Abstract
Key points
Both uncoupling protein 1 (UCP1) and UCP3 are important for mammalian thermoregulation.
UCP1 and UCP3 in brown adipose tissue mediate early and late phases of sympathomimetic thermogenesis, respectively.
Lipopolysaccharide thermogenesis requires skeletal muscle UCP3 but not UCP1.
Acute noradrenaline‐induced hyperthermia requires UCP1 but not UCP3.
Loss of both UCP1 and UCP3 accelerate the loss of body temperature compared to UCP1KO alone during acute cold exposure.
Abstract
Uncoupling protein 1 (UCP1) is the established mediator of brown adipose tissue‐dependent thermogenesis. In contrast, the role of UCP3, expressed in both skeletal muscle and brown adipose tissue, in thermoregulatory physiology is less well understood. Here, we show that mice lacking UCP3 (UCP3KO) have impaired sympathomimetic (methamphetamine) and completely abrogated lipopolysaccharide (LPS) thermogenesis, but a normal response to noradrenaline. By comparison, UCP1 knockout (UCP1KO) mice exhibit blunted methamphetamine and fully inhibited noradrenaline thermogenesis, but an increased febrile response to LPS. We further establish that mice lacking both UCP1 and 3 (UCPDK) fail to show methamphetamine‐induced hyperthermia, and have a markedly accelerated loss of body temperature and survival after cold exposure compared to UCP1KO mice. Finally, we show that skeletal muscle‐specific human UCP3 expression is able to significantly rescue LPS, but not sympathomimetic thermogenesis blunted in UCP3KO mice. These studies identify UCP3 as an important mediator of physiological thermogenesis and support a renewed focus on targeting UCP3 in metabolic physiology.
Keywords: brown adipose tissue, LPS, methamphetamine, skeletal muscle, sympathomimetic, thermogenesis, uncoupling protein
Key points
Both uncoupling protein 1 (UCP1) and UCP3 are important for mammalian thermoregulation.
UCP1 and UCP3 in brown adipose tissue mediate early and late phases of sympathomimetic thermogenesis, respectively.
Lipopolysaccharide thermogenesis requires skeletal muscle UCP3 but not UCP1.
Acute noradrenaline‐induced hyperthermia requires UCP1 but not UCP3.
Loss of both UCP1 and UCP3 accelerate the loss of body temperature compared to UCP1KO alone during acute cold exposure.
Abbreviations
- BAT
brown adipose tissue
- LPS
lipopolysaccharide
- METH
methamphetamine
- NA
noradrenaline
- SKM
skeletal muscle
- UCP
uncoupling protein
Introduction
Mammalian thermogenic mechanisms are classified as obligatory and facultative. The former represents the sum total of biochemical inefficiency of all reactions in the body, whereas the latter represents thermogenic mechanisms that can be induced. Apart from shivering, the molecular mechanisms of non‐shivering facultative thermogenesis are poorly understood. Dysregulation of thermogenesis leads to a spectrum of metabolic disorders ranging from obesity to hyperthermia. Currently, therapeutically targeting thermogenesis for intervening in metabolic illness has been confounded by incomplete understanding of thermogenic mechanisms.
Mitochondrial uncoupling protein 1 (UCP1) is the primary physiological mediator of cold‐induced thermogenesis in brown adipose tissue (BAT), an organ specialized for heat production (Nicholls et al. 1978). UCP1 is activated in response to sympathetic nervous system stimulation and noradrenaline (NA) release, leading to the liberation, uptake, and oxidation of fatty acids in BAT (Locke et al. 1982). Fatty acids are the endogenous ligands for UCP1‐dependent proton translocation (Rial et al. 1983), a process that ‘uncouples’ the proton pumping activities of the electron transport chain from ATP synthesis. In this ‘uncoupled’ state the chemical energy stored in the mitochondrial proton gradient is released as heat (Brand et al. 1999). The failure of UCP1 knockout mice (UCP1KO) to survive an acute bout of cold led to a ‘UCP1‐centric’ view of mammalian thermoregulation and to the notion that UCP1 was the predominant, if not only, protein able to mount a thermogenic response to cold, and perhaps any other thermogenic stimulus, in vivo (Enerback et al. 1997; Matthias et al. 1999; Feldmann et al. 2009).
Despite recent discoveries suggesting that humans may have significant amounts of BAT, the contribution of classical BAT to global body temperature responses and energy dissipation in most large mammals has long been thought of as negligible in the majority of subjects relative to rodents and hibernators However, from a thermogenic perspective, clinical research continues to raise questions about the capacity of even the most BAT‐replete of individuals to have sufficient BAT amounts and oxygen delivery to account for more than just a small fraction of the total oxygen uptake during a global thermogenic response (Blondin et al. 2015). Further challenging the UCP1‐centric view of thermoregulation, UCP1 lacks a significant role in inflammatory fever involving interleukin‐1 beta (Okamatsu‐Ogura et al. 2007). BAT has been reported to be important in sympathomimetic drug‐induced thermogenesis but UCP1 has yet to be identified as a causal mediator (Sanchez‐Alavez et al. 2013). Moreover, UCP1KO mice are able to generate sufficient heat to protect body temperature in response to cold when adapted over a period of several days to weeks (Shabalina et al. 2010).
Uncoupling protein 3 (UCP3) is a UCP1 homologue expressed in BAT and skeletal muscle (SKM) that has been reported to regulate SKM mitochondrial coupling efficiency (Vidal‐Puig et al. 2000) and increase fatty acid oxidation (Bézaire et al. 2001). Its presumptive role in thermogenesis was originally ruled out because UCP3KO mice adapted normally to an acute bout of cold, unlike their UCP1KO counterparts (Enerback et al. 1997; Gong et al. 2000). In contrast, we previously demonstrated that UCP3KO mice exhibited strongly blunted peak thermogenic and lethal responses to treatments with the hyperthermia‐inducing sympathomimetic drugs 3,4‐methylenedioxymethamphetamine (MDMA) and methamphetamine (METH) (Mills et al. 2003, 2004; Sprague et al. 2004).
Here, we aimed to build on those observations by defining the comparative, causal roles of UCP1 and UCP3 for thermogenesis in response to physiological thermogenic stimuli cold, lipopolysaccharide (LPS) and NA administration. The data indicate that both UCP1 and UCP3 play essential but temporally distinct roles in sympathomimetic thermogenesis, with UCP1 mediating an early and UCP3 a delayed component of the heat response. Moreover, UCP3KO animals completely lack the febrile response to LPS observed in WT mice, whereas UCP1KO mice had increased LPS‐induced thermogenesis. Finally, whereas UCP3KO animals maintained WT body temperature and survival in response to cold, UCP1KO animals fail to maintain normal body temperature and survival. Strikingly, however, UCP1/3 double knockout mice (UCPDK) have a dramatically accelerated loss of body temperature and survival compared to UCP1KO. Together the data show that both UCP1 and UCP3 play essential and complementary roles in thermogenic responses in the mouse and suggest that UCP3‐dependent thermogenesis is an under‐appreciated mode of thermogenic energy dissipation.
Methods
Ethical approval
All animal husbandry and experimental procedures were carried out in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin (IACUC, AUP‐2015‐00175). All procedures were performed with ethical principles under which the Journal of Physiology operates.
Animals
All experiments were performed in male mice between the ages of 7 and 10 weeks. All animals were maintained on standard breeder chow diet with access to food and water ad libitum. Upon conclusion of the experiments all mice were killed via CO2 inhalation followed by secondary cervical dislocation unless otherwise noted. C57BL/6J wild‐type mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). UCP3KO mice in the C57BL/6J background were a gift from Dr Marc Reitman, formerly of the National Institutes of Health. UCP1KO mice were a gift from Dr Leslie Kozak (Maine Medical Research Institute, Scarborough, ME, USA). UCP3KO mice were crossed with the UCP1KO mice to generate our UCP1 and UCP3 double knockout line (UCPDK) transgenic mice overexpressing human UCP3 in SKM generated from human alpha1‐actin promoter targeting construct (TgSKM UCP3) and were a gift from Dr Mary‐Ellen Harper of the University of Ottawa. TgSKM UCP3 were then crossed with the UCP3KO and UCPDK lines to generate a transgenic mouse overexpressing human UCP3 in SKM in the UCP3KO and UCPDK background (TgSKM‐UCP3KO and TgSKM‐UCPDK). All TgSKM‐UCP3 strains were kept as hemizygous breeder colonies. All interbred strains displayed proper Mendelian ratios.
Reagents
LPSs (Escherichia coli, 0111:B4), METH and NA were purchased from Sigma Aldrich (St Louis, MO, USA).
Surgical procedures
Male mice 6–7.5 weeks of age, weighing 20–28 g were used for all experiments. All surgical tools and operating areas were prepared under sterile technique procedures. The right dorsal flank of each mouse was shaved and cleaned with sterile povidine solution. The mice were anaesthetized with isoflurane (1–2%) via nose cone and administered a preoperative subcutaneous injection of 5 mg kg–1 carprofen. A transverse oblique incision was made dorsally above the iliac crest, followed by an incision through the peritoneal wall. A wireless temperature probe (Starr Life Sciences Corp., Oakmont, PA, USA) was inserted into the peritoneal cavity. The incision on the peritoneal wall was closed with non‐absorbable Vicryl sutures. Lastly, the skin was closed with sterile skin clips. Mice were administered a postoperative subcutaneous injection of carprofen (5 mg kg–1). After surgical procedures all mice were individually housed and monitored for signs of distress or infection during recovery.
Mouse telemetry
All temperature measurements were performed at an ambient temperature of 25 ± 0.2°C based on published literature establishing the role of ambient temperature in the regulation of sympathomimetic hyperthermia (Green et al. 2005). After 1 week of recovery mice were placed in a lighted temperature‐controlled room for 30 min prior to drug administration. During experiments mice had access food and water ad libitum. All experiments were initiated between 10:00 and 12:00 h. All drugs were prepared in non‐pyrogenic saline and diluted to final concentrations immediately prior to administration. METH (20 mg kg–1) and NA (1 mg kg–1) administered subcutaneously, LPS (50 μg kg–1) and saline were administered via intraperitoneal injection. Temperatures were recorded for 10 min prior to drug administration and were monitored every minute for 3.5–5 h using VitalView Software and implantable Mini mitter temperature probes (Starr Life Sciences, Oakmont, PA, USA).
Cold exposure
Mice were individually house and placed in a lighted room maintained at 4 ± 0.3°C. Baseline temperature was recorded 30 min prior to cold exposure and temperature was recorded every hour for 4 h with a rectal temperature probe (Physitemp, Clifton, NJ, USA). Mice were removed from cold and killed if temperature dropped below 31°C.
Statistics
Analysis of variance was calculated between groups using a two‐tailed Student's test with Welsh's correction or one‐way ANOVA followed by Tukey's post hoc test corrected for multiple comparisons. A survival curve log‐rank test was calculated using Graphpad Prism software. Results were considered significant at P ≤ 0.05.
Results
UCP1 and UCP3 mediate early and late phases of sympathomimetic thermogenesis, respectively
We previously reported that UCP3KO mice are significantly protected from the hyperthermic effects of sympathomimetic drugs using rectal thermometry to assess body temperature every 30 min for 3 h (Mills et al. 2003). We began the present studies by confirming those results with greater temporal resolution using intraperitoneal telemeters and continuous monitoring. As shown in Fig. 1 A, UCP3KO and WT mice exhibited a similar rapid rise in body temperature for the first 40 min after METH administration (20 mg kg–1, s.c.), but UCP3KO had a significantly blunted maintenance phase of the thermogenic response 70–150 min after administration compared to WT (Fig. 1 A). By contrast, UCP1KO mice lacked the early peak rise in body temperature in response to METH, but maintained the later thermogenic component and demonstrated a similar rate of return to baseline temperature in a manner comparable to WT (Fig. 1 B). The thermogenic responses to METH in both the early (30 min) and later time points (70–150 min) in both genotypes were also significantly different between UCP3KO and UCP1KO (Fig. 1 C). Finally, at no time points over the 2 h measurement period did the UCPDK mice exhibit a significant rise in body temperature in response to METH over baseline compared to WT (Fig. 1 D). Together, the data demonstrate that UCP3 and UCP1 each participates in sympathomimetic hyperthermia and that their combined activities fully account for heat production in this drug‐induced thermogenesis model.
Figure 1. Complementary roles of UCP1 and UCP3 in METH‐induced hyperthermia.
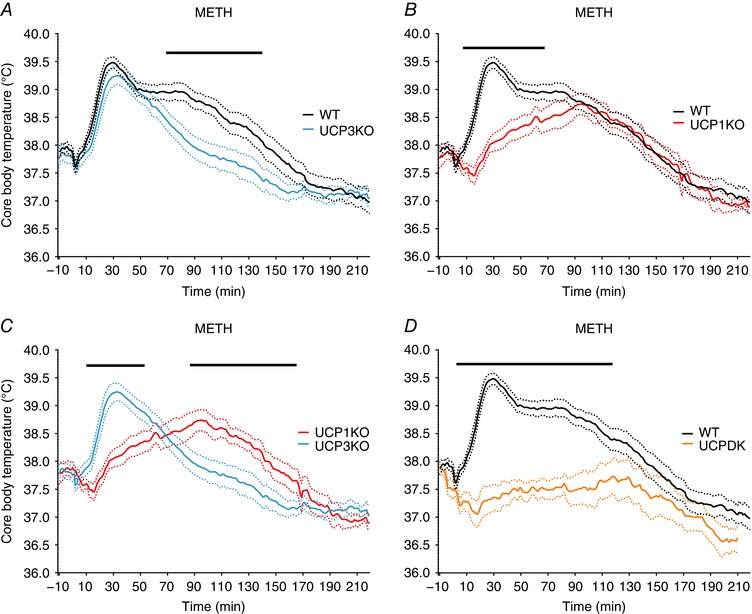
METH (20 mg kg–1, s.c.) administered at 0 min. A, changes in core body temperature of wild‐type (black, n = 20) and UCP3KO (blue, n = 17) mice. B, changes in core body temperature of wild‐type and UCP1KO (red, n = 12) mice. C, changes in core body temperature of UCP1KO and UCP3KO mice. D, changes in core body temperature of WT and UCPDK (orange, n = 12) mice. Dotted lines represent ± SEM. Black bars represent significance between groups with Welsh's two‐tailed t test, P ≤ 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
LPS thermogenesis requires UCP3 but not UCP1
To more fully define the physiological relevance of UCP‐mediated thermogenesis, we assessed the role of UCP3 in the thermoregulatory responses to endotoxin (LPS). WT and UCP3KO mice were administered LPS (50 μg kg–1, i.p.) and body temperature was monitored. WT and UCP3KO mice each exhibited an overlapping hypothermia (approximately 1°C) at 70 min after injection, but UCP3KO animals failed to exhibit the recovery thermogenesis observed in WT animals over the 300 min assessment period (Fig. 2 A). LPS‐mediated fever is mediated via interleukin‐1 beta (IL‐1β), and UCP1KO mice were previously reported to have normal IL‐1β thermogenesis (Okamatsu‐Ogura et al. 2007). Consistent with those results, and in stark contrast to UCP3KO mice, we found that UCP1KO mice had significantly elevated LPS thermoregulatory responses compared to WT mice (Fig. 2 B).
Figure 2. Loss of UCP3 blunts second phase of thermogenesis in LPS‐induced fever.
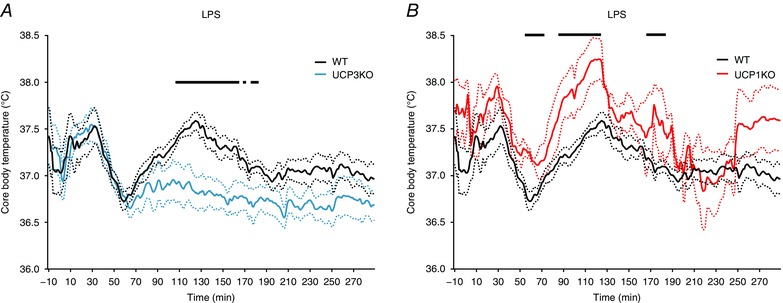
LPS (50 μg kg–1, i.p.) administered at 0 min. A, wild‐type (black, n = 9) and UCP3KO (blue, n = 8) mice. B, wild‐type and UCP1KO (red, n = 3) mice. Dotted lines represent ± SEM. Black bars represent significance between groups with Welsh's two‐tailed t test, P ≤ 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
Differential influence of SKM‐specific expression in sympathomimetic and LPS thermogenesis
Given that UCP3 is expressed in both SKM and BAT (Affourtit et al. 2007), we tested the capacity for UCP3 to mount a sympathomimetic thermogenic response when expressed exclusively in muscle. UCP3KO and UCPDK mice were interbred with those expressing an SKM‐specific alpha‐1 actin human UCP3 transgene (TgSKM) previously shown to drive UCP3 protein expression at physiological levels of induction in SKM mitochondria (Bezaire et al. 2005). Surprisingly, skeletal muscle‐selective UCP3 expression failed to rescue any component of the UCP3‐dependent METH thermogenesis (20 mg kg–1, s.c.) lost in UCP3KO (Fig. 3 A) and UCPDK mice (Fig. 3 B). These data suggest the possibility that UCP3 in BAT may be the predominant mediator of the late phase of sympathomimetic hyperthermia. Unlike sympathomimetic thermogenesis that lacked an SKM UCP3 component, SKM‐selective UCP3 expression was able to significantly rescue the WT LPS thermoregulatory responses lost in UCP3KO animals (Fig. 3 C). An important additional consideration is sex; we used males for these studies given the established effects of sex in sympathomimetic hyperthermia (Wyeth et al. 2009). Nonetheless, the data indicate that UCP3 activity in SKM is an important component of endotoxin‐mediated changes in body temperature.
Figure 3. Skeletal muscle UCP3 expression has differential consequences for METH‐ and LPS‐induced thermogenesis.
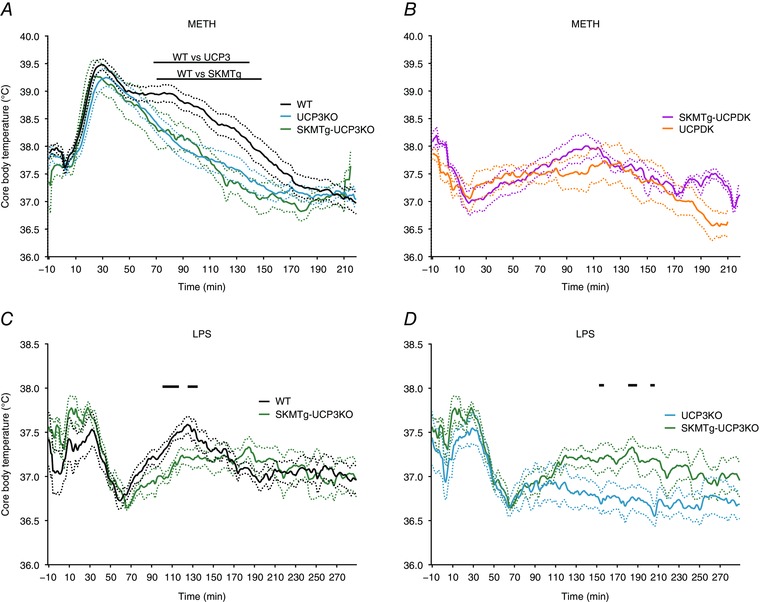
A, METH (20 mg kg–1, s.c.) induced change in core body temperature of WT (black) UCP3KO (blue) and SKMTg‐UCP3KO (green, n = 9) mice. B, METH (20 mg kg–1, s.c.) induced change in core body temperature of UCPDK (orange) and SKMTg‐UCPDK (magenta, n = 4) mice. C, LPS (50 μg kg–1, i.p.) induced change in core body temperature of wild‐type (black line) and SKMTg‐UCP3KO (green, n = 4) mice. D, LPS (50 μg kg–1, i.p.) induced change in core body temperature of SKMTg‐UCP3KO (green) and UCP3KO (blue) mice. Dotted lines represent ± SEM. Black bars represent significance between groups with Welsh's two‐tailed t test, P ≤ 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
Acute NA‐induced hyperthermia requires UCP1 but not UCP3
Next, we determined the roles of UCP3 and UCP1 in NA thermogenesis following subcutaneous administration (1 mg kg–1, s.c.). UCP3KO mice had identical and rapid thermogenic (peak ∼ 30 min) and subsequent hypothermic responses (Fig. 4 A), but UCP1KO mice completely lacked an NA‐driven thermogenic response. Furthermore, UCPDK mice displayed a thermoregulatory response profile to NA that phenocopied UCP1KO mice. These data indicate that UCP3 may not be involved in acute NE‐dependent thermoregulation.
Figure 4. UCP1 selectively controls NA‐induced thermogenesis.
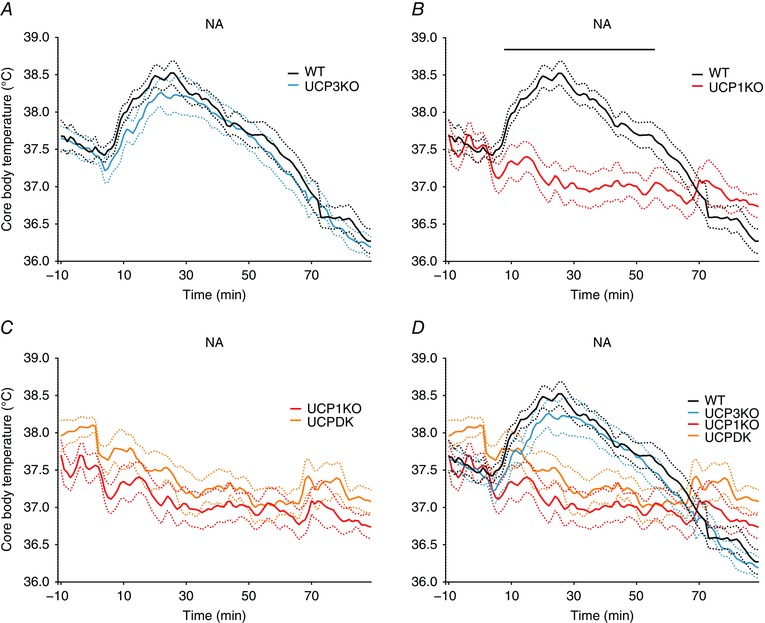
NA (1 mg kg–1, s.c.) administered at 0 min. A, change in core body temperature of wild‐type (black, n = 11) and UCP3KO (blue, n = 12) mice. B, change in core body temperature of wild‐type and UCP1KO (red, n = 8) mice. C, change in core body temperature of UCP1KO and UCPDK (orange, n = 5) mice. D, change in core body temperature of all genotypes tested. Dotted lines represent ± SEM. Black bars represent significance between groups with Welsh's two‐tailed t test, P ≤ 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
UCP3 participates in cold thermogenesis when UCP1 is absent
Finally, we sought to further scrutinize the conclusions drawn from the few observations available in the literature that UCP3 lacks a role in cold‐mediated, physiological thermogenesis by making use of the UCPDK mouse model. WT, UCP3KO, UCP1KO and UCPDK mice were exposed to cold (4°C) and body temperatures and survival were monitored over time. As previously reported, cold‐naïve UCP1‐null animals were markedly cold intolerant compared to WT mice, and UCP3‐null mice were nearly identical to WT in their capacities to protect body temperature (Fig. 5 A; Enerback et al. 1997). Notably, UCPDK mice exhibited a dramatically accelerated loss of body temperature and induction of terminal hypothermia compared to UCP1KO mice in response to cold, indicating that the presence of UCP1 may mask the role of UCP3 in response to acute cold challenge in WT mice (Fig. 5).
Figure 5. UCP1‐UCP3 double null animals fail to maintain temperature after acute cold exposure.
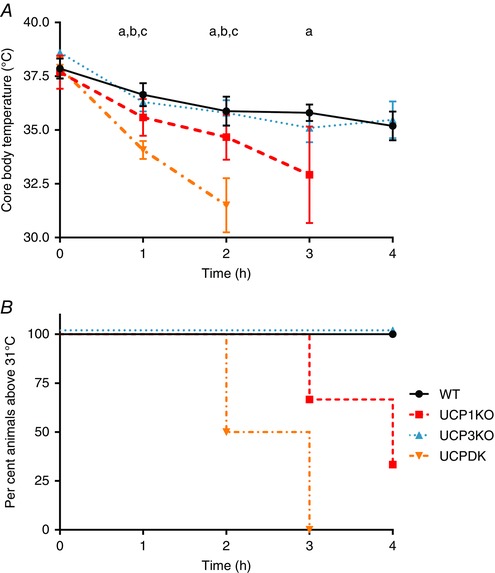
A, change in core body temperature as measured by rectal temperature each hour for a total of 4 h. Error bars represent ± SEM. a represents significant difference between wild‐type and UCP1KO, b represents significant difference between UCPDK and WT, and c represents significant difference between UCP1 and UCPDK. Significance was determined by one‐way ANOVA corrected for multiple comparisons followed by Tukey's post hoc analysis. P ≤ 0.05. B, survival curve where death is considered a core body temperature < 31°C. UCP1–UCPDK P = 0.0068 (log‐rank test): wild‐type (black, n = 9), UCP3KO (green, n = 4), UCP1KO (red, n = 9) and UCPDK (orange, n = 4). [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
UCP3 plays a role in thermoregulatory physiology
UCP3KO mice were first reported to have normal thermogenic responses to cold, LPS, thyroid hormone and beta‐3 adrenergic agonist administration (Gong et al. 2000). When combined with observations that brown adipocytes from UCP1KO mice failed to show significant fatty acid‐induced changes in respiration despite expression of UCP3 and UCP2 (Matthias et al. 2000), the consensus in the UCP field that persisted was that only UCP1 had a thermoregulatory function. Vidal‐Puig et al. (2000) also reported that UCP3KO SKM mitochondria were more coupled than WT, suggesting that UCP3 may control basal thermogenesis in SKM mitochondria, paradoxically not reflected in a basal or inducible thermogenic phenotype in vivo under the conditions used. Here, we provide extensive evidence that supports a physiological thermogenic role for UCP3 and extend previous observations regarding the roles of UCPs in toxic sympathomimetic drug‐induced hyperthermia (Mills et al. 2003; Sprague et al. 2004). We show that (a) UCP1 and UCP3 each participates in sympathomimetic and cold thermogenesis (Figs 1 and 5), (b) UCP3, but not UCP1, mediates LPS fever (Fig. 2), (c) UCP1, but not UCP3, regulates rapid, NA‐dependent hyperthermia (Fig. 4) and (d) SKM‐specific hUCP3 expression could significantly rescue LPS but not sympathomimetic thermogenesis on a UCP3KO background (Fig. 3). When combined, the data support a hypothetical model where UCP1 activity may control rapid, NA‐dependent BAT thermogenesis, and UCP3 may be required for sustaining a more protracted thermogenic response in SKM and BAT.
BAT UCP3 and SKM UCP3 participate in thermogenic responses in a stimulus‐specific manner
UCP3 protein is primarily expressed in SKM, BAT and heart, but among these only SKM and BAT are established to generate sufficient heat to rapidly control body temperature. At the molecular level, UCP3 activation is associated with fatty acid oxidation and the control of reactive oxygen species generation (Anderson et al. 2007), but its functions as a thermogenic protein are debated. We show here that UCP3 plays an integral role in mammalian thermogenesis, probably in both SKM and BAT. SKM is a well‐established site of thermogenesis regulated by catecholamines (Astrup et al. 1985), adrenaline (Astrup et al. 1989), thyroid hormones (Lombardi et al. 2015) and leptin (Dulloo et al. 2002), all of which have been shown to regulate the expression of UCP3. SKM calcium‐handling mechanisms regulated by sarcolipin, sarco(endo)plasmic reticulum ATPase (SERCA) and the ryanodine receptor are the predominant mechanisms of SKM heat production in response to cold and diet (Sahoo et al. 2013; Rowland et al. 2015). However, the ryanodine receptor inhibitor dantrolene and skeletal muscle relaxants fail to affect sympathomimetic hyperthermia (Rusyniak et al. 2004; Rusyniak & Sprague, 2005), suggesting that sympathomimetic hyperthermia arises at least in part from BAT‐dependent mechanisms. Additionally, it has been shown that LPS induces expression of UCP3 and directly uncouples skeletal muscle mitochondria, while chronic administration attenuates adaptive thermogenesis (Frisard et al. 2015; Okla et al. 2015). To our knowledge, no tissue‐specific UCP3KO mice exist. While little is known regarding the molecular metabolic functions of UCP3 in BAT in most models, Nau et al. (2008) reported on a colony of Djungarian hamsters harbouring a BAT‐selective loss of UCP3 expression (but normal UCP1) with reduced cold tolerance. The authors concluded that UCP3 is necessary for sustaining high metabolic rates in BAT. Furthermore, Gong et al. (2000) showed that the loss of UCP3 plays a differential role in mitochondrial proton leak, with significantly lower maximal uncoupled respiration in BAT and increased proton‐motive force during non‐phosphorylating conditions in SKM. These observations, combined with the lack of increased sympathomimetic thermogenesis in hUCP3 transgenic mice, imply that UCP3‐dependent sympathomimetic hyperthermia may arise primarily from BAT, whereas LPS‐induced UCP3 thermogenesis appears to derive from SKM.
Potential mechanisms of UCP3 thermogenesis
UCP3 was originally dubbed ‘a mitochondrial carrier in search of a function’ (Lowell, 1999) in part because of the continuous debate over its roles as a bona fide uncoupler and in resting metabolism (Cadenas et al. 2002; Anderson et al. 2007). The observations presented here are consistent with the possibility that UCP3 may act as an inducible thermogenin in vivo. However, UCP3 could indirectly mediate thermogenesis by increasing fatty acid oxidation (Bezaire et al. 2005) and metabolite transport, as we have shown for UCP2 and UCP4 (Pfeiffer et al. 2011; Vozza et al. 2014). Recent work has demonstrated that UCP activities are redox‐regulated by sulfenylation (UCP1) (Chouchani et al. 2016) and glutathionylation (UCP2–3) (Mailloux et al. 2011), adding yet another layer of complexity pertaining to the regulation of uncoupling. The data presented here offer new experimental opportunities for better understanding of how UCP3 functions and is regulated in vivo and provide new insights into the basic mechanisms of thermoregulation mediated by uncoupling proteins.
Additional information
Competing interests
The authors do not have any competing interests.
Author contributions
All experiments reported were performed at the University of Texas at Austin. MNS, JES and EMM conceived the project. CLR, CKD, MAK and EMM designed the experiments. CLR, CKD, LM, SK, SMN, AS and ZL acquired the data. CLR, JES and EMM analysed the results. CLR, GF, JES and EMM interpreted the results. CLR and EMM drafted the manuscript. All authors revised the manuscript for intellectual content.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases 5R01DK089224‐05.
Acknowledgements
We would like thank Gloria Fang for her help with animal procedures.
Linked articles This article is highlighted by a Perspective by Harper. To read this Perspective, visit http://dx.doi.org/10.1113/JP273485.
This is an Editor's Choice article from the 15 December 2016 issue.
References
- Affourtit C, Crichton PG, Parker N & Brand MD (2007). Novel uncoupling proteins. Novartis Found Symp 287, 70–80; discussion 80–91. [PubMed] [Google Scholar]
- Anderson EJ, Yamazaki H & Neufer PD (2007). Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid‐supported respiration. J Biol Chem 282, 31257–31266. [DOI] [PubMed] [Google Scholar]
- Astrup A, Bülow J, Madsen J & Christensen NJ (1985). Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am J Physiol 248, E507–515. [DOI] [PubMed] [Google Scholar]
- Astrup A, Simonsen L, Bülow J, Madsen J & Christensen NJ (1989). Epinephrine mediates facultative carbohydrate‐induced thermogenesis in human skeletal muscle. Am J Physiol 257, E340–345. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Spriet LL, Campbell S, Sabet N, Gerrits M, Bonen A & Harper ME (2005). Constitutive UCP3 overexpression at physiological levels increases mouse skeletal muscle capacity for fatty acid transport and oxidation. FASEB J 19, 977–979. [DOI] [PubMed] [Google Scholar]
- Blondin DP, Labbé SM, Phoenix S, Guérin B, É Turcotte, Richard D, Carpentier AC & Haman F (2015). Contributions of white and brown adipose tissues and skeletal muscles to acute cold‐induced metabolic responses in healthy men. J Physiol 593, 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Brindle KM, Buckingham JA, Harper JA, Rolfe DF & Stuart JA (1999). The significance and mechanism of mitochondrial proton conductance. Int J Obes Relat Metab Disord 23 Suppl 6, S4–11. [DOI] [PubMed] [Google Scholar]
- Bézaire V, Hofmann W, Kramer JK, Kozak LP & Harper ME (2001). Effects of fasting on muscle mitochondrial energetics and fatty acid metabolism in Ucp3(–/–) and wild‐type mice. Am J Physiol Endocrinol Metab 281, E975–982. [DOI] [PubMed] [Google Scholar]
- Cadenas S, Echtay KS, Harper JA, Jekabsons MB, Buckingham JA, Grau E, Abuin A, Chapman H, Clapham JC & Brand MD (2002). The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein‐3. J Biol Chem 277, 2773–2778. [DOI] [PubMed] [Google Scholar]
- Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik‐Bogoslavski D, Vetrivelan R, Clish CB, Robinson AJ, Gygi SP & Spiegelman BM (2016). Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo AG, Stock MJ, Solinas G, Boss O, Montani JP & Seydoux J (2002). Leptin directly stimulates thermogenesis in skeletal muscle. FEBS Lett 515, 109–113. [DOI] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, & Kozak LP (1997). Mice lacking mitochondrial uncoupling protein are cold‐sensitive but not obese. Nature 387, 90–94. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B & Nedergaard J (2009). UCP1 ablation induces obesity and abolishes diet‐induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9, 203–209. [DOI] [PubMed] [Google Scholar]
- Frisard MI, Wu Y, McMillan RP, Voelker KA, Wahlberg KA, Anderson AS, Boutagy N, Resendes K, Ravussin E & Hulver MW (2015). Low levels of lipopolysaccharide modulate mitochondrial oxygen consumption in skeletal muscle. Metabolism 64, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong DW, Monemdjou S, Gavrilova O, Leon LR, Marcus‐Samuels B, Chou CJ, Everett C, Kozak LP, Li C, Deng C, Harper ME & Reitman ML (2000). Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein‐3. J Biol Chem 275, 16251–16257. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Saadat KS, Elliott JM & Colado MI (2005). Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol 146, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke RM, Rial E, Scott ID & Nicholls DG (1982). Fatty acids as acute regulators of the proton conductance of hamster brown‐fat mitochondria. Eur J Biochem 129, 373–380. [DOI] [PubMed] [Google Scholar]
- Lombardi A, Moreno M, de Lange P, Iossa S, Busiello RA & Goglia F (2015). Regulation of skeletal muscle mitochondrial activity by thyroid hormones: focus on the “old” triiodothyronine and the “emerging” 3,5‐diiodothyronine. Front Physiol 6, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB (1999). Uncoupling protein‐3 (UCP3): a mitochondrial carrier in search of a function. Int J Obes Relat Metab Disord 23 Suppl 6, S43–45. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S & Harper ME (2011). Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem 286, 21865–21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias A, Jacobsson A, Cannon B & Nedergaard J (1999). The bioenergetics of brown fat mitochondria from UCP1‐ablated mice. UCP1 is not involved in fatty acid‐induced de‐energization (“uncoupling”). J Biol Chem 274, 28150–28160. [DOI] [PubMed] [Google Scholar]
- Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J & Cannon B (2000). Thermogenic responses in brown fat cells are fully UCP1‐dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid‐induced thermogenesis. J Biol Chem 275, 25073–25081. [DOI] [PubMed] [Google Scholar]
- Mills EM, Banks ML, Sprague JE & Finkel T (2003). Pharmacology: uncoupling the agony from ecstasy. Nature 426, 403–404. [DOI] [PubMed] [Google Scholar]
- Mills EM, Rusyniak DE & Sprague JE (2004). The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4‐methylenedioxymethamphetamine. J Mol Med (Berl) 82, 787–799. [DOI] [PubMed] [Google Scholar]
- Nau K, Fromme T, Meyer CW, von Praun C, Heldmaier G & Klingenspor M (2008). Brown adipose tissue specific lack of uncoupling protein 3 is associated with impaired cold tolerance and reduced transcript levels of metabolic genes. J Comp Physiol B 178, 269–277. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Bernson VS & Heaton GM (1978). The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl 32, 89–93. [DOI] [PubMed] [Google Scholar]
- Okamatsu‐Ogura Y, Kitao N, Kimura K & Saito M (2007). Brown fat UCP1 is not involved in the febrile and thermogenic responses to IL‐1beta in mice. Am J Physiol Endocrinol Metab 292, E1135–1139. [DOI] [PubMed] [Google Scholar]
- Okla M, Wang W, Kang I, Pashaj A, Carr T & Chung S (2015). Activation of Toll‐like receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. J Biol Chem 290, 26476–26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer M, Kayzer EB, Yang X, Abramson E, Kenaston MA, Lago CU, Lo HH, Sedensky MM, Lunceford A, Clarke CF, Wu SJ, McLeod C, Finkel T, Morgan PG & Mills EM (2011). Caenorhabditis elegans UCP4 protein controls complex II‐mediated oxidative phosphorylation through succinate transport. J Biol Chem 286, 37712–37720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial E, Poustie A & Nicholls DG (1983). Brown‐adipose‐tissue mitochondria: the regulation of the 32000‐Mr uncoupling protein by fatty acids and purine nucleotides. Eur J Biochem 137, 197–203. [DOI] [PubMed] [Google Scholar]
- Rowland LA, Bal NC, Kozak LP & Periasamy M (2015). Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem 290, 12282–12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak DE, Banks ML, Mills EM & Sprague JE (2004). Dantrolene use in 3,4‐methylenedioxymethamphetamine (ecstasy)‐mediated hyperthermia. Anesthesiology 101, 263; author reply 264. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE & Sprague JE (2005). Toxin‐induced hyperthermic syndromes. Med Clin North Am 89, 1277–1296. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC & Periasamy M (2013). Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem 288, 6881–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Alavez M, Conti B, Wood MR, Bortell N, Bustamante E, Saez E, Fox HS & Marcondes MC (2013). ROS and sympathetically mediated mitochondria activation in brown adipose tissue contribute to methamphetamine‐induced hyperthermia. Front Endocrinol (Lausanne) 4, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina IG, Hoeks J, Kramarova TV, Schrauwen P, Cannon B & Nedergaard J (2010). Cold tolerance of UCP1‐ablated mice: a skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up‐regulation not associated with increased basal, fatty acid‐ or ROS‐induced uncoupling or enhanced GDP effects. Biochim Biophys Acta 1797, 968–980. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Mallett NM, Rusyniak DE & Mills E (2004). UCP3 and thyroid hormone involvement in methamphetamine‐induced hyperthermia. Biochem Pharmacol 68, 1339–1343. [DOI] [PubMed] [Google Scholar]
- Vidal‐Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM & Lowell BB (2000). Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem 275, 16258–16266. [DOI] [PubMed] [Google Scholar]
- Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D, Paradies E, Scarcia P, Palmieri F, Bouillaud F & Fiermonte G (2014). UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A 111, 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth RP, Mills EM, Ullman A, Kenaston MA, Burwell J & Sprague JE (2009). The hyperthermia mediated by 3,4‐methylenedioxymethamphetamine (MDMA, ecstasy) is sensitive to sex differences. Toxicol Appl Pharmacol 235, 33–38. [DOI] [PubMed] [Google Scholar]


