Abstract
Background:
The role of fine-needle aspiration cytology (FNAC) in thyroid nodules has been well-studied but there is a paucity of studies solely involving papillary thyroid carcinoma (PTC). The diagnostic criteria for PTC are established but still there is a worrisome possibility of false positive and false-negative results, which can have a serious impact on patient care. This article correlates the cytological and histological diagnosis of PTC with an eventual aim of analyzing the cause of false positive and false negative results in order to prevent misdiagnosis. An increase in the incidence of PTC in the younger population has been noted.
Aims:
To analyze the epidemiological trends and cytohistological correlation of PTC and evaluate the discordant cases and pitfalls of FNAC.
Materials and Methods:
Seventy-two cases who had undergone both FNAC and histopathological examination (HPE) of the thyroid gland were selected. Age and sex distribution as well as cytohistological correlation were done for all the cases.
Results:
Cytohistological correlation was 81.94%. False positives were 5.56% and the false negative rate was 13.2%. Sensitivity was 86.7% and the positive predictive value was 93.6%. The peak age was 31-40 years among females and 41-60 years among males. Seven of our patients were <20 years of age (10%).
Conclusion:
FNAC is an indispensible tool for the early diagnosis of PTC. However, certain conditions of the thyroid gland can cause diagnostic dilemma. Awareness of pathologists regarding these pitfalls can prevent misdiagnosis and provide better patient care. Increasing the incidence of PTC with a more striking increase in the younger population makes early diagnosis all the more important owing to better prognosis in this age group.
Keywords: Cytohistological correlation, fine-needle aspiration cytology (FNAC), incidence of thyroid cancer, papillary carcinoma
Introduction/Background
Thyroid cancer is the most common endocrine malignancy.[1] Papillary thyroid carcinoma (PTC) is the most common malignant tumor among all thyroid cancers, accounting for an estimated 75-85% of thyroid cancers.[2] In India and worldwide, it appears to be a major health problem. In the USA, the estimated new cases in 2015 was 62,450.[3] In India, the National Cancer Registry Programme has reported the thyroid gland as the leading site of cancer accounting for 1.5% of all cancers in men and 3.3% in women.[4] The increasing incidence of thyroid cancer, especially PTC, is of concern.[2]
PTC is more common in females and the peak age group is 30-50 years,[5] with some variations in different populations.[6,7] Such age-specific data are not available for our population. In the last few years, we have noticed it to be occurring more often in younger patients. This study was conducted to find out the epidemiological pattern of this tumor in our population and to observe and analyze the shift in the peak age for this tumor, if present. In contrast to the high incidence, death from PTC is rare and most patients respond well to surgery and targeted therapy with radioactive iodine. PTC in the younger age group has a better prognosis as compared to the middle age group. Hence, early diagnosis can be greatly beneficial.
The established diagnostic criteria of PTC on fine-needle aspiration cytology (FNAC) and histopathology are characteristic. FNAC is the first-line diagnostic test for the evaluation of swellings of the thyroid gland. It is an efficient method of selecting patients who require surgical treatment owing to its simplicity, low cost, high sensitivity, and specificity. Despite these advantages, FNAC can be limited by the quality of the material. There is a worrisome possibility of false positive and false negative results on cytology, which can have a serious effect on patient care. The most common reasons for false-positive diagnosis are the presence of similar features in various other diseases of the thyroid gland.[8] A false negative diagnosis could have been given in cases where the characteristic features of papillary carcinoma are absent or these features are present less frequently in cytology smears. Another aim of our study was to analyze the correlation between the cytological diagnosis and histopathological (HP) diagnosis of PTC. This would allow the pathologist to be more vigilant when dealing with such cases in order to avoid misdiagnosis.
Materials and Methods
This is a retrospective analysis conducted between 2009 and 2014 at our institution and it was approved by the institution's ethical committee. It included all the cases reported as PTC either on cytology or histopathology. The data were searched from cytology and histopathology records. The information gathered were the age and sex of the patient, and cytology and histopathology diagnoses so as to study the age distribution of this tumor and correlation between the cytology and histopathology reports. An attempt was made to find out if there was any frequent cause of misdiagnosis.
Results
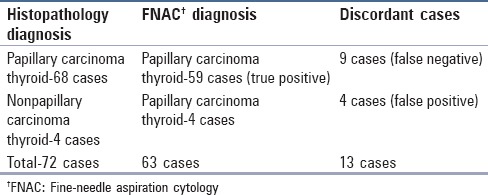
In the database, there were a total of 72 cases reported as PTC on cytology or histopathology. The cytological diagnosis of PTC was given in 53 cases and “suspicious for PTC” in 10 cases. All the 53 cases were confirmed to be PTC on HPE. Out of 10 cases reported as “suspicious for PTC” on cytology, six cases were confirmed on HPE. In four cases, the cytological diagnosis did not correlate with the HPE diagnosis amounting to false positives of 5.56% [Figure 1]. The HPE diagnosis in these cases was adenomatous goiter in two cases, Hashimoto's thyroiditis in one case, and nodular goiter in one case [Table 1 and Figure 2a–c].
Figure 1.
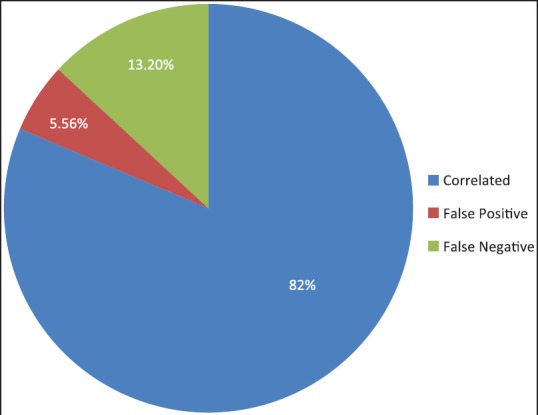
Cytology–histopathology correlation among the cases analyzed
Table 1.
Details of false positive cases on cytology
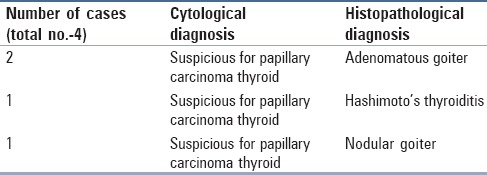
Figure 2.
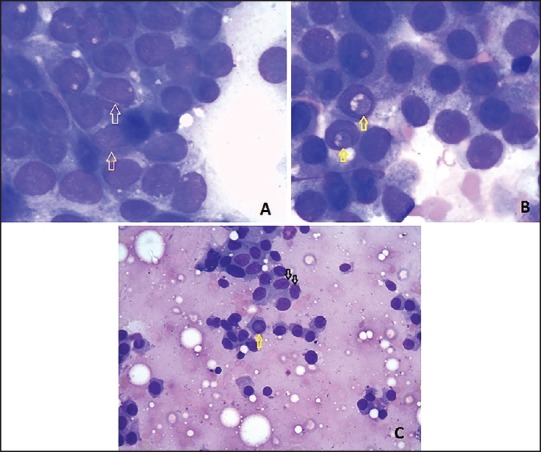
Cytology of cases reported as “suspicious of papillary carcinoma”: (a) Showing nuclear grooves (arrows). This case on histopathology was found to be of adenomatous goiter. (MGG stain, ×100) (b) Showing intranuclear cytoplasmic inclusions (arrows). This case on histopathology was found to be nodular goiter. (MGG stain, ×100) (c) Showing nuclear grooves (black arrows) and intranuclear cytoplasmic inclusions (yellow arrow). This case on histopathology was found to be Hashimoto's thyroiditis. (MGG stain, ×40)
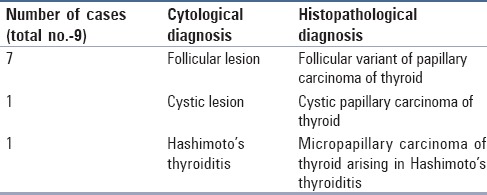
The HPE diagnosis of PTC was given in 68 cases, out of which in nine cases the cytology reports differed. This amounted to a false negative rate of 13.2%. The cytology diagnosis in seven out of these nine cases was follicular neoplasm, in one case cystic lesion of the thyroid gland, and Hashimoto's thyroiditis in one case. The HPE diagnosis for these nine cases was a follicular variant of PTC in seven cases, cystic PTC in one case, and micropapillary carcinoma arising in Hashimoto's thyroiditis in one case [Table 2 and Figure 1].
Table 2.
Details of false negative cases on cytology
The sensitivity (true positive/true positive + false negative) of FNAC for detecting PTC was 86.7% and positive predictive value (true positive/true positive + false positive) was 93.6% [Table 3]. The false positive rate, specificity, and negative predictive values could not be calculated as the study included only PTC cases and there were no true negative cases.
Table 3.
Cytohistopathology correlation
The age and sex distribution of PTC was calculated for 68 cases and was confirmed to be PTC on histopathology. There were 52 females and 16 males (female:male ratio-3.25:1). The age distribution among our cases is shown in Table 4 and Figure 3. The peak incidence was noted in the age range of 31-40 years among females and 41-60 years among males.
Table 4.
Age distribution of papillary carcinoma of thyroid in our study
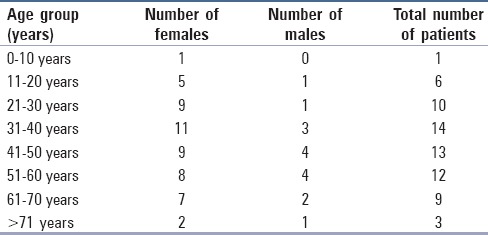
Figure 3.
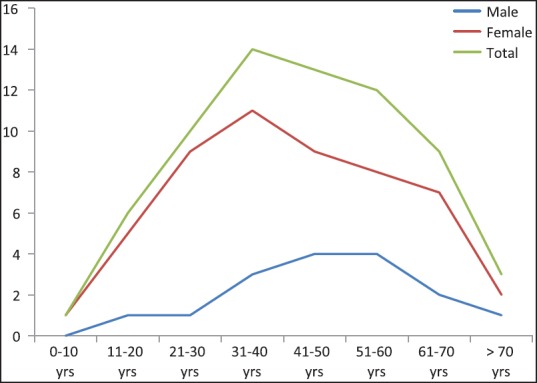
Age distribution of the cases in the present series
Discussion
Thyroid diseases are among the most common endocrine disorders worldwide. In India too, there is a significant burden of thyroid diseases.[1] Papillary carcinoma is the most common type of thyroid malignancy. According to Surveillance, Epidemiology, and End Results (SEER) stat fact sheets, the number of estimated new cases of thyroid cancer in 2014 was 62,980 and it accounted for 3.8% of all the new cancer cases with the estimated deaths amounting to 1,890.[7] In India, the National Cancer Registry Programme has reported thyroid as a leading site of cancer accounting for 1.5% of all cancers in men and 3.3% in women.[4]
The literature shows a clear preponderance of PTC in females. The reported female:male ratio varies between 2:1 and 4:1.[2,5] In our study, the ratio was 3.25:1. This is in concordance with the previous studies. The Indian Council of Medical Research established the National Cancer Registry Programme, which has collected the data of more than 300,000 cancer patients between 1984 and 1993. Among these patients, the National Cancer Registry Programme (NCRP) noted 5,614 cases of thyroid cancer, and this included 3,617 females and 2,007 males.
Most papillary carcinomas are diagnosed in patients in the third to fifth decades of life.[5] Carcangiu et al. from Italy noted the peak incidence in the age group of 31-40 years.[9] Their incidence gradually decreased till >70 years age group. According to the cancer research statistics of the UK, age-specific incidence rates in males rise gradually from around the age of 1-14 years.[6] The rates in females rise sharply from around the age of 10-14 years, reaching a peak at the age of 35-39 years and then remaining high.[6] Incidence rates are higher for females than for males for most age groups.[6] In our study also, the peak age group was 31-40 years in females and 41-60 years in males. The age-specific incidence in females remained relatively high till the age of 70 years. These observations are similar to those of the UK cancer society.[6] Unlike the UK cancer society, we noted a decline in incidence after 70 years of age, which was probably because of a lesser life expectancy in our Indian population.
According to SEER, between 2007 and 2011, the peak age for thyroid cancer was 45-54 years with 24.2% cases.[7] The age group with patients less than 20 years accounted for only 1.8% of the cases.[7] In the present series, which included only PTC, there were seven cases who were less than 20 years of age accounting for 10% of the total cases. Six of the seven patients were in the 11-20 years age group and only one patient was less than 10 years of age (7 years at the time of diagnosis). This indicates a much higher incidence of this tumor in our younger population as compared to the American population. However, our incidence in <20 years age group appears to be similar to the publication from Italy.[9] A trend toward an increase in pediatric thyroid cancer has been noticed by other researchers as well.[10]
The incidence of clinically palpable thyroid nodules in children is estimated to be around 1-1.5%. However, in teenagers this prevalence is 13%.[11] When compared to adults, children have four times greater risk of malignancy when a thyroid nodule is diagnosed.[12] Despite this, pediatric thyroid cancers have a much better prognosis than their adult counterparts with an almost 100% disease-free survival.[13] Hence, all the thyroid nodules in children should be screened with high suspicion.
FNAC is regarded as a gold standard in the initial diagnosis of thyroid nodules. It is simple, reliable, time-saving, minimally invasive, and cost-effective. One of the major advantages is that FNAC can be done as an outpatient procedure. The diagnostic value and accuracy FNAC in the evaluation of thyroid nodules is well-established. FNAC is relied upon to distinguish benign from neoplastic or malignant thyroid nodules and therefore, has led to a dramatic decrease in thyroid surgeries.[14] However, fine-needle aspiration has some limitations such as specimen inadequacy, sampling techniques, worrisome histologic alterations following fine-needle aspiration of the thyroid (WHAFFT) changes, and false negative and false positive results.[15,16] The cytopathologist should be aware of these potential limitations of FNA interpretation. Histopathological examination (HPE) of surgically excised thyroid gland swelling is the most accurate way to determine the pathology.
In the present study, cytology diagnosis clearly correlated with histopathology diagnosis in 53 out of 72 cases. In 10 cases, cytology diagnosis was given as “suspicious for papillary carcinoma”; these cases were also included in the papillary carcinoma group on cytology in this study because all the cases “suspicious for papillary carcinoma” of the thyroid gland are considered as papillary carcinoma as far as treatment is concerned. Out of 10 cases of “suspicious for papillary carcinoma,” six cases were confirmed as papillary carcinoma in HP. In the remaining four cases, the cytological diagnosis did not correlate with HP, resulting in false positives of 5.56%. The false positive rate, specificity, and negative predictive values could not be calculated as the study included only PTC cases and there were no true negative cases. The HP diagnoses in these four cases were adenomatous goiter in two cases, Hashimoto's thyroiditis in one case, and nodular goiter in another.
It is noteworthy that all the cases, which were reported as papillary carcinoma on cytology proved to be so on HP. This indicates that when sampling was adequate and there was presence of frequent characteristic features on cytology, the diagnosis was confirmatory, whereas four out of 10 lesions that were reported as “suspicious of papillary carcinoma” were not found to be papillary carcinoma on HP. This emphasizes the pitfall of FNAC in rendering confirmed diagnosis in cases where there is a paucity of characteristic features of PTC.
The cytohistological correlation including the suspicious cases came to 81.94%. The literature review for similar studies has reported a correlation ranging from 72% to 88%.[14,17,18,19,20]
Although cytologic diagnosis of papillary carcinoma has a high specificity, it is the most common false positive diagnosis when compared to other thyroid malignancies because of the presence of papillary carcinoma-like features in other lesions as well. The percentage of false positive cytological diagnosis in our study was 5.56% as compared to the literature percentage ranging from 5.25% to 11.60% in various studies.[15,17,18,20,21]
The reason accountable for false positive diagnosis in our study and also in the literature was the presence of features diagnostic of PTC, which can also be seen in benign conditions of the thyroid gland. There is evidence in the literature that the characteristic features of PTC such as nuclear grooves, nuclear pseudoinclusions, and many more features can be seen in benign conditions of the thyroid gland such as adenomatous goiter, Hashimoto's thyroiditis, nodular goiter, and follicular neoplasm.[8,22] However, studies have stated that the occurrence of these features in benign lesions is very less as compared to PTC.[23] In our study also, we observed the same reasons for false positive cases. Figures 2a and c show nuclear grooves on the cytology of cases, which were found to be adenomatous goiter and Hashimoto's thyroiditis on histopathology, respectively. Similarly, Figures 2b and c show intranuclear cytoplasmic inclusions on the cytology of cases, which were found to be nodular goiter and Hashimoto's thyroiditis on histopathology, respectively. Owing to the presence of these characteristic features of PTC, these cases were reported as “suspicious of PTC” on cytology. However, all the four cases showed the presence of nuclear grooves and intranuclear cytoplasmic inclusions in less number of cells and in addition, the other characteristic features of PTC such as metaplastic cytoplasm, psammoma bodies, and three-dimensional fragments were absent. Hence, the awareness of pathologists in this context and strict adherence to adequacy criteria can reduce the false positive rate.
The false negative FNAC results may occur because of sampling error or misinterpretation of cytology, and are of great concern because they indicate the potential to miss a malignant lesion. An adequate cellular aspirate is indispensable for an accurate diagnosis.
In present study, nine cases with a diagnosis of PTC on HPE had been misdiagnosed on cytology. Out of these, for seven cases a diagnosis of follicular neoplasm had been rendered on cytology and the corresponding HPE diagnosis was a follicular variant of PTC (FVPTC). Out of the remaining two cases, one was Hashimoto's thyroiditis and the other was cystic lesion of the thyroid gland. The false negative rate in the present study was 13.2% as compared to 4-19% reported in other comparable studies.[17,18,20,21]
Studies in the past have highlighted the fact that in FVPTC, the nuclear features essential for a cytologic diagnosis of PTC are infrequent.[24,25,26,27] One of the most common and clinically significant pitfalls in the assessment of an aspirate of a follicular lesion is the failure to recognize the nuclear features of the FVPTC, the most common variant of papillary carcinoma.[28] Cytological diagnosis is difficult because of the paucity of nuclear changes of papillary carcinoma.[20] This indicates that the sensitivity of FNA in establishing a diagnosis of FVPTC is low.[29] These concerns in the literature are further affirmed in our observation study where FVPTC cases accounted for the high false negative rate (seven out of nine false negative cases). From this, we infer that the presence of infrequent nuclear features with a follicular pattern in cytology should alert the pathologist to consider the diagnosis of FVPTC, which is a major diagnostic challenge.
False negative diagnosis can also occur due to inadequate sampling or due to the aspiration of fluid in cystic lesions of the thyroid gland with an underlying malignancy, as happened in one of our cases (reported as cystic lesion on cytology). Among all the thyroid cancers, PTC tends to undergo marked hemorrhagic degenerative changes. Sampling of this hemorrhagic fluid with sparse tumor cells may result in false interpretation as a benign cyst.[30] In the present case, suggestion for surgical excision had been given by the pathologist in order to rule out an underlying malignancy, which led to timely surgery and saved the patient of undue complications of PTC.
Although fine-needle biopsy is the best predictor of malignancy in either cystic or solid thyroid lesions, it is slightly less reliable when a thyroid lesion is fluid-filled rather than solid.[31] A close follow-up or thyroid lobectomy for diagnosis should be strongly considered in these patients even when FNA cytologic finding is interpreted as benign.[32,33] Meticulous examination of all the smears is of paramount importance in reducing discrepant cases.[20] Any recurrent cystic lesion should raise a strong suspicion for malignancy and should be treated accordingly.
One of the other false negative cases was reported as Hashimoto's thyroiditis on cytology. This case on HPE was found to be papillary microcarcinoma of the thyroid gland. The term papillary microcarcinoma is used when the papillary carcinoma is an incidental finding and measures less than 1 cm in diameter. Papillary microcarcinoma arising in Hashimoto's thyroiditis or in any other benign lesion has high chances of being missed because FNAC is a blind procedure and as a result of which the aspirated sample may not be fully representative of the existing lesion. Papillary microcarcinomas of the thyroid (i.e., papillary carcinomas <1 cm in size) are frequent; autopsy surveys have revealed a prevalence of up to 30% in high-risk groups.[34] However, their clinical impact is limited in the vast majority of cases and do not cause a significant reduction in the survival.[35]
In our study, we found that FNAC is a reliable method of screening thyroid nodules for PTC with a sensitivity as high as 86.7% and a positive predictive value of 93.6%. However, there are some studies in the literature reporting a sensitivity of as low as 55.3% and a false negative as low as 44.7%.[36] Our results are comparable to other reports in the literature.[14,17,21,35,37,38] The likely reason reported for lower sensitivity in a series of Mistry et al. was a combination of operator variability, low number (56 cases), and diagnostic difficulty of using FNAC in certain thyroid pathologies.[36] In our series of 72 cases where FNACs were conducted by trained personnel and cytology smears studied by an experienced pathologist, the FNAC had high sensitivity and positive predictive value.
Conclusion
PTC is the most common thyroid malignancy and its incidence seems to be on a rise. There appears to be an increase in its incidence in the younger population, causing an epidemiological shift in the age distribution. Early diagnosis is extremely essential for timely treatment and good prognosis. FNAC serves as a gold standard and an indispensable tool for early diagnosis. However, FNAC has pitfalls that lead to false positive and false negative diagnosis. False positive diagnosis can be caused due to the presence of characteristic features of PTC in benign conditions of the thyroid gland. False negative diagnosis occurs as a result of inadequate sampling and the presence of infrequent nuclear features of PTC, which can cause diagnostic dilemma such as in FVPTC. Cystic PTC is another diagnostic challenge and can be missed on FNA. Therefore, despite a benign cytology clinically suspicious lesions should have close clinical follow-up and should probably be excised. Papillary microcarcinoma is the most common form of PTC but it is an incidental diagnosis as most of the cases are missed owing to its small size. Awareness of pathologists regarding these pitfalls of FNAC and meticulous examination of smears with strict adherence to the diagnostic criteria of PTC can reduce false positive and false negative diagnoses and thus, provide better patient care.
Financial support and sponsorship
Nil.
Conflicts of interest
No financial support of any form was obtained by any of the authors in relation to this study.
References
- 1.Unnikrishnan AG, Menon UV. Thyroid disorders in India: An epidemiological perspective. Indian J Endocrinol Metab. 2011;15(Suppl 2):S78–81. doi: 10.4103/2230-8210.83329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maitra A, Abbas AK. The endocrine system. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Phildelphia: Saunders; 2004. pp. 1155–226. [Google Scholar]
- 3.American Cancer Society: Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Last accessed on 2015 Mar 28]. Available from: http://www.cancer.org/research/cancerfactsstatistics . [Google Scholar]
- 4.Consolidated report of hospital based cancer registries 2007-11. New Delhi, India: National cancer registry program (ICMR); [Last accessed on 2015 Mar 12]. Available from: http://www.icmr.nic.in/ncrp/HBRC_Report_2007_2011/Main.htm . [Google Scholar]
- 5.Jayaram G. Atlas and Text of Thyroid Cytology. New Delhi: Arya Publications; 2006. Papillary carcinoma; pp. 35–48. [Google Scholar]
- 6.Thyroid cancer incidence statistics: Cancer Research UK-2014. [Last accessed on 2015 Apr 1]. Available from: http://www.cancerresearchuk.org/cancer.../cancerstats/.../thyroid/incidence/uk-t .
- 7.National cancer institute-USA-SEER Stat Fact Sheet-2012. USA: [Last accessed on 2015 Mar 28]. Available from: http://www.Seer.cancer.gov/statfacts/html/thyro.html . [Google Scholar]
- 8.Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology-Definition, Criteria and Explanatory Notes. New York: Springer; 2010. [Google Scholar]
- 9.Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer. 1985;55:805–28. doi: 10.1002/1097-0142(19850215)55:4<805::aid-cncr2820550419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: Incidence and outcomes in 1753 patients. J Surg Res. 2009;156:167–72. doi: 10.1016/j.jss.2009.03.098. [DOI] [PubMed] [Google Scholar]
- 11.Josefson J, Zimmerman D. Thyroid nodules and cancers in children. Pediatr Endocrinol Rev. 2008;6:14–23. [PubMed] [Google Scholar]
- 12.Vaisman F, Corbo R, Vaisman M. Thyroid carcinoma in children and adolescents-systematic review of the literature. J Thyroid Res 2011. 2011 doi: 10.4061/2011/845362. 845362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kacar A, Paker I, Bayram GK, Demirel F, Senel E, Kizilgun M. Fine needle aspiration cytology of pediatric thyroid nodules. Turk Patoloji Derg. 2010;26:147–52. [Google Scholar]
- 14.Borgohain R, Lal RK, Chatterjee P, Brahma N, Khanna S. A study of cyto-histological correlation in the diagnosis of thyroid swelling. IOSR J Dent Med Sci. 2014;13:46–9. [Google Scholar]
- 15.Kumar SK, Seetharamaiah T, Rampure D, Ramakrishna C, Devi RY. Thyroid nodule: Cytohistological correlation. Scholar J Appl Med Sci. 2013;1:745–7. [Google Scholar]
- 16.Sharma C, Krishnanand G. Histologic analysis and comparison of techniques in fine needle aspiration-induced alterations in thyroid. Acta Cytol. 2008;52:56–64. doi: 10.1159/000325435. [DOI] [PubMed] [Google Scholar]
- 17.Sidawy MK, Del Vecchio DM, Knoll SM. Fine-needle aspiration of thyroid nodules: Correlation between cytology and histology and evaluation of discrepant cases. Cancer. 1997;81:253–9. doi: 10.1002/(sici)1097-0142(19970825)81:4<253::aid-cncr7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Bakhos R, Selvaggi SM, DeJong S, Gordon DL, Pitale SU, Herrmann M, et al. Fine-needle aspiration of the thyroid: Rate and causes of cytohistopathologic discordance. Diagn Cytopathol. 2000;23:233–7. doi: 10.1002/1097-0339(200010)23:4<233::aid-dc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Choudhury M, Singh S, Agarwal S. Diagnostic utility of Ki67 and p53 immunostaining on solitary thyroid nodule — A cytohistological and radionuclide scintigraphic study. Indian J Pathol Microbiol. 2011;54:472–5. doi: 10.4103/0377-4929.85077. [DOI] [PubMed] [Google Scholar]
- 20.Pandey P, Dixit A, Mahajan NC. Fine-needle aspiration of the thyroid: A cytohistologic correlation with critical evaluation of discordant cases. Thyroid Res Pract. 2012;9:32–9. [Google Scholar]
- 21.Jarwani PB, Patel S. Fine-needle aspiration cytology (FNAC) of the thyroid: A cytohistologic correlation with critical evaluation of discordant cases. GCSMC J Med Sci. 2013;2:5–12. [Google Scholar]
- 22.Tahlan A, Dey P. Nuclear grooves. How specific are they? Acta Cytol. 2001;45:48–50. doi: 10.1159/000327186. [DOI] [PubMed] [Google Scholar]
- 23.Ocque R, Khalbuss WE, Monaco SE, Michelow PM, Pantanowitz L. Cytopathology of extracranial ectopic and metastatic meningiomas. Acta Cytol. 2014;58:1–8. doi: 10.1159/000355284. [DOI] [PubMed] [Google Scholar]
- 24.Harach HR, Zusman SB. Cytologic findings in the follicular variant of papillary carcinoma of the thyroid. Acta Cytol. 1992;36:142–6. [PubMed] [Google Scholar]
- 25.Shih SR, Shun CT, Su DH, Hsiao YL, Chang TC. Follicular variant of papillary thyroid carcinoma: Diagnostic limitations of fine needle aspiration cytology. Acta Cytol. 2005;49:383–6. doi: 10.1159/000326170. [DOI] [PubMed] [Google Scholar]
- 26.Yang YJ, Demirci SS. Evaluating the diagnostic significance of nuclear grooves in thyroid fine needle aspirates with a semiquantitative approach. Acta Cytol. 2003;47:563–70. doi: 10.1159/000326569. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–15. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 28.Clark DP, Faquin WC. Thyroid cytopathology. In: Dorothy LR, editor. Essentials in Cytopathology. Series 1. New York: Springer; 2005. pp. 71–9. [Google Scholar]
- 29.Kesmodel SB, Terhune KP, Canter RJ, Mandel SJ, LiVolsi VA, Baloch ZW, et al. The diagnostic dilemma of follicular variant of papillary thyroid carcinoma. Surgery. 2003;134:1005–12. doi: 10.1016/j.surg.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen GK, Lee MW, Ginsberg J, Wragg T, Bilodeau D. Fine-needle aspiration of the thyroid: An overview. Cytojournal. 2005;2:12. doi: 10.1186/1742-6413-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de los Santos ET, Keyhani-Rofagha S, Cunningham JJ, Mazzaferri EL. Cystic thyroid nodules. The dilemma of malignant lesions. Arch Intern Med. 1990;150:1422–7. doi: 10.1001/archinte.150.7.1422. [DOI] [PubMed] [Google Scholar]
- 32.Meko JB, Norton JA. Large cystic/solid thyroid nodules: A potential false-negative fine-needle aspiration. Surgery. 1995;118:996–1004. doi: 10.1016/s0039-6060(05)80105-9. [DOI] [PubMed] [Google Scholar]
- 33.Müller N, Cooperberg PL, Suen KC, Thorson SC. Needle aspiration biopsy in cystic papillary carcinoma of the thyroid. AJR Am J Roentgenol. 1985;144:251–3. doi: 10.2214/ajr.144.2.251. [DOI] [PubMed] [Google Scholar]
- 34.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–8. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Ravetto C, Colombo L, Dottorini ME. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinoma: A retrospective study in 37,895 patients. Cancer. 2000;90:357–63. [PubMed] [Google Scholar]
- 36.Mistry SG, Mani N, Murthy P. Investigating the value of fine needle aspiration cytology in thyroid cancer. J Cytol. 2011;28:185–90. doi: 10.4103/0970-9371.86345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinna EA, Ezzat N. Diagnostic accuracy of fine needle aspiration cytology in thyroid lesions. J Egypt Natl Canc Inst. 2012;24:63–70. doi: 10.1016/j.jnci.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine-needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125:484–8. doi: 10.5858/2001-125-0484-AOFNAO. [DOI] [PubMed] [Google Scholar]


