Abstract
Thiamin pyrophosphate (ThDP), the active form of thiamin (vitamin B1), is believed to be an essential cofactor for all living organisms1,2. Here, we report the unprecedented result that thiamin is dispensable for the growth of the Lyme disease pathogen Borrelia burgdorferi (Bb)3. Bb lacks genes for thiamin biosynthesis and transport as well as known ThDP-dependent enzymes4, and we were unable to detect thiamin or its derivatives in Bb cells. We showed that eliminating thiamin in vitro and in vivo using BcmE, an enzyme that degrades thiamin, has no impact on Bb growth and survival during its enzootic infectious cycle. Finally, high-performance liquid chromatography analysis reveals that the level of thiamin and its derivatives in Ixodes scapularis ticks, the enzootic vector of Bb, is extremely low. These results suggest that by dispensing with use of thiamin, Borrelia, and perhaps other tick-transmitted bacterial pathogens, are uniquely adapted to survive in tick vectors before transmitting to mammalian hosts. To our knowledge, such a mechanism has not been reported previously in any living organisms.
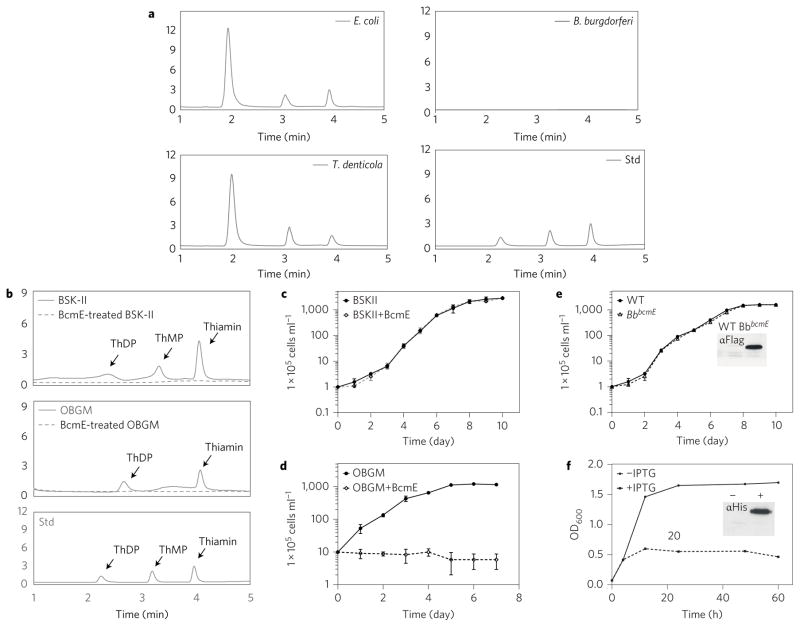
Thiamin pyrophosphate (ThDP) is a cofactor in many biological processes including central carbohydrate metabolism and amino acid biosynthesis2,5. Animals cannot synthesize thiamin and must obtain it from their diets6. Most bacteria acquire thiamin and its phosphorylated derivatives (that is, thiamin monophosphate (ThMP) and ThDP) either through de novo biosynthesis pathways or through uptake via specific transporters2,7. Some bacteria synthesize ThDP via salvage pathways8,9. The biosynthesis, uptake and use of thiamin by microorganisms have been studied extensively in two prototype bacteria, Escherichia coli and Bacillus subtilis, and most of the genes involved have been characterized1,2. Surprisingly, genomic mining and phylogenetic analyses reveal that the known thiamin biosynthesis proteins and transporters are absent in Borrelia burgdorferi (Bb) (Fig. 1), the causative agent of Lyme disease, which is the most commonly reported tick-borne illness in the USA and Europe3,4,10. In addition to the Thi genes identified in the enteric bacteria (Fig. 1), we also searched Thi homologues or orthologues (that is, ThiO, Thi5 and ThiXYZ) identified in other organisms8,11. However, none of these genes was found in the genome of Bb. Even more surprising, Bb also lacks genes encoding known ThDP-dependent enzymes (Fig. 1) such as pyruvate dehydro-genase (PDH) and transketolase (TK), two ThDP-dependent enzymes involved in carbon metabolism. Consistent with the bio-informatics data, there were no PDH and TK activities detected in Bb, as revealed by biochemical assays (Supplementary Figs 1 and 2). Based on these observations, we reasoned that Bb has either developed a new route of acquiring thiamin or evolved an alternative strategy to survivewithout thiamin. To test this hypothesis, we used high-performance liquid chromatography (HPLC) to directly measure intracellular thiamin, ThMP and ThDP in Bb and two other bacterial species, E. coli and the oral spirochaete Treponema denticola. Thiamin, ThMP and ThDP could be detected easily in E. coli and T. denticola cells. The intracellular level of thiamin and its derivatives ranged from 3.6 × 104 to 4.8 × 104 molecules per cell in E. coli and 2.4 × 104 to 2.8 × 104 molecules per cell in T. denticola (Fig. 2 and Supplementary Table 2). In contrast, no trace of thiamin, ThMP or ThDP was detected in Bb cells, even using a high density (1011 cells per ml) of cells for the HPLC analysis (Fig. 2a). This result is consistent with the absence of a thiamin biosynthesis pathway and transporter in the genome, suggesting that thiamin is dispensable for the growth of Bb.
Figure 1. Loss of genes for thiamin synthesis and transport and ThDP-dependent enzymes in Borrelia and Rickettsia.
A phylogeny (left: all visible branches are well-supported with >90% bootstrap values) of representative bacterial genomes was inferred using concatenated alignment of 24 single-copy genes (see Methods). Homologues to 12 E. coli thiamin synthesis and transport proteins (‘Thi homologues’) and two thiamin-binding domains (‘PYR’ and ‘PP’ domains; number of domain-containing open reading frames listed) are present in all genomes except for those of Borrelia and Rickettsia. Note: (1) ThiF is a member of the large HesA/MoeB/ThiF family (PF00899) and a ThiF homologue is present in B. garinii, B. valaisiana and B. afzelii genomes with relatively low (~25% versus ~40% in other spirochaetes) sequence identity. This orphan protein is probably a ThiF paralogue serving functions other than thiamin synthesis. (2) Wolbachia appears to have lost all Thi genes but contains a thiamin-utilization gene (GenBank accession no. NC_006833).
Figure 2. Bb is free of thiamin, ThMP and ThDP and able to grow in a thiamin-free medium.
a, Detection of intracellular thiamin, ThMP and ThDP in E. coli (Ec, 1 × 1010 cells ml−1), T. denticola (Td, 1 × 1010 cells ml−1) and B. burgdorferi (Bb, 1 × 1011 cells ml−1) using HPLC. Std: standard calibrators of ThDP, ThMP and thiamin. b, Detection of thiamin, ThMP and ThDP in BSK-II (top, solid grey line), BcmE-treated BSK-II (top, dashed grey line), OBGM (middle, solid grey line) and BcmE-treated OBGM (middle, dashed grey line) media using HPLC. Data shown in a,b are representative of three independent experiments. c,d, Growth curves of Bb and Td in normal and BcmE-treated growth media. e,f, Overexpression of BcmE had no impact on Bb growth. Growth curves were repeated in triplicate (n = 3) from at least three independent experiments. The results were expressed as means ± s.e.m. WT: B31A3-68; BbbcmE: B31A3-68 that overexpresses bcmE; Flag antibody (αFlag); His antibody (α His); E. coli: B21 Star (DE3) strain; −, no IPTG; +, 1 mM IPTG.
Bb is a fastidious bacterium that grows in nutrient-enriched media such as BSK-II, a modified Barbour–Stoenner–Kelly (BSK) medium3. HPLC analysis revealed that BSK-II contains thiamin (109.15 ± 1.26 nM), ThMP (73.86 ± 9.45 nM) and ThDP (59.61 ± 1.14 nM) (Fig. 2b). To confirm that thiamin is dispensable for Bb growth, we attempted to develop a thiamin-free BSK-II medium using both physical (heat and ultraviolet irradiation) and chemical (oxidation with alkaline) methods12,13. However, all these attempts failed to completely deplete thiamin and its derivatives from the medium. Sikowitz et al. recently reported that BcmE, a thia-minase I of Clostridium botulinum, degrades thiamin and its derivatives14. To remove thiamin from BSK-II medium, recombinant BcmE (rBcmE) enzyme was prepared from E. coli at 15 °C and co-incubated with the medium at 25 °C overnight. After the treatment, no trace of thiamin, ThMP or ThDP was detected by the HPLC analysis (Fig. 2b), indicating that thiamin and its derivatives were eliminated from the medium. When cultivated in this medium, Bb grew at the same rate as in normal BSK-II medium (Fig. 2c). T. denticola needs exogenous thiamin for growth15. As a control, its growth medium, oral bacterial growth medium (OBGM), was treated with rBcmE (Fig. 2b). In contrast to Bb, T. denticola failed to grow in the rBcmE-treated medium (Fig. 2d). These results indicate that the elimination of thiamin and its derivatives from the medium has no effect on Bb growth. To further substantiate that Bb does not require thiamin, the bcmE gene was cloned and transformed into Bb cells to deplete endogenous thiamin and its derivatives, if any. BcmE was overexpressed in Bb; however, it had no effect on cell growth (Fig. 2e). In contrast, the overexpression of BcmE inhibited the growth of E. coli (Fig. 2f), even though it has both a thiamin biosynthesis pathway and a transporter. This result confirms that thiamin is dispensable for Bb growth in vitro.
As a tick-borne pathogen, Bb cycles between the Ixodes tick vector and mammals in its natural environment10,16. It is possible that Bb requires thiamin for its survival in vivo, but it lacks a role for it in vitro. To rule out this possibility, we infected mice and ticks with the bcmE-expressing Bb strains and examined its effect on infectivity and survival in mice and ticks, as well as its influence on the transmission of Bb from ticks to mice. The results showed that bcmE was highly expressed in infected mouse tissues and ticks (Fig. 3b,c), but it had no impact on Bb infectivity in mice (Fig. 3a and Supplementary Table 3), survival in ticks (Fig. 3c), or transmission from the infected ticks to mice (Supplementary Table 3). These results suggest that Bb has evolved an alternative strategy to bypass the need for ThDP both in vitro and in vivo, which is unprecedented among bacteria.
Figure 3. Overexpression of bcmE in Bb had no impact on its survival and infectivity in mice and nymphs.
a, Measurement of bacterial burden (copies of flaB mRNA relative to that of mouse β-actin) in infected mouse tissues using qRT–PCR. b, Measurement of bcmE expression in mouse tissues using qRT–PCR. c, Measurement of bacterial burden ( flaB mRNA) and bcmE expression in nymphs. Data are presented as the means of relative levels of flaB transcript ± s.e.m. from three mice or three groups of ticks (each group containing four nymphs). Overexpression of BcmE had no impact on the bacterial burden in mice tissues and nymphs (P > 0.05). d,e, Detection of thiamin, ThMP and ThDP in mouse plasma (n = 4) (d) and unfed nymphs (n = 100) (e) using HPLC. Std: standard calibrators of ThMP, ThDP and thiamin. Of note, the elution times of thiamin, ThMP and ThDP in mouse plasma samples were different from those in ticks. This discrepancy is due to the use of different HPLC columns.
To explore why Bb has evolved this unusual mechanism, we measured the levels of thiamin and its derivatives in mice and unfed Ixodes scapularis nymphs. As expected, thiamin, ThMP and ThDP were easily detected in mouse plasma (Fig. 3d). Surprisingly, no ThDP was detected, and only trace amounts of thiamin (1.15 pmol per tick, n = 100 ticks) and ThMP (2.02 pmol per tick, n = 100 ticks) were detected in unfed nymphs (Fig. 3e and Supplementary Table 4), indicating that the level of thiamin and its derivatives in the ticks is extremely low. Under such a circumstance, it would be impossible for Bb, an organism that lacks a thiamin biosynthesis pathway and transporter, to acquire sufficient thiamin from the tick vectors if its growth depends on this key nutrient. In support of this proposition, the parsimony analysis suggests that the relapsing fever spirochaete B. hermsii and Rocky Mountain spotted fever Rickettsia also lack thiamin biosynthesis pathways and ThDP-dependent enzymes (Fig. 1). Collectively, these results indicate that Bb and probably other tick-borne pathogens have evolved an alternative strategy to circumvent the shortage of thiamin in ticks, allowing the pathogens to survive, complete their infectious cycles between mammalian hosts and tick vectors, and cause disease.
The discovery in this report provides insights into our understanding of microbial metabolisms and host microbe interactions. Dispensing with thiamin seems like an insurmountable evolutionary challenge for any organism considering the nearly universal occurrence of ThDP-dependent enzymes and their important roles in energy metabolism. How does Bb bypass a need for thiamin? Bb has a small genome. Consistent with this, Bb has a highly restricted metabolic capacity; for example, it lacks a tricarboxylic acid (TCA) cycle, oxidative phosphorylation or any pathways for de novo biosynthesis of carbohydrates, amino acids or lipid4,17,18. Genomic and metabolic pathway analyses suggest that Bb has evolved a unique mechanism to bypass the lack of ThDP-dependent enzymes (Fig. 4). For instance, Bb lacks PDH and pyruvate oxidase (POX), two ThDP-dependent enzymes. Thus, it cannot generate acetyl-CoA through pyruvate oxidation. Instead, it converts pyruvate to lactate using a lactate dehydrogenase (BB_0087) and produces acetyl-CoA from metabolism of acetate, mediated by acetate kinase (ACK; BB_0622) and phosphate acetyltransferase (PTA; BB_0589). This unique carbon metabolic pathway may well be the key adaptation that allows Bb to survive in the tick vector in response to thiamin deficiency. The other intriguing question is that how unfed ticks acquire thiamin. Recent studies have suggested that vitamin supplementation by gut symbionts ensures metabolic homeostasis in insects19. Therefore, it is possible that ticks acquire thiamin from their gut microbiome. Investigations are needed regarding how the gut microbiome affects arthropod nutrient homeostasis and, in turn, the competency of ticks as the vector of Bb.
Figure 4. Bb requires no ThDP-dependent enzymes for ATP and acetyl-CoA generation.

The metabolic pathways were proposed based on previous reports4,17,18 and the information listed in BioCyc (http://biocyc.org/BBUR224326/NEW-IMAGE?type=PATHWAY&object=PWY0-1312). Dashed lines and no entry signs are pathways found in other bacteria but absent in Bb. ACK, acetate kinase; ADH, alcohol dehydrogenase; LDH, lactate dehydrogenase; PDC, pyruvate decarboxylase; PDH, pyruvate dehydrogenase; PFL, pyruvate formate lyase; POX, pyruvate oxidase; PTA, phosphate acetyltransferase. Enzymes in red (PDC, PDH and POX) are ThDP-dependent. *Bb generates three ATP per molecule of glucose using a phosphofructokinase (BB_0020).
Methods
Bacterial strains and growth conditions
Infectious clone A3-68 derived from Borrelia burgdorferi (Bb) sensu stricto B31A3-68 was used in this study20. Cells were grown in BSK-II medium supplemented with 6% rabbit serum at 34 °C in the presence of 3.4% CO2. The strains were grown in the appropriate antibiotic(s) for selective pressure as needed: kanamycin (300 μg ml−1) and/or gentamicin (40 μg ml−1). T. denticola (Td) ATCC 35405 strain was grown in OBGM supplemented with 10% heat-inactivated rabbit serum at 37 °C in an anaerobic chamber AS-580 (Anaerobe Systems) as previously described15. E. coli TOP10 strain (Invitrogen) was used for DNA cloning, and B21 Star (DE3) strain was used to express C. botulinum thiaminase I, BcmE (ref. 14). The E. coli strains were grown in lysogeny broth (LB) supplemented with appropriate concentrations of antibiotics.
PDH and TK assays
E. coli DH5α (positive control) was grown to exponential phase in LB amended with 0.4% glucose. Cell number (colony-forming units, c.f.u.) was enumerated by the conventional plate count method. Bb B31A3-68 was grown in BSK-II to log phase and then directly enumerated using a Petroff–Hausser counting chamber. Bacterial cells were collected by centrifugation at 8,000g for 8 min. The resultant cell pellets were washed three times with cold PBS buffer before being lysed by sonication on ice. The PDH activities of E. coli and Bb cells were measured using a colorimetric assay kit (BioVision, catalogue no. K679-100) according to the manufacturer’s protocol. Cell lysates without pyruvate served as negative controls. Blank control contained all the reagents but no cell lysates. One enzyme unit is defined as the amount of enzyme that generates 1.0 μmol of NADH per min from pyruvate at 37 °C at pH 7.5.
A previously documented colorimetric method21,22 was used to measure TK activity in E. coli and Bb cells. The cell lysates were prepared as described above. To increase the assay sensitivity, methanal was used as an aldehyde acceptor because TK showed higher activity towards methanal. The reaction mixture (200 μl) consisted of ThDP (2.4 mM), MgCl2 (9.0 mM), lithium hydroxylated pyruvate (LiHPA, 25 mM), methanol (25 mM) and 100 μl of cell lysate in a gly-gly buffer (50 mM, pH 7.3). The reaction was incubated at room temperature for 48 h. After that, 90 μl of the reaction solution was added to an equal volume of methanol to quench the reaction, and then incubated with 25 mg of MP-Carbonate resin (Biotage AB, catalogue no. 800493) and 40 μl of water for 5 h with shaking to remove any remaining LiHPA. For colour development, 100 μl of the solution was mixed with 70 μl of tetrazolium red solution (0.1% 2,3,5-triphenyltetrazolium chloride in methanol) and 10 μl NaOH (3 M). The absorbance was read at 485 nm. The blank control contained all reagents but no cell lysates. Cell lysate controls contained everything except for methanal.
Detection of thiamin and its phosphorylated derivatives using HPLC
The concentrations of thiamin and its phosphate esters in bacterial cells, growth media, ticks and mouse plasma were measured by HPLC, as previously described with some modifications8,15. Briefly, up to 50 ml of stationary-phase Bb, Td and E. coli cultures were collected by centrifugation at 6,000g for 10 min. The cell pellets were washed twice with phosphate-buffered saline (PBS, pH 7.4), then resuspended in 1 ml PBS, lysed by sonication and then fractionated by centrifugation at 10,000g for 10 min. The cell supernatants were subjected to HPLC analysis. I. scapularis nymph ticks (Oklahoma State University) whole bodies and guts were crushed in liquid nitrogen, extracted with 100 μl 0.1 M hydrochloric acid and fractionated by centrifugation 10,000g for 10 min. The soluble fractions were collected for HPLC analysis. Mouse venous blood samples were drawn from 3- to 4-week-old mice and collected into EDTA-containing tubes. Before the HPLC analysis, the supernatants of Bb, Td, E. coli, tick extractions and mouse plasma were first treated with the same volume of 10% trichloroacetic acid (TCA) to precipitate proteins. The samples were vortex-mixed vigorously for 15 s, left standing on ice for 15 min, and then centrifuged for 10 min at 4 °C to remove precipitated proteins. The obtained supernatants were transferred into new tubes and washed twice with water-saturated methyl tert-butyl ether (750 μl) to remove TCA. Aliquots were added to a threefold volume of methanol and then centrifuged at 16,000g for 10 min. After the centrifugation, 100 μl of the supernatant was treated with a freshly prepared derivatization buffer (0.2 mM potassium ferricyanide and 5% sodium hydroxide) to oxidize thiamin and its phosphate esters to the thiochrome derivatives that can be fluorimetrically detected by HPLC. A 20 μl volume of the obtained samples was processed for HPLC analysis. The standard calibrators of ThDP, ThMP and thiamin were prepared in 0.1 M hydrochloric acid to concentrations of 0, 10, 50, 100 and 200 nmol l−1. The calibrators were treated and detected in the same way as other biological samples. The peak areas of calibrators were used to establish the standard curves corresponding to their initial concentrations.
Preparations of recombinant C. botulinum BcmE (rBcmE)
The recombinant protein was expressed and purified as previously described14. Briefly, 5 ml of the overnight seed culture was added to 1 l of sterile LB medium shaking at 37 °C. After the cultures reached an OD600 of 0.6, the incubator temperature was reduced to 15 °C. After reaching an OD600 of 0.8, isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to the cultures with a final concentration of 0.5 mM. After 18 h induction, the cells were collected and subjected to protein purification. The protein was purified using Ni-nitrilotriacetic acid (NTA) beads (Qiagen) under native conditions, as previously described14. The final purified protein was dialysed in a buffer of 10 mM Tris-HCl and 150 mM KCl, pH 8.0 at 4 °C overnight and then concentrated to 3 mg ml−1 using a Spin-X UF (Corning) with a molecular weight cutoff of 20 kDa. The purified protein was aliquoted, flash frozen in liquid nitrogen and stored at −80 °C.
Preparation of thiamin-free media using rBcmE
To remove thiamin, ThMP and ThDP from the growth media, 100 μl (500 μg) rBcmE was added to 10 ml BSK-II and OBGM and incubated at 25 °C for 2 h. In parallel, the media were treated with the same amount of heat-inactivated rBcmE, which was used as a negative control. Following incubation, 100 μl of treated media was taken and subjected to the HPLC analysis described above.
Expression of bcmE in Bb
To consecutively express bcmE in Bb, flgBp, a promoter of Bb (ref. 23) and the full-length bcmE gene were PCR amplified using primers FflgB (with a BamHI site)/RflgB and FbcmE/RbcmE (PstI site), respectively. Of note, RbcmE contains a sequence encoding a FLAG tag (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys). The resulting two DNA fragments were fused together by PCR. The fused flgBp–bcmE was cloned into pBSV2G (a shuttle vector of Bb) at its BamHI and PstI sites, yielding flgBp–bcmE/pBSV2G (ref. 24). The primers used here are described in Supplementary Table 1. The resultant plasmid and pBSV2G (a control) were transformed into the strain of A3-68 via electroporation25. Twelve gentamicin-resistant colonies were first screened by PCR for the presence of the bcmE gene, and the positive colonies were further confirmed by immunoblotting using αFlag, an antibody specific to the Flag tag. In addition, the plasmid profiles of those positive colonies were detected by PCR, as previously documented26. Only the clones containing the same plasmid profile as A3-68 were used for the in vitro and in vivo experiments described below. One of these clones was designated as BbbcmE.
Measuring growth rates of Bb and Td
To measure the impact of thiamin on Bb growth, 5 μl of the stationary-phase spirochaete culture (1 × 108 cells per ml) was inoculated into 5 ml of either normal BSK-II or the rBcmE-treated BSK-II medium (described above) and then incubated at 34 °C in the presence of 3.4% CO2. As a parallel control, the same amount of Td cells was inoculated into 5 ml of either normal OBGM or the rBcmE-treated OBGM medium and incubated at 37 °C in an anaerobic chamber as described above. The bacterial cells in the cultures were enumerated every 24 h for up to 10 days using a Petroff–Hausser counting chamber. Counts were repeated in triplicate with three independent samples and the results are expressed as mean ± standard error of the mean (s.e.m.). The same method was used to measure the growth rates of BbbcmE and its parental A3-68 strain. In addition, the cultures were sampled at given times to monitor the expression of bcmE by immunoblotting using αFlag.
Measuring the impact of BcmE on E. coli growth
A 5 ml volume of overnight E. coli culture was added to 500 ml sterile LB medium and cultivated at 37 °C with shaking. After reaching an OD600 of 0.4, the culture was separated into two flasks: one with 0.5 mM IPTG and the other without IPTG. The cells were cultivated at 16 °C with shaking. The cell densities were monitored every 12 h at OD600 and the levels of BcmE were examined by immunoblotting using anti-His antiserum (αHis). In parallel, 10 ml aliquots of the cell cultures were collected at the indicated time points, washed and subjected to HPLC to detect intracellular ThDP, ThMP and thiamin, as described above.
SDS–PAGE and immunoblotting
Equal amounts of bacterial whole-cell lysates were separated in SDS–PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories). The immunoblots were probed with either αHis or anti-Flag and developed using horseradish peroxidase-conjugated secondary antibody with an enhanced chemiluminescence (ECL) luminol assay, as previously described27.
Quantitative reverse transcription PCR (qRT–PCR)
RNA samples for the qRT–PCR analysis were prepared as previously described27. Briefly, total RNA from mouse tissues and ticks was isolated using TRIzol reagent (Invitrogen), and contaminated genomic DNA was removed using Turbo DNase (Ambion). The DNase-treated RNA samples were purified and converted to cDNA using an AffinityScript Multiple Temperature cDNA Synthesis Kit (Agilent Technologies) according to the manufacturer’s instructions. The amounts of cDNAs were measured using iQ SYBR Green Supermix (Bio-Rad). The spirochaete burdens within infected mice and ticks were expressed as the flaB transcript levels relative to the copy number of either mouse or tick β-actin transcript levels.
Mouse infection studies
All animal experimentation was conducted following the National Institutes of Health (NIH) guidelines for the housing and care of laboratory animals. The protocols for using mice and ticks were approved by the Committees on the Ethics of Animal Experiments and the Institutional Animal Care and Use Committees of State University of New York at Buffalo (approval no. ORB08046Y) and University of Maryland (permit number R-12-33). The mouse infection studies were carried out as previously described27. Briefly, randomly grouped (three mice per bacterial strain) 4- to 6-week-old female BALB/c mice (Jackson Laboratory) were given a single subcutaneous injection of 1 × 104 spirochaetes and killed three weeks post infection. Tissues from ear, skin, joint, heart and spleen were collected and placed into 1 ml BSK-II medium. The samples were incubated at 34 °C for up to 2 weeks and microscopically monitored for the presence of spirochaetes.
Experimental tick infection and tick-to-mouse infection
I. scapularis nymphal ticks (Oklahoma State University) were microinjected with the wild-type and BbbcmE strains, as previously described27. After injection, ticks were allowed to remain in the incubator for 24 h for recovery and were subsequently placed on mice (three animals per group) and allowed to engorge. Post-fed ticks were collected and analysed for the presence of bacteria using qRT–PCR as previously described27. Parallel groups of microinjected unfed ticks were kept in the incubator for 5 days and used to assess the persistence of spirochaetes using qRT–PCR. For assessment of pathogen transmission from ticks to the mammalian host, mice were killed following 2 weeks of tick engorgement. The heart, bladder, skin and joints were collected and subjected to qRT–PCR analysis to measure bacterial burdens. Mouse spleen tissues were collected and assessed for the presence of viable spirochaetes using culture analysis.
Bacterial genomes and phylogeny
Whole genomes of selected bacterial species closely related to Borrelia and Rickettsia, two tick-transmitted obligate parasites, were downloaded from NCBI GenBank. Genomes closely related to Rickettsia (details in Fig. 1) included six genera of α-Proteobacteria and four genera of intracellular parasites or symbionts of arthropods28. Genomes closely related to Borrelia included representatives of all six major spirochaete genera (details in Fig. 1). A phylogeny of these genomes, including an E. coli genome used as an outgroup, was reconstructed based on protein sequences at 24 single-copy conserved loci, including infB (encoding an initiation factor), lepA (encoding a GTP-binding protein), pheS (encoding phenylalanyl-tRNA synthetase subunit alpha), rplB/C/D/E/F/K/N/O/P (encoding 50S ribosomal proteins) and rpsB/C/E/G/H/I/J/K/L/M/Q/S (encoding 30S ribosomal proteins)29. The protein sequences were aligned using MUSCLE (version 3.8.31)30, and the resulting alignments were concatenated using a customized BioPerl-based script31 and the tree was inferred with FastTree (version 2.1.7)32.
BLAST searches
For identification of thiamin synthesis genes in spirochaete genomes, protein sequences of 12 genes from the E. coli K12 genome (GenBank accession no. NC_000913), identified as key components of the thiamine biosynthesis pathway, were extracted and used as the query sequences, including thiB, thiC, thiD, thiE, thiF, thiG, thiH, thiI, thiK, thiL, thiM and thiS (ref. 1). The search for homologues was performed using Position-Specific Initiated BLAST (Psi-Blast, version 2.2.25+) with an E-value cutoff of 10e-5 (ref. 33). For identification of thiamin-dependent enzymes in spirochaete genomes, we extracted sequences of the pyrophosphate (PP, residues 323–538) and pyrimidine (Pyr, residues 1–350) domains from the E. coli protein EcoTK (Protein Data Bank accession no. 1QGD. pdb). These two domains are the conserved features of ThDP-dependent enzymes across prokaryotes and eukaryotes34. The search for homologues was performed using Psi-Blast version 2.2.25+ with an E-value cutoff of 10e-5 (ref. 33). Bacterial phylogeny and the presence or absence of thiamin-related genes are displayed using the R package APE (ref. 35).
Statistical analysis
For the growth curves and mouse and tick infection studies, the results are expressed as means ± s.e.m. The significance of the difference between different experimental groups was evaluated using an unpaired Student t-test (P < 0.01).
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grants DE023080 and AI078958 to C.H.L., AI107955 to W.G.Q., T34GM007823 to R.E.N., AI080615 to U.P. and DK67081 to S.E.E.). The authors thank L. Kinsland and L. Di for assistance in manuscript and figure preparations and J. Leadbetter for assistance with the discussion.
Footnotes
Author contributions
K.Z., J.B., Y.D., A.S., R.E.N., M.B.L., U.P. and A.Y. conducted the experiments and data analyses. W.Q., S.E.E., and C.L. designed the experiments and prepared the manuscript. All authors contributed to the interpretation of the results and writing of the manuscript.
Additional information
Supplementary information is available for this paper. Reprints and permissions information is available at www.nature.com/reprints.
Competing interests
The authors declare no competing financial interests.
References
- 1.Jurgenson CT, Begley TP, Ealick SE. The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem. 2009;78:569–603. doi: 10.1146/annurev.biochem.78.072407.102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begley TP, et al. Thiamin biosynthesis in prokaryotes. Arch Microbiol. 1999;171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorfer W, et al. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 4.Fraser CM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 5.Downs DM. Understanding microbial metabolism. Annu Rev Microbiol. 2006;60:533–559. doi: 10.1146/annurev.micro.60.080805.142308. [DOI] [PubMed] [Google Scholar]
- 6.Eijkman C. Anti-neuritis vitamin and beriberi. Nobel prize paper. 1929. Ned Tijdschr Geneeskd. 1990;134:1654–1657. [PubMed] [Google Scholar]
- 7.Webb E, Claas K, Downs D. thiBPQ encodes an ABC transporter required for transport of thiamine and thiamine pyrophosphate in Salmonella typhimurium. J Biol Chem. 1998;273:8946–8950. doi: 10.1074/jbc.273.15.8946. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins AH, Schyns G, Potot S, Sun G, Begley TP. A new thiamin salvage pathway. Nat Chem Biol. 2007;3:492–497. doi: 10.1038/nchembio.2007.13. [DOI] [PubMed] [Google Scholar]
- 9.Bazurto JV, Farley KR, Downs DM. An unexpected route to an essential cofactor: Escherichia coli relies on threonine for thiamine biosynthesis. MBio. 2016;7:e01840–15. doi: 10.1128/mBio.01840-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bale S, Rajashankar KR, Perry K, Begley TP, Ealick SE. HMP binding protein thiY and HMP-P synthase THI5 are structural homologues. Biochemistry. 2010;49:8929–8936. doi: 10.1021/bi101209t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Button DK. Selective thiamine removal from culture media by ultraviolet irradiation. Appl Microbiol. 1968;16:530–531. doi: 10.1128/am.16.3.530-531.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwivedi BK, Arnold RG. Chemistry of thiamine degradation in food products and model systems. Review. J Agric Food Chem. 1973;21:54–60. doi: 10.1021/jf60185a004. [DOI] [PubMed] [Google Scholar]
- 14.Sikowitz MD, Shome B, Zhang Y, Begley TP, Ealick SE. Structure of a Clostridium botulinum C143S thiaminase I/thiamin complex reveals active site architecture. Biochemistry. 2013;52:7830–7839. doi: 10.1021/bi400841g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bian J, Shen H, Tu Y, Yu A, Li C. The riboswitch regulates a thiamine pyrophosphate ABC transporter of the oral spirochete Treponema denticola. J Bacteriol. 2011;193:3912–3922. doi: 10.1128/JB.00386-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caimano MJ, Drecktrah D, Kung F, Samuels DS. Interaction of the Lyme disease spirochete with its tick vector. Cell Microbiol. 2016;18:919–927. doi: 10.1111/cmi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corona A, Schwartz I. Borrelia burgdorferi: carbon metabolism and the tick–mammal enzootic cycle. Microbiol Spectr. 2015 doi: 10.1128/microbiolspec.MBP-0011-2014. http://dx.doi.org/10.1128/microbiolspec.MBP-0011-2014. [DOI] [PMC free article] [PubMed]
- 18.Gherardini FC. In: Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Samuels DS, Radolf JD, editors. Caister Academic; 2010. pp. 103–138. [Google Scholar]
- 19.Snyder AK, Deberry JW, Runyen-Janecky L, Rio RV. Nutrient provisioning facilitates homeostasis between tsetse fly (Diptera: Glossinidae) symbionts. Proc Biol Sci. 2010;277:2389–2397. doi: 10.1098/rspb.2010.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rego RO, Bestor A, Rosa PA. Defining the plasmid-borne restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J Bacteriol. 2011;193:1161–1171. doi: 10.1128/JB.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MEB, Kaulmann U, Ward JM, Hailes HC. A colorimetric assay for screening transketolase activity. Bioorg Med Chem. 2006;14:7062–7065. doi: 10.1016/j.bmc.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Cázares A, et al. Non-α-hydroxylated aldehydes with evolved transketolase enzymes. Org Biomol Chem. 2010;8:1301–1309. doi: 10.1039/b924144b. [DOI] [PubMed] [Google Scholar]
- 23.Ge Y, Charon NW. Identification of a large motility operon in Borrelia burgdorferi by semi-random PCR chromosome walking. Gene. 1997;189:195–201. doi: 10.1016/s0378-1119(96)00848-7. [DOI] [PubMed] [Google Scholar]
- 24.Elias AF, et al. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J Mol Microbiol Biotechnol. 2003;6:29–40. doi: 10.1159/000073406. [DOI] [PubMed] [Google Scholar]
- 25.Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol. 1995;47:253–259. doi: 10.1385/0-89603-310-4:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias AF, et al. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sze CW, Zhang K, Kariu T, Pal U, Li C. Borrelia burgdorferi needs chemotaxis to establish infection in mammals and to accomplish its enzootic cycle. Infect Immun. 2012;80:2485–2492. doi: 10.1128/IAI.00145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunning Hotopp JC, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu M, Eisen JA. A simple, fast, and accurate method of phylogenomic inference. Genome Biol. 2008;9:R151. doi: 10.1186/gb-2008-9-10-r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stajich JE, et al. The Bioperl toolkit: Perl modules for the life sciences. Genome Res. 2002;12:1611–1618. doi: 10.1101/gr.361602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30:1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costelloe SJ, Ward JM, Dalby PA. Evolutionary analysis of the TPP-dependent enzyme family. J Mol Evol. 2008;66:36–49. doi: 10.1007/s00239-007-9056-2. [DOI] [PubMed] [Google Scholar]
- 35.Popescu AA, Huber KT, Paradis E. ape 3.0: New tools for distance-based phylogenetics and evolutionary analysis in R. Bioinformatics. 2012;28:1536–1537. doi: 10.1093/bioinformatics/bts184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.