] [Google Scholar]
- 2.Lioe TF, Elliott H, Allen DC, Spence RA. The role of fine needle aspiration cytology (FNAC) in the investigation of superficial lymphadenopathy; uses and limitations of the technique. Cytopathology. 1998;10:291–7. doi: 10.1046/j.1365-2303.1999.00183.x. [DOI] [PubMed] [Google Scholar]
- 3.Pilotti S, Di Palma S, Alasio L, Bartoli C, Rilke F. Diagnostic assessment of enlarged superficial lymph nodes by fine needle aspiration. Acta Cytol. 1993;37:853–66. [PubMed] [Google Scholar]
- 4.Stewart CJ, Duncan JA, Farquharson M, Richmond J. Fine needle aspiration cytology diagnosis of malignant lymphoma and reactive lymphoid hyperplasia. J Clin Pathol. 1998;51:197–203. doi: 10.1136/jcp.51.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavatkar AN, Nagwanshi CA, Dabak SM. Study of a manual method of liquid-based cervical cytology. Indian J Pathol Microbiol. 2008;51:190–4. doi: 10.4103/0377-4929.41678. [DOI] [PubMed] [Google Scholar]
- 6.Hoda RS. Non-gynecologic cytology on liquid-based preparations: A morphologic review of facts and artifacts. Diagn Cytopathol. 2007;35:621–34. doi: 10.1002/dc.20698. [DOI] [PubMed] [Google Scholar]
- 7.Mygdakos N, Nikolaidou S, Tzilivaki A, Tamiolakis D. Liquid Based Preparation (LBP) cytology versus conventional cytology (CS) in FNA samples from breast, thyroid, salivary glands and soft tissues. Our experience in Crete (Greece) Rom J Morphol Embryol. 2009;50:245–50. [PubMed] [Google Scholar]
- 8.Ryu HS, Park IA, Park SY, Jung YY, Park SH, Shin HS. A pilot study evaluating liquid-based fine needle aspiration cytology of breast lesions: A cytomorphological comparison of SurePath ® liquid-based preparations and conventional smears. Acta Cytol. 2013;57:391–9. doi: 10.1159/000351306. [DOI] [PubMed] [Google Scholar]
- 9.Dey P, Luthra UK, George J, Zuhairy F, George SS, Haji BI. Comparison of ThinPrep and conventional preparations on fine needle aspiration cytology material. Acta Cytol. 2000;44:46–50. doi: 10.1159/000326224. [DOI] [PubMed] [Google Scholar]
- 10.Köybaşioğlu F, Önal B, Şimşek GG, Yilmazer D, Han U. Comparison of ThinPrep and conventional smears in head and neck fine needle aspiration cytology. Turk Patoloji Derg. 2008;24:159–65. [Google Scholar]
- 11.Garbar C, Remmelink M, Mascaux C. Fine needle aspiration cytology of lymph node: Experience of 2 university hospitals with conventional smears and liquid-based cytology. Acta Cytol. 2008;52:418–23. doi: 10.1159/000325546. [DOI] [PubMed] [Google Scholar]
- 12.Lee KR, Papillo JL, St John T, Eyerer GJ. Evaluation of the ThinPrep processor for fine needle aspiration specimens. Acta Cytol. 1996;40:895–9. doi: 10.1159/000333999. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Seo DW, Moon S-H, Gong G. Utility of liquid-based cytology in the evaluation of endoscopic ultrasound-guided fine-needle aspiration: Comparison with the conventional smears. Basic and Applied Pathology. 2010;3:57–62. [Google Scholar]
- 14.Ford L, Rasgon BM, Hilsinger RL, Jr, Cruz RM, Axelsson K, Rumore GJ, et al. Comparison of ThinPrep versus conventional smear cytopreparatory techniques for fine-needle aspiration specimens of head and neck masses. Otolaryngol Head Neck Surg. 2002;126:554–61. doi: 10.1067/mhn.2002.124704. [DOI] [PubMed] [Google Scholar]
- 15.Wildi SM, Judson MA, Fraig M, Fickling WE, Schmulewitz N, Varadarajulu S, et al. Is endosonography guided fine needle aspiration (EUS-FNA) for sarcoidosis as good as we think? Thorax. 2004;59:794–9. doi: 10.1136/thx.2003.009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung CK, Lee A, Jung ES, Choi YJ, Jung SL, Lee KY. Split sample comparison of a liquid-based method and conventional smears in thyroid fine needle aspiration. Acta Cytol. 2008;52:313–9. doi: 10.1159/000325513. [DOI] [PubMed] [Google Scholar]


