Abstract
A certain number of studies have showed that p53 gene transfer has an anti-tumor activity in vitro and in vivo. This study was to evaluate the efficacy and safety of thoracic perfusion of recombinant human adenovirus p53 (rAd-p53, Gendicine) for controlling malignant pleural effusion (MPE). We searched for the relevant studies from the database of MEDLINE, Web of Science, EMBASE, Cochrance Library and CNKI to collect the trials concerning the efficacy and safety of rAd-p53 to treat MPE. Fourteen randomised controlled trials (RCTs) with 879 patients were involved in this analysis. The rAd-p53 combined with chemotherapeutic agents significantly improved the overall response rate (ORR) (P < 0.001; odds ratio = 3.73) and disease control rate (DCR) (P < 0.001; odds ratio = 2.32) of patients with MPE as well as the quality of life (QOL) of patients (P < 0.001; odds ratio = 4.27), compared with that of chemotherapeutic agents alone. In addition, the participation of rAd-p53 did not have an obvious impact on the most of incidence of adverse reactions (AEs) (P < 0.05) except the fever (P < 0.001). However, the fever was self-limited and could be tolerated well. The application of rAd-p53 through thoracic perfusion for treating MPE had a better efficacy and safety, which could be a potential choice for controlling MPE.
Malignant pleural effusion (MPE) is one of the common complications of lung cancer, which mainly caused by the hyperpermeability of microvascular tissue or invasion of cancer cells into lymphatic vessels1. Research show that the incidence of malignant effusion in patients with lung cancer is about 7–23%2,3 and occurs in 15% of cancer-related deaths1, which usually leads to debilitating dyspnoea, worsening quality of life and a poor survival. However, its treatment is quite difficult2. The present treatments of MPE included the drainage of pleural effusion, intrapleural chemotherapy and systemic chemotherapy. Unfortunately, not all patients with MPE can benefit from quasi chemotherapy and treatment1,2,3.
The mutation of the p53 gene is one of the most common gene mutations in malignant tumors. It locates on the short arm of human chromosome 17 and encodes a nuclear phosphoprotein4. It is reported that the p53 gene is mutated in 50% to 70% of patients with lung cancer5, which plays a crucial function in cell cycle regulation, genomic stability, stress induced reaction and DNA repairing4. The point mutations on exons 3–9 and 5–8 are mainly common mutations of p53 gene in lung cancer; especially the point mutation on exons 5–8, has been observed in 40–50% of non-small cell lung cancer (NSCLC)6. So far, the gene mutation of p53 and overexpression of p53 protein have been investigated to be closely related to the differentiation level, malignant biological behavior and poor prognosis of lung cancer6,7,8. A certain number of studies have been showed that p53 gene transfer has an anti-tumor activity in vitro and in vivo5,9,10,11,12,13,14. Moreover, a series of studies on p53-based gene therapy have been performed from fundamental research (molecule and cell) to clinical applications (human)1,9,11,12; for example, a p53-based gene therapy has been approved as part of biological cancer therapy in China15.
Recombinant human adenovirus p53 (rAd-p53; Gendicine) applies type 5 adenovirus to carry the exogenetic p53 into malignant tumor cells to express wild type-p53 protein that inhibits the cell division and induces the apoptosis of tumor cells12. The rAd-p53 injection (Gendicine) was first developed by SiBiono Gene Technology Co., Ltd, Shenzhen, China, and was approved by the China State Food and Drug Administration (SFDA) for a gene therapy administered intratumorally in 200315. So far, some clinical randomized controlled trials (RCTs) evaluated the efficacy and safety of rAd-p53 by thoracic perfusion in controlling MPE. However, whether or not rAd-p53 has the definite curative effects in treating MPE is still unclear. In addition, its safety still needs to be further evaluated. The aim of this study is to do a systematic evaluation in order to assess the clinical benefits and toxicities of rAd-p53 in treating MPE.
Results
Selection and identification of studies
Originally, we searched 86 relevant reports that talked about the therapy of rAd-p53 in malignant tumors from a series of network databases mentioned above. Through the careful screening, we found 35 studies pertaining to the efficacy and safety of rAd-p53 by thoracic perfusion in treating MPE. However, we had to abandon 13 studies because eight investigations belonged to preclinical studies and five were not the first hand materials (reviews and meeting records). After that, 22 studies were considered as the clinical randomized controlled trials (RCTs) on thoracic perfusion of rAd-p53 for treating MPE. Of them, we abolished eight trials once again because of the following reasons: too small cases (n = 3), duplicate of another study (n = 1) and low quality of statistical design (n = 4). Finally, 1416,17,18,19,20,21,22,23,24,25,26,27,28,29 studies published between 2008 and 2015 completely fulfilled the inclusion criteria (Fig. 1A).
Figure 1. Selection and assessment of literature.
(A) Studies were retrieved from the electronic bibliographic databases such as PubMed, Embase, Cochrane Library and SCI database. (B,C) According to the criteria made by the Cochrane Handbook (Version 5.0.1), these results suggested that no heterogeneity existed in eligible RCTs. Overall, these studies had moderate to higher quality.
Description of included studies
Table 1 listed the detailed study characteristics of 14 studies, which involved in a total of 879 patients, and the patients cases of researches oscillated between 3524 and 18018 patients. The age of patients ranged from 32 to 82 years old, and the vast majority of MPE were caused by lung cancer, and fewer MPE were from patients with breast cancer21,28,29, digestive cancer21, osteosarcoma21 and mesothelioma of pleura28. All of studies provided the effective endpoint event of observation, which included ORR (overall response rate), DCR (disease control rate), SI (symptom improvement) and AEs (adverse effects). Ten studies designed the thoracic perfusion project of rAd-p53 combined with paclitaxel versus paclitaxel alone for controlling MPE19,20,21,22,23,24,25,26,27,28,29, one compared the rAd-p53 with Group A streptococcus17, and other two assessed the differences between with and without rAd-p5316,19. The patients of five studies accepted the intravenous chemotherapy besides the therapy of thoracic perfusion at the same time, which included vinorelbine24, paclitaxel27, gemcitabine23, nedaplatin16, paclitaxel20, these information was shown in Table 1 in detail.
Table 1. Patient characteristics of the clinical trials reviewed.
| Study | N | M/F | Age (Years) | Sources of tumor (N) | Histology of Lung cancer LAC/LSCC/SCLC/Other | Quality of Life | End point |
|---|---|---|---|---|---|---|---|
| Dong M19 | 48 | 29/19 | 35–82 | MPE (48) | NA | KPS ≥ 60 | RR, DCR, SI |
| Weizhu Z24 | 35 | 19/16 | 37–75 | Lung cancer (35) | 19/14/0/2 | KPS ≥ 60 | RR, DCR, SI, AEs |
| Yue W28 | 95 | 50/45 | 34–75 | Lung cancer (46) Breast cancer (21) Mesothelioma of pleura (9) Others (19) | NA | KPS ≥ 60 | RR, DCR, SI, AEs |
| Nanchang Y22 | 46 | 24/22 | 40–70 | NSCLC (46) | 20/18/0/8 | KPS ≥ 60 | RR, DCR, SI, AEs |
| Yimei Q27 | 43 | 25/18 | 38–75 | Lung cancer (43) | 24/15/0/4 | KPS ≥ 60 | RR, DCR, SI, AEs |
| Yanliang Z26 | 40 | 23/17 | 34–75 | Lung cancer (40) | 31/9 | ZPS ≤ 2 | RR, DCR, SI, AEs |
| Rong Z23 | 39 | 21/17 | 37–45 | Lung cancer (39) | 17/20/0/2 | KPS ≥ 30 | RR, DCR, SI, AEs |
| Ling T21 | 40 | 24/16 | 39–73 | Lung cancer (29) Breast cancer (5) Digestive cancer (4) Osteosarcoma (1) | NA | KPS ≥ 60 | RR, DCR, SI, AEs |
| Chengzhi Z17 | 96 | 67/29 | 29–77 | Lung cancer (96) | 71/12/13 | ZPS ≤ 2 | RR, DCR, SI, AEs |
| Zongyuan Z29 | 80 | 44/36 | 32–65 | Lung cancer (48) Breast cancer (32) | NA | NA | RR, DCR, AEs |
| Dong L18 | 180 | 119/61 | 61.4 ± 9.1 | Lung cancer (180) | 108/45/27 | NA | RR, DCR, SI, AEs |
| Xiaohong W25 | 60 | 43/17 | 38–73 | NSCLC (60) | NA | KPS ≥ 70 | RR, DCR, SI, AEs |
| Bo T16 | 36 | NA | NA | Lung cancer (36) | NA | NA | RR, DCR, SI, AEs |
| Lin Z20 | 41 | 24/17 | 69.2 ± 10.2 | NSCLC (41) | 8/20/0/13 | KPS ≥ 60 | RR, DCR, SI, AEs |
N = number of patients; M/F = male/female; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; SCLC, small cell lung cancer; MPE = malignant pleural effusion; NA = not available; KPS, Karnofsky performance scale index; ZPS, performance scale index made by Eastern Cooperative Oncology Group; RR = response rate; DCR = disease control rate; SI = symptom improvement; AEs = adverse effects.
Study quality of included RCTs and heterogeneity analysis
We assessed the quality of included RCTs according to the criteria shaped by the Cochrane Handbook, which was specialized in evaluating the systematic reviews of Interventions (Version 5.0.1). After assessing the studies, we found ten17,18,19,20,22,25,26,27,28,29 of the 14 RCTs (71%) displayed low risk of bias, and remaining four16,21,23,24 RCTs showed unclear risk of bias (28.6%) (Table 2 and Fig. 1B,C). The heterogeneity analysis showed that the heterogeneity chi-squared was 3.56 (d.f. = 13) and p = 0.995; also I-squared (variation in OR attributable to heterogeneity) was 0.0%; these results suggested that no heterogeneity existed in included RCTs. Overall, these studies had moderate to higher quality. Therefore, we applied the fixed effects model of meta-analysis to calculate the overall effects.
Table 2. Raw data and methodological quality of included trials.
| Studies | Region | Sequence generation | Allocation concealment | Blind | Outcome data | Selective outcome reporting | Other sources of bias | ITT | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Dong M19 | Single center | Random number table (SPSS) | Insufficient | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Weizhu Z24 | Single center | NA | Insufficient | Unclear | Yes | No | Unclear | Unclear | Unclear risk of bias |
| Yue W 201022 | Single center | Random number table (SAS) | Clear | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Nanchang Y22 | Single center | Random number table (SAS) | Clear | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Yanliang Z26 | Single center | Random number table (SPSS) | Insufficient | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Yimei Q27 | Single center | Random number table (SPSS) | Insufficient | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Rong Z23 | Single center | NA | Insufficient | Unclear | Yes | No | Unclear | Unclear | Unclear risk of bias |
| Ling T21 | Single center | NA | Insufficient | Unclear | Yes | No | Unclear | Unclear | Unclear risk of bias |
| Chengzhi Z17 | Single center | Random number table (SPSS) | Insufficient | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Zongyuan Z29 | Single center | Random number table (SAS) | Clear | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Dong L18 | Single center | Random number table (SPSS) | Insufficient | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Xiaohong W25 | Single center | Random number table (SPSS) | Clear | Clear | Yes | No | Unclear | Yes | Low risk of bias |
| Bo T16 | Single center | NA | Insufficient | Unclear | Yes | Unclear | Unclear | Unclear | Unclear risk of bias |
| Lin Z20 | Single center | Random number table (SPSS) | Insufficient | Clear | Yes | No | Unclear | Yes | Low risk of bias |
ITT, intention-to-treat.
Thoracic perfusion of rAd-p53 combined with other agents had a higher ORR compared with other agents alone
As shown in Table 3, fourteen RCTs16,17,18,19,20,21,22,23,24,25,26,27,28,29 recruited in this meta-analysis offered the data on comparison of ORR between rAd-p53 combined other agents and other agents alone by thoracic perfusion for treating MPE. The results from the fixed effects model of meta-analysis displayed that odds ratio was 3.73 (95% CI 2.70 to 5.16; Z value = 7.96, P < 0.001), which indicated that the ORR of rAd-p53 combined other agents was remarkably higher than that of other agents alone (Fig. 2A).
Table 3. Efficacy of recombinant human Ad- p53 injection in treating malignant pleural effusion.
| Study | |
Intravenous chemotherapy simultaneously | |
Efficacy of therapy |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design (N) |
Pleural perfusion (N) |
Group 1 |
Group 2 |
Improvement of SI (N, %) |
|||||||||||
| Group 1 | Group 2 | Group 1 | Group 2 | CR | PR | SD | PD | CR | PR | SD | PD | Group 1 | Group 2 | ||
| Dong M19 | 27 | 21 | No | rAd-p53 + P | P | 6 | 11 | 4 | 6 | 4 | 5 | 8 | 4 | NA | NA |
| Weizhu Z24 | 17 | 18 | Vinorelbine | rAd-p53 + P | P | 8 | 6 | 2 | 1 | 6 | 3 | 5 | 4 | 11 | 6 |
| Yue W 201022 | 31 | 32 | No | rAd-p53 + P | P | 2 | 23 | 4 | 2 | 0 | 15 | 13 | 4 | 27 | 14 |
| Nanchang Y22 | 15 | 16 | No | rAd-p53 + P | P | 3 | 10 | 2 | 1 | 1 | 5 | 5 | 4 | 15 | 6 |
| Yanliang Z26 | 20 | 20 | No | rAd-p53 + P | P | 4 | 12 | 4 | 2 | 7 | 11 | 16 | 11 | ||
| Yimei Q27 | 22 | 21 | Paclitaxel | rAd-p53 + P | P | 11 | 8 | 2 | 1 | 9 | 3 | 7 | 2 | 19 | 11 |
| Rong Z23 | 21 | 17 | Gemcitabine | rAd-p53 + P | P | 6 | 11 | 2 | 2 | 2 | 6 | 5 | 4 | 14 | 5 |
| Ling T21 | 20 | 20 | No | rAd-p53 + P | P | 2 | 13 | 4 | 1 | 1 | 9 | 8 | 2 | NA | NA |
| Chengzhi Z17 | 46 | 50 | GP | rAd-p53 | Group A streptococcus | 15. | 18 | 3 | 10 | 18 | 21 | 2 | 9 | 36 | 29 |
| Zongyuan Z29 | 40 | 40 | No | rAd-p53 + P | P | 5 | 28 | 5 | 2 | 2 | 18 | 16 | 4 | NA | NA |
| Dong L18 | 90 | 90 | TP | rAd-p53 | None | 34 | 46 | 10 | 0 | 10 | 44 | 27 | 9 | NA | NA |
| Xiaohong W25 | 30 | 30 | DP | rAd-p53 + P | P | 3 | 18 | 8 | 1 | 2 | 11 | 14 | 3 | 24 | 13 |
| Bo T16 | 20 | 16 | Nedaplatin | rAd-p53 | None | 10 | 7 | 2 | 1 | 4 | 4 | 5 | 3 | 16 | 12 |
| Lin Z20 | 20 | 21 | Paclitaxel | rAd-p53 + P | P | 6 | 8 | 5 | 1 | 2 | 6 | 7 | 6 | 13 | 7 |
N = cases; rAd-p53 = recombinant human Ad- p53 injection; Group 1 = recombinant human Ad- p53 injection combined with other therapy; Group 2 = other therapy alone; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; P = cisplatin; TP = Docetaxel + cisplatin; GP = Gemcitabine + cisplatin; NA = not available.
Figure 2. Efficacy comparison of rAd-p53 combined with another agent versus another agent alone by thoracic perfusion for controlling MPE.
(A) Thoracic perfusion of rAd-p53 combined with other agents had a higher ORR compared with other agents alone. (B) Thoracic perfusion of rAd-p53 combined with other agents had a higher DCR compared with other agents alone. (C) Thoracic perfusion of rAd-p53 combined with other agents improved the QOL of patients with MPE compared with other agents alone. ORR, overall response rate; DCR, disease control rate; OR, odds ratio; QOL, quality of life.
Thoracic perfusion of rAd-p53 combined with other agents had a higher DCR compared with other agents alone
As shown in Table 3, the data on comparison of overall DCR between rAd-p53 combined other agents and other agents alone by thoracic perfusion for treating MPE was provided by the all included studies16,17,18,19,20,21,22,23,24,25,26,27,28,29. By the fixed effects model of meta-analysis, we calculated that the odds ratio was 2.32 (95% CI 1.49 to 5.16; Z value = 3.73, P < 0.001), suggesting that therapy of thoracic perfusion of rAd-p53 combined other agents had a far more benefit of DCR than other agents alone (Fig. 2B).
Thoracic perfusion of rAd-p53 combined with other agents improved the quality of life of patients with MPE compared with other agents alone
Ten of 14 trials16,17,20,22,23,24,25,26,27,28 compared the quality of life (QOL) between rAd-p53 combined other agents and other agents alone by thoracic perfusion for treating MPE (Table 3). The results from the fixed effects model of meta-analysis exhibited that odds ratio of two different projects was 4.27 (95% CI 2.85 to 6.40; Z = 7.01, P < 0.001), which demonstrated that the combination therapy of rAd-p53 and other agents remarkably improved the QOL of patients with MPE (Fig. 2C).
Adverse effects comparison of rAd-p53 combined with other agents versus other agents alone
Table 4 listed all common adverse effects of rAd-p53 combined with other agents versus other agents alone, including fever, chest pain, myelosuppression and digestive reaction. Fourteen trials compared the incidence rate of fever between rAd-p53 combined with other agents and other agents alone16,17,18,19,20,21,22,23,24,25,26,27,28,29. The rAd-p53 combination therapy displayed a higher incidence rate of fever than the project of other agents alone (OR = 4.92, 95% CI 3.44 to 7.03, P < 0.001) (Fig. 3A). Nine trials17,18,20,21,22,23,24,26 compared the incidence rate of chest pain and showed that rAd-p53 combined with other agents and other agents alone had a similar incidence of chest pain (OR = 0.90, 95% CI 0. 60 to 1.35, P = 0.598) (Fig. 3B). Results from 14 trials17,18,19,20,21,22,23,24,25,26,27,28,29 that were included in our analysis revealed that no difference in incidence rate of myelosuppression (OR = 0.80, 95% CI 0. 57 to 1.13, P = 0.208) (Fig. 3C) was testified between rAd-p53 combined with other agents and other agents alone. Moreover, the digestive reaction of rAd-p53 combination therapy had the same occurrence probability compared with the other agents alone (OR = 0.79, 95% CI 0. 56 to 1.12, P = 0.183) (Fig. 3D).
Table 4. Comparison of adverse events between rAd-p53 with other therapy and other therapy alone.
| Study | Fever (N) |
Chest pain (N) |
Myelosuppression (N) |
Digestive reaction (N) |
||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | |
| Weizhu Z24 | 10/17 | 2/18 | 2/17 | 3/18 | 3/17 | 4/18 | 5/17 | 7/18 |
| Yue W 201022 | 11/31 | 6/32 | ‒ | ‒ | 12/31 | 11/32 | 10/31 | 8/32 |
| Nanchang Y22 | 6/16 | 3/15 | 4/16 | 3/15 | 7/16 | 6/15 | 6/16 | 4/15 |
| Yanliang Z26 | 15/20 | 3/20 | 5/20 | 3/20 | 3/20 | 3/20 | 9/20 | 8/20 |
| Yimei Q27 | 14/22 | 2/21 | 8/22 | 9/21 | 4/22 | 6/21 | ||
| Rong Z23 | 11/21 | 3/17 | 3/21 | 3/17 | 4/21 | 3/17 | 4/21 | 6/17 |
| Ling T21 | 6/20 | 2/20 | 3/20 | 2/20 | 2/20 | 1/20 | 3/20 | 4/20 |
| Chengzhi Z17 | 5/46 | 8/50 | 2/46 | 10/50 | 7/46 | 8/50 | 4/46 | 5/50 |
| Zongyuan Z29 | 4/20 | 5/20 | 3/20 | 4/20 | 3/20 | 2/20 | 4/20 | 3/20 |
| Dong L18 | 47/90 | 10/90 | 42/90 | 43/90 | 25/90 | 38/90 | 16/90 | 28/90 |
| Xiaohong W25 | 24/30 | 3/30 | ‒ | ‒ | 15/30 | 16/30 | 9/30 | 10/30 |
| Lin Z20 | 10/20 | 3/21 | 4/20 | 2/21 | 4/20 | 3/21 | 6/20 | 5/21 |
| P < 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |||||
Group 1 = recombinant human Ad- p53 injection combined with other therapy; Group 2 = other therapy alone.
Figure 3. Safety evaluation of rAd-p53 combined with another agent versus another agent alone by thoracic perfusion for controlling MPE.
(A) The rAd-p53 combination therapy displayed a higher incidence rate of fever than the project of other agents alone. (B) The rAd-p53 combined with other agents and other agents alone had a similar incidence of chest pain. (C) No difference in incidence rate of myelosuppression was testified between rAd-p53 combined with other agents and other agents alone. (D) The digestive reaction of rAd-p53 combination therapy had the same occurrence probability compared with the other agents alone. ORR, overall response rate; DCR, disease control rate; OR, odds ratio.
Sensitivity and publication bias analysis of included trials
Sensitivity analysis of 14 trials clearly revealed that omitting any trial did not shake the pooled effect of meta-analysis (Fig. 4A). Begg’s test showed that Std. Dev. of Score was 18.27 and value of P was 0.07, and the funnel plot seems to be nearly symmetrical (Fig. 4B). Moreover, the result of the Egger’s test was t = 0.33 (P = 0.746) (Fig. 4C). Through comprehensive analysis, we knew that each of 14 included trials did not have a potential impact on the pooled effect of present meta-analysis.
Figure 4. Sensitivity and publication bias analysis.
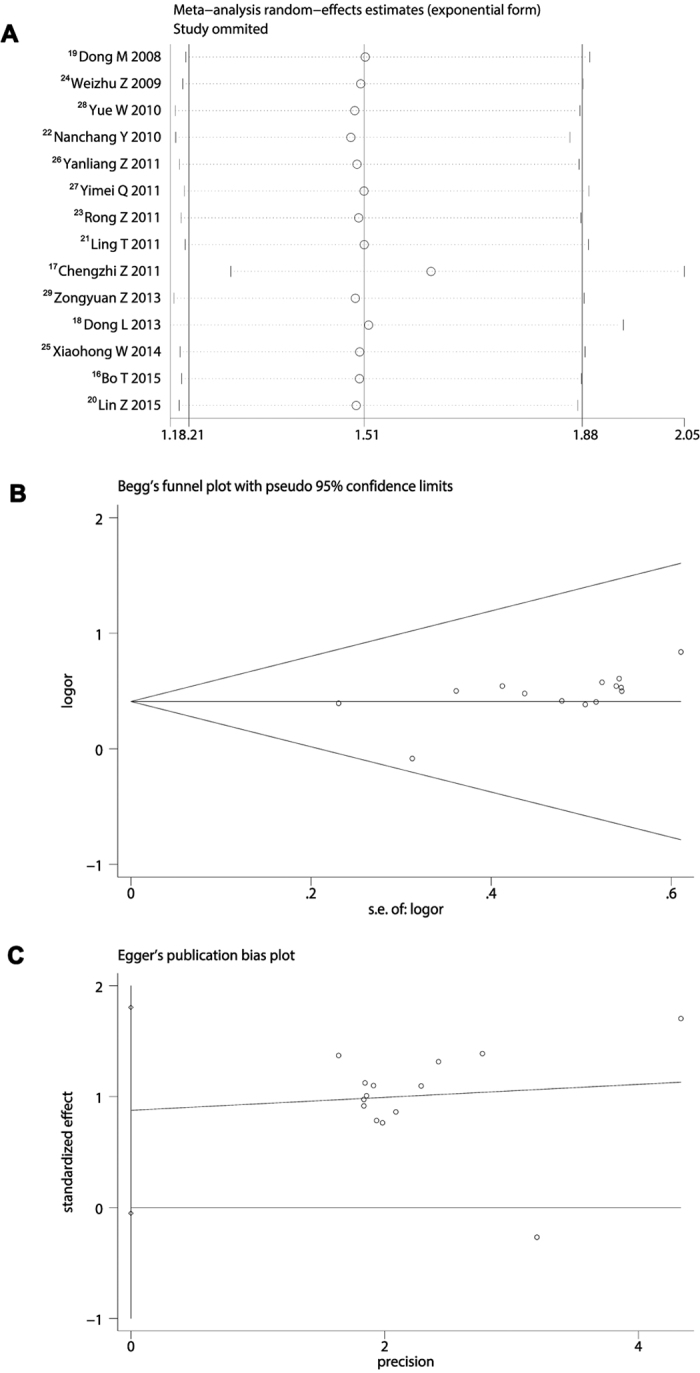
(A) Omitting any trial did not shake the pooled effect of meta-analysis. (B) Begg’s test showed that P was 0.07, and the funnel plot seems to be nearly symmetrical. (C) Egger’s test showed that P value was 0.746, suggesting included trials did not have a potential impact on the pooled effect of present meta-analysis.
Discussion
In China, lung cancer has become a highly fatal disease with limited therapeutic options due to aggravation of environmental disruption and the absence of food safety. Researches show various factors affect the prognosis and survival of lung cancer patients. Malignant pleural effusion (MPE), a common complication of lung cancer, has been identified to be a crucial negative factor to remarkably bring down the quality of life (QOL) of patients and reduce the survival30. At present, although a number of therapeutic methods are developed to control the MPE, most effective strategies just include local treatment of the chest and platinum based chemotherapy31. Today, with the rapid development of genomics and proteomics, various novel targeted drugs based gene mutations have been discovered and developed to cure lung cancer, they have therapeutic selectivity to kill tumor cells but have not significant toxicity to normal cells, and some of them have been used for therapy of MPE32,33. However, a variety of agents achieve clinical effects to treat MPE, no ideal agent has been identified as having a considerable efficacy. Therefore, the discovery or development of more effective agents and treat strategies has become a major focus in studies investigating possible malignant pleural effusion treatment strategies31.
Studying on the relationship between P53 gene and malignant tumors has been disclosed that P53 gene is a tumor suppressing gene, which exerts multiple anti-cancer activity including impeding the cell cycle, promoting apoptosis of tumor cells, suppressing the angiogenesis of tumors34. Recombinant adenoviral human p53 gene (rAd-p53) has been shown to be effective for many types of solid malignant tumors35. As an important part of the combined therapy for the cancer patients, the p53 gene therapy mediated adenovirus has been blossomed to be a promising treatment strategy36. Now, a number of clinical studies about Adp53-based therapies have been reported in many countries and districts. Gendicine is a recombinant human serotype 5 adenovirus in which the E1 region is replaced by a human wild-type p53 expression cassette. Then the adenovirus will carry the exogenetic p53 into malignant tumor cells to express wild type-p53 protein and exert the action in inhibiting the cell division and inducing the apoptosis of tumor cells. Up to now, it has been used in a number of clinical trials for treating various malignant tumors37. We searched the relevant studies on the clinical benefits and toxicities of rAd-p53 in treating MPE to disclose the clinical benefits and safety. A total of 14 studies with 879 patients were involved in our analysis. We assessed the quality of included RCTs using the criteria shaped by the Cochrane Handbook and found these studies had a very good clinical homogeneity. Therefore, we applied the fixed effects model of meta-analysis to calculate the overall effects. We made a comparison of overall response rate (ORR) between rAd-p53 combined other agents and other agents alone by thoracic perfusion for treating MPE. The results displayed an odds ratio of 3.73 (95% CI 2.70 to 5.16; P < 0.001), which responded an absolute promotion of 26.4% in ORR, indicating that the rAd-p53 combination had a better benefit on ORR for treating MPE. Moreover, the comparison of overall DCR showed that the odds ratio was 2.32 (95% CI 1.49 to 5.16; P < 0.001), suggesting that therapy of thoracic perfusion of rAd-p53 combined other agents had a far more benefit of DCR than that of other agents alone, which displayed an absolute increase of 7.85% in DCR. Since it is approved in 2003 by the State FDA of China, many RCTs of rAd-p53 have been performed for treating malignant tumors, including lung cancer, liver cancer, malignant glioma and ovarian carcinoma. The results of clinical trials all showed the overall response rates and survival rates were better in the rAd-p53 treatment groups than the control groups36.
Health-related quality of life (QOL) has been proposed as an important end-point in studies of outcomes in oncology. Studies of QOL have several benefits when they show evidence that the measurements were conducted and reported appropriately38. Indeed, the aim of assessing the impact of disease and treatment on QOL is increasingly stressed as crucial for evaluating the overall treatment effectiveness in cancer clinical trials. Moreover, cancer patients require information not only relate to survival estimates, but also regarding QOL issues39. In our analysis, we compared the QOL between rAd-p53 combined other agents and other agents alone by thoracic perfusion for treating MPE, and found that odds ratio of two different projects was 4.27 (95% CI 2.85 to 6.40; Z = 11.02, P < 0.001), suggesting that the combination therapy of rAd-p53 remarkably improved the QOL of patients with MPE. In conclusion, for those patients with advanced MPE, rAd-p53 gene therapy combined with drugs (cisplatin mainly) can enhance their immune function against tumors and it offers a potential better promise of a new and effective therapy. The pleural surface is often invaded by tumor cells, by infusion of rAd-p53 through thoracocentesis and closed drainage into the pleural space, making the exogenetic p53 genes to suppress the growth and proliferation of tumor cells. It seems that adenovirus is a good exogenous p53 gene carrier31. The suppression of tumor cells by infusion of rAd-p53 may also have controlled the local and systemic tumor lesions, further improving the QOL of patients with MPE.
In our analysis, more adverse effects (AEs) showed by rAd-p53 combination therapy were fever (46.2%), chest pain (25.6%), myelosuppression (26.3%) and digestive reaction (22.7%). The rAd-p53 combination therapy displayed a higher incidence rate of fever than the project of other agents alone (OR = 4.92, P < 0.001). The application of adenoviral vector has been reported to beget a clear immune response in those who accepted gene therapy, and self-limited fever is always manifested in the process of treatment. Although the fever caused by rAd-p53 is viewed as a obvious adverse effect in clinical treatment, sometimes it also indicates the possibility of efficacy and benefits of rAd-p53 therapy, suggesting that rAd-p53 can effectively mobilize the immune systems of human body to kill the tumor cells37. Fortunately, the incidence rate of chest pain, myelosuppression and digestive reaction of rAd-p53 combination therapy had the same occurrence compared with other agents alone (P > 0.05). Get together, though some such common AEs have been reported in the treatment of rAd-p53, no severe AEs have been showed in patients with MPE when accepted the treating with rAd-p53 (Gendicine). Especially, so far no rAd-p53 related fatalities have been clearly reported. In fact, more data have exhibited that the adenovirus-mediated p53 gene therapy could be well tolerated and had a better safety for clinical application.
However, there are some flaws existed in included trials. First, none of included studies focused on the P53 mutation in patients with MPE, so we did not assess whether P53 gene mutation has an influence on the treatment response of Gendicine. But previous studies have shown that Ad-p53 can simultaneously inhibit the growth of human lung adenocarcinoma cell line containing mutant p53 gene and wild-type p53 gene as well. Moreover, Ad-p53 can promote the apoptosis of cancer cells and its anti-tumor effect does not depend on endogenous P53 status40,41. Second, sample size of some studies is relatively small. Third, all studies were performed in China (because Gendicine was approved by the China State Food and Drug Administration), which may lead to geographical and ethnic differences. In spite of this, these studies still propose a credible suggestion pointing toward that the rAd-p53 (Gendicine) is effective and safe for treating MPE. However, rAd-p53 still needs to be investigated for treating MPE in the future. Anyway, rigorously randomized control trials with large sampler size and multi-centered cooperation should be done before it could be recommended in clinic extensively.
Conclusions
Thoracic perfusion of rAd-p53 (Gendicine) combined with chemotherapeutic agents has a better benefit of ORR and DCR for treating MPE and improves the QOL of MPE patients, compared with chemotherapeutic agents alone. However, self-limited fever is shown as a special adverse effect for the participation of rAd-p53, but it could be tolerated well. Nevertheless, rigorously randomized control trials should be required before it is used widely.
Methods and Materials
Searching and identification of studies
We searched the previous published literature concerning the efficacy and safety of rAd-p53 by thoracic perfusion in treating MPE from the following databases: Medline/PubMed, SpringerLink, Embase, Ovid, Cochrance Library, Web of Science, and CNKI (China National Knowledge Infrastructure). The searching time we defined was from January 2000 to July 2016. The MeSH terms and key words that we defined were as follows: “recombinant human adenovirus p53 injection,” “rAd-p53,” “AD-P53 gene therapy,” “Gendicine,” “malignant pleural effusions,” and “MPE.” We also further searched the relative literature from the reference lists of having been included studies and identified them whether they were available.
Criteria of inclusion and exclusion for selecting studies
Inclusion criteria: (1) patients who were included in selected studies must be diagnosed with MPE by cytology and histology; (2) study design must be clinical randomized controlled trial comparing rAd-p53 plus another drug to another drug alone; (3) the basic treatment of two groups must be completely equal; (4) the clinical baseline of two groups must be basically equal, such as the scores of life quality, size of MPE, basic laboratory index and vital signs; (5) the rAd-p53 and another drug must be given through thoracic perfusion; and (6) included studies must have reported the outcome measures, which included response, (RR), disease control rate (DCR), symptom improvement (SI) and adverse effects (AEs). Exclusion criteria: (1) non-original articles, such as abstract, meeting record, editorial, review and correspondence; (2) non-human studies; (3) used excessive other adjutant drugs; (4) the funding and expenditure of studies were provided by the producer of rAd-p53; (5) control design was not balanced; (6) lost of follow up rate of patients was above 15%; and (7) the study quality of the literature is too low when assessed by the evaluation criteria from the Cochrane Handbook (Version 5.0.1); and (8) lack of ethics statement.
Extraction of study variables
The variables we shaped included: (1) authors of study, years of publication, the number of patients who participated each RCT; (2) gender and histology of lung cancer patients; (3) the quality of life of patients; (4) the specific process of clinical intervention; (5) RR and DCR of clinical intervention; and (6) improvement of SI and AEs.
Supervision of clinical intervention
Trial design: clinical RCTs of rAd-p53 combined with another agent versus another agent alone by thoracic perfusion for controlling MPE. Type of clinical interventions: the dosage of rAd-p53 was defined in accordance with the instruction of producer and the frequency of dosing at least two times. Indicators of efficacy that evaluated rAd-p53 included the first outcome ORR and DCR, and secondary quality of life (QOL) and AEs.
Quality assessment of included studies
The Cochrane Handbook (Version 5.1.0) shaped the criteria for evaluating the clinical randomized controlled trials. We acted in accordance with the criteria to assess the quality of included studies. The criteria included the following aspects: (1) random sequence generation of patients; (2) setting blinding; (3) allocation concealment; (4) outcome data; (5) reporting of selective outcome; (6) other bias factors; and 7) intention-to-treat (ITT).
Statistics process
We employed two different statistical models, fixed effects model and random effects model, to measure the safety and efficacy of rAd-p53 thoracic perfusion for treating MPE. We utilized the chi-square test and I2 value to determine whether the heterogeneity existed in included studies. If no heterogeneity existed, the method of fixed effects model was adopted, or using the random effects model. Relevant variables were calculated by the statistics estimation of odds ratios (OR) and a calculated 95% confidence interval (CI). The pooled effect was measured through a value of Z-scores and P < 0.05 was considered as having a significance of statistics. We also performed a sensitivity analysis of meta-analysis, which omitted each study one by one in order to know clearly whether any study could shake the pooled effect of meta-analysis. Further, we drew a funnel plot of included studies, and conducted the Begg’s test and Egger’s test to evaluate the publication biases. We used the following statistics software to do all statistics process: SPSS (version 19.0, Chicago, USA), Stata 13.0 (StataCorp LP, USA) and RevMan 5.2 (Cochrane Collaboration). We defined the p-value was two-sided, and P value less than 0.05 indicated a statistical significance.
Additional Information
How to cite this article: Biaoxue, R. et al. Evaluation of efficacy and safety for recombinant human adenovirus-p53 in the control of the malignant pleural effusions via thoracic perfusion. Sci. Rep. 6, 39355; doi: 10.1038/srep39355 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We appreciate the great help of Mr. Xu Xiaoweng, and Miss Gao Jin as interviewers.
Footnotes
Author Contributions Rong B.X., Pan Hui, Gao W.L. and Yang S.Y. participated in the design and coordination of the study, carried out the critical appraisal of studies, statistical analysis of studies and wrote the manuscript.
References
- Ebata T. et al. Retrospective analysis of unknown primary cancers with malignant pleural effusion at initial diagnosis. Thorac Cancer 7, 39–43, doi: 10.1111/1759-7714.12271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. et al. Diagnostic and prognostic significance of receptor-binding cancer antigen expressed on SiSo cells in lung-cancer-associated pleural effusion. Clin Respir J, doi: 10.1111/crj.12527 (2016). [DOI] [PubMed] [Google Scholar]
- Wong W. M. et al. Managing malignant pleural effusion with an indwelling pleural catheter: factors associated with spontaneous pleurodesis. Hong Kong Med J, doi: 10.12809/hkmj154673 (2016). [DOI] [PubMed] [Google Scholar]
- Mir R. et al. Clinical implications of cytosine deletion of exon 5 of P53 gene in non small cell lung cancer patients. South Asian J Cancer 5, 33–36, doi: 10.4103/2278-330x.179701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappori A. A., Soliman H., Janssen W. E., Antonia S. J. & Gabrilovich D. I. INGN-225: a dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed association between immune response and enhanced chemotherapy effect. Expert Opin Biol Ther 10, 983–991, doi: 10.1517/14712598.2010.484801 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebitekin C., Bayram A. S., Tunca B. & Balaban S. A. Clinical significance of p53 gene mutation in T1-2N0 non-small cell lung cancer. Asian Cardiovasc Thorac Ann 15, 35–38 (2007). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Association between smoking and p53 mutation in lung cancer: a meta-analysis. Clin Oncol (R Coll Radiol) 26, 18–24, doi: 10.1016/j.clon.2013.09.003 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou C. Z., Li Y. & Xu J. Correlation between p53 gene mutation and the expression of tumor drug resistance genes in lung cancer and its clinical significance. Zhonghua Jie He He Hu Xi Za Zhi 27, 678–682 (2004). [PubMed] [Google Scholar]
- Swisher S. G. & Roth J. A. Clinical update of Ad-p53 gene therapy for lung cancer. Surg Oncol Clin N Am 11, 521–535 (2002). [DOI] [PubMed] [Google Scholar]
- Cun Y. et al. Combined use of adenoviral vector Ad5/F35-mediated APE1 siRNA enhances the therapeutic efficacy of adenoviral-mediated p53 gene transfer in hepatoma cells in vitro and in vivo. Oncol Rep 29, 2197–2204, doi: 10.3892/or.2013.2384 (2013). [DOI] [PubMed] [Google Scholar]
- Koom W. S. et al. Combination of radiotherapy and adenovirus-mediated p53 gene therapy for MDM2-overexpressing hepatocellular carcinoma. J Radiat Res 53, 202–210 (2012). [DOI] [PubMed] [Google Scholar]
- Ma J. T. et al. Synergistic cytotoxic effects of recombinant human adenovirus p53 and radiation at various time points in A549 lung adenocarcinoma cells. Oncol Lett 4, 529–533, doi: 10.3892/ol.2012.747 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J. S., Wang W. H. & Li F. Q. Combination of interventional adenovirus-p53 introduction and ultrasonic irradiation in the treatment of liver cancer. Oncol Lett 9, 1297–1302, doi: 10.3892/ol.2014.2811 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Ad-p53 enhances the sensitivity of triple-negative breast cancer MDA-MB-468 cells to the EGFR inhibitor gefitinib. Oncol Rep 33, 526–532, doi: 10.3892/or.2014.3665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Z. et al. Quality control of clinical-grade recombinant adenovirus used in gene therapy. Zhonghua Yi Xue Za Zhi 84, 849–852 (2004). [PubMed] [Google Scholar]
- Bo T. et al. Recombined adenovirus expressing P53 in the treatment of malignant pleural effusion with lung cancer (in Chinese). J Reg Anat Oper Surg 24, 192–194 (2015). [Google Scholar]
- Chengzhi Z., Ouyuan M., Shiyue L. & Huo T. Clinical study on treatment of 96 lung cancer patients with malignant pleural effusion (in Chinese). Guide of China Medicine 9, 5–7 (2011). [Google Scholar]
- Dong L. Clinical observation of gene therapy combined with chemotherapy in the treatment of malignant pleural effusion caused by lung carcinoma (in Chinese). Journal of Clinical Pulmonary Medicine 18, 1481–1482 (2013). [Google Scholar]
- Dong M. et al. Advanced malignant pleural or peritoneal effusion in patients treated with recombinant adenovirus p53 injection plus cisplatin. J Int Med Res 36, 1273–1278 (2008). [DOI] [PubMed] [Google Scholar]
- Lin Z., Jiqian W. & Li W. Efficacy of recombinant human Ad-p53 injection combined hyperthermia chemotherapy in treating 41 cases malignant pleural effusion of NSCLC (in Chinese). Chinese Journal of Gerontology 35, 213–214 (2015). [Google Scholar]
- Ling T. & Ying X. Efficacy observation on recombinant human Ad- p53 injection combined with cisplatin for malignant pleural effusion (in Chinese). Chongqing Medicine 40, 2996–2997 (2011). [Google Scholar]
- Nanchang Y. & Xiaodong W. Efficacy observation on recombinant human Ad- p53 injection combined with cisplatin for malignant pleural effusion of non- small- cell lung cancer with intracavitary administration (in Chinese). China Pharmacy 21, 1120–1122 (2010). [Google Scholar]
- Rong Z., Liang C. & Zili M. Clinical research on recombinant human Ad-p53 pleural injection combined with cisplatin in treatment of non-small cell lung cancer with malignant pleural effusion (in Chinese). Journal of Clinical Pulmonary Medicine 16, 896–898 (2011). [Google Scholar]
- Weizhu Z., Jikun W., Wei L. & Xiuli Z. Clinical research on recombinant human Ad-p53 injection combined with cisplatin in treatment of malignant pleural effusion induced by lung cancer (in Chinese). Chinese Journal of Cancer 28, 1324–1327 (2009). [DOI] [PubMed] [Google Scholar]
- Xiaohong W., Juanquan Y. & Guohuan L. Clinical observation of recombinational human p53 adenovirus injection combined with chemotherapy in treatment of malignant pleural effusion (in Chinese). Chinese Journal of Coal Industry Medicine 17, 188–191 (2014). [Google Scholar]
- Yanliang Z. & Yun M. Efficacy observation on recombinant human Ad- p53 injection combined with cisplatin for lung cancer with malignant pleural effusion (in Chinese). Practical Journal of Cancer 26, 87–88 (2011). [Google Scholar]
- Yimei Q., Guoqing L. & Hongmei W. Clinical research on recombinant human Ad p53 injection combined with continuous heperthermic perfusion in treatment of malignant pleural effusion induced by lung cancer (in Chinese). Chin J Diffic and Compl Cas 10, 594–596 (2011). [Google Scholar]
- Yue W., Zhe C., Jian C. & Wei L. Efficacy observation on recombinant human Ad- p53 injection combined with cisplatin in treating 99 cases of malignant pleural effusion (in Chinese). Guangdong Medical Journal 31, 2988–2990 (2010). [Google Scholar]
- Zongyuan Z. & Xia L. Clinical efficacy of recombinant adenovirus-p53 injection in the treatment of malignant pleural effusions (in Chinese). Sichuan Medical Journal 34, 695–696 (2013). [Google Scholar]
- Biaoxue R., Shuxia M., Wenlong G. & Shuanying Y. Thoracic perfusion of matrine as an adjuvant treatment improves the control of the malignant pleural effusions. World J Surg Oncol 13, 329, doi: 10.1186/s12957-015-0729-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. L. et al. Efficacy of recombinant adenoviral human p53 gene in the treatment of lung cancer-mediated pleural effusion. Oncol Lett 9, 2193–2198, doi: 10.3892/ol.2015.3054 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaoxue R., Shuanying Y., Wei L., Wei Z. & Zongjuan M. Maintenance therapy of gefitinib for non-small-cell lung cancer after first-line chemotherapy regardless of epidermal growth factor receptor mutation: a review in Chinese patients. Curr Med Res Opin 28, 1699–1708, doi: 10.1185/03007995.2012.728525 (2012). [DOI] [PubMed] [Google Scholar]
- Rong B., Yang S., Li W., Zhang W. & Ming Z. Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World J Surg Oncol 10, 170, doi: 10.1186/1477-7819-10-170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Brown C. J., Verma C. & Cheok C. F. New insights into p53 based therapy. Discov Med 12, 107–117 (2011). [PubMed] [Google Scholar]
- Zhang X. et al. Efficacy of recombinant adenoviral human p53 gene in treatment of malignant pleural or peritoneal effusions. Zhongguo Fei Ai Za Zhi 16, 153–156, doi: 10.3779/j.issn.1009-3419.2013.03.07 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li B., Li C. J. & Li L. J. Key points of basic theories and clinical practice in rAd-p53 (Gendicine) gene therapy for solid malignant tumors. Expert Opin Biol Ther 15, 437–454, doi: 10.1517/14712598.2015.990882 (2015). [DOI] [PubMed] [Google Scholar]
- Chen G. X. et al. Clinical utility of recombinant adenoviral human p53 gene therapy: current perspectives. Onco Targets Ther 7, 1901–1909, doi: 10.2147/ott.s50483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 7, 102, doi: 10.1186/1477-7525-7-102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidou Z. et al. Impact of response shift on time to deterioration in quality of life scores in breast cancer patients. PLoS One 9, e96848, doi: 10.1371/journal.pone.0096848 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liqun Y., Ruilin W., Hui W. & Yuimei P. P53 gene status and its therapeutic effect. Chinese and Foreign Medical 28, 16–17 (2009). [Google Scholar]
- Wang Z., Lu B., Wang T., De W. & Shu Y. Effect of recombinant adenovirus-p53 on growth and chemosensitivity of human lung adenocarcinoma cell lines. Zhongguo Fei Ai Za Zhi 9, 127–131, doi: 10.3779/j.issn.1009-3419.2006.02.06 (2006). [DOI] [PubMed] [Google Scholar]





