Abstract
Calorie restriction (CR) in the absence of malnutrition exerts a multitude of physiological benefits with ageing in model organisms and in humans including improvements in vascular function. Despite the well‐known benefits of chronic CR, long‐term energy restriction is not likely to be a feasible healthy lifestyle strategy in humans due to poor sustained adherence, and presents additional concerns if applied to normal weight older adults. This review summarizes what is known about the effects of CR on vascular function with ageing including the underlying molecular ‘energy‐ and nutrient‐sensing’ mechanisms, and discusses the limited but encouraging evidence for alternative pharmacological and lifestyle interventions that may improve vascular function with ageing by mimicking the beneficial effects of long‐term CR.
Keywords: arterial stiffness, CR‐mimetics, endothelial dysfunction, energy sensing, intermittent fasting
Abbreviations
- AGE
advanced glycation end product
- AMPK
AMP‐activated protein kinase
- BH4
tetrahydrobiopterin
- CR
calorie restriction
- CVD
cardiovascular diseases
- EDD
endothelium‐dependent dilatation
- eNOS
endothelial nitric oxide synthase
- IF
intermittent fasting
- MMP
matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- NFκB
nuclear factor kappa B
- NMN
nicotinamide mononucleotide
- NR
nicotinamide riboside
- NO
nitric oxide
- ROS
reactive oxygen species
- SIRT‐1
sirtuin 1
- SOD
superoxide dismutase
- TGFβ
transforming growth factor‐β
- TRF
time restricted feeding
Cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality in developed nations. Advancing age is the primary risk factor for the development of CVD, with more than 80% of all CVD‐related deaths occurring in adults above the age of 65 years (Mozaffarian et al. 2016). The burden of CVD‐related morbidity and mortality among older adults is compounded by current demographic trends of developed and developing nations towards increasingly older populations (Olshansky et al. 2009), thus setting the stage for a new epidemic of CVD in the near future if effective interventions are not established (Seals & Melov, 2014). The link between advancing age and the risk of developing CVD is largely attributable to the dysfunction of arteries (Lakatta & Levy, 2003). Although many adverse changes occur to arteries during ageing, two key expressions of arterial ageing have been most associated with risk for CVD: the development of vascular endothelial dysfunction and stiffening of the large elastic arteries (i.e. the aorta and carotid arteries) (Lakatta & Levy, 2003; Seals et al. 2011). As such, these domains of vascular function remain high‐priority therapeutic targets for delaying or reversing age‐related CVD. In this regard, lifestyle modifications including short‐ and long‐term calorie restriction (CR) have been shown to be effective at improving vascular function during ageing in animal models; however, adherence to such interventions remains poor for the general population, and may even be unsafe for normal weight middle‐aged and older adults. The purpose of this review is to summarize the primary mechanisms by which vascular dysfunction occurs with ageing, and to present what is known about the benefits and mechanisms of regular CR for reversing age‐related vascular dysfunction. Finally, several promising ‘CR‐mimicking’ strategies that have recently emerged and their potential for reducing vascular ageing will be discussed as practical alternatives to regular CR, particularly for normal weight middle‐aged and older humans.
Vascular endothelial dysfunction
The vascular endothelium is a single monolayer of cells that lines the interior lumen of all blood vessels and plays a particularly critical role in maintaining the health and function of arteries, as well as their surrounding tissues. Endothelial cells synthesize and release a wide variety of vasoactive molecules that act within the endothelium and surrounding vascular smooth muscle cells in an autocrine and/or paracrine manner to regulate arterial function and tone (Luscher & Barton, 1997; Cines et al. 1998). Among the most important of these vasoactive molecules is nitric oxide (NO), which is synthesized by endothelial nitric oxide synthase (eNOS) from the amino acid l‐arginine (Palmer et al. 1988). Endothelium‐derived NO diffuses into the surrounding smooth muscle cells, initiating a signalling cascade that results in vasodilatation. Endothelial dysfunction, characterized by a decrease in this ‘endothelium‐dependent dilation (EDD)’ in response to mechanical (e.g. shear stress associated with increased blood flow) or chemical (e.g. acetylcholine) stimuli, occurs with ageing and is primarily attributed to a reduction in endothelial NO bioavailability (Seals et al. 2011).
Mechanisms of impaired NO bioavailability with ageing
NO bioavailability declines in arteries with ageing as a result of rapid NO degradation and/or decreased NO synthesis by the endothelium (Fig. 1; top panel). The mechanism by which these two events occur is predominately the result of oxidative stress, which is defined as an increase in the bioactivity of reactive oxygen species (ROS) relative to endogenous antioxidant defences (Seals et al. 2011). The production of ROS occurs naturally during fuel oxidation by the mitochondria and is typically counterbalanced by a host of endogenous antioxidant enzymes that protect cells from excessive oxidative damage. During ageing, oxidative stress develops within endothelial cells due to a disruption of this balance in favour of excess production of the ROS superoxide by pro‐oxidant enzymes (e.g. NADPH oxidase), along with decreased abundance (or lack of compensatory increase) of endogenous anti‐oxidant enzymes (e.g. superoxide dismutase; SOD). The excessive supply of superoxide reacts quickly with NO to form the secondary free radical, peroxynitrite (ONOO−), thus reducing the bioavailability of NO and contributing further to oxidative stress. Peroxynitrite, in turn, contributes to the nitration of tyrosine residues on proteins (i.e. ‘nitrotyrosine’), thus serving as an important cellular marker of oxidative damage.
Figure 1. Primary mechanisms of vascular ageing.
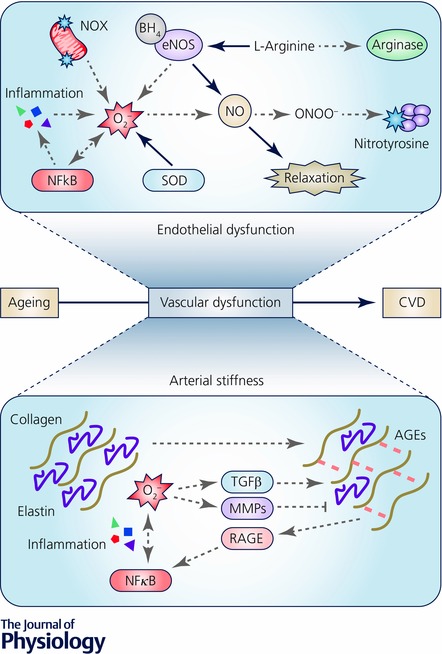
Top panel, healthy endothelial function (continuous black arrows) is characterized by adequate production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS) from the amino acid l‐arginine. Endothelial dysfunction (dashed grey arrows) occurs with ageing as a result of increased superoxide (O2 −) generation by mitochondrial NADPH oxidases (NOX), impaired superoxide scavenging by endogenous antioxidants (e.g. superoxide dismutase; SOD) and increased cytokine production via the up‐regulation of NFκB. O2 − combines with NO to form peroxynitrite (ONOO−) resulting in nitrotyrosine formation and reduced NO bioavailability. Oxidation of tetrahydrobiopterin (BH4) or depletion of l‐arginine by arginase results in eNOS uncoupling and further O2 − production. Bottom panel, large elastic artery stiffening occurs with ageing as a result of increased collagen deposition, fragmentation/loss of elastin and formation of advanced glycation end products (AGEs). Collagen deposition is mediated by activation of transforming growth factor beta (TGFβ) whereas elastin degradation occurs primarily through the activation of matrix metalloproteinases (MMPs), both of which are triggered by oxidative stress. AGEs bind to the receptor for AGEs (RAGE) resulting in exacerbation of inflammation and oxidative stress through activation of NFκB.
In addition to rapidly sequestering available NO, superoxide also reacts with tetrahydrobiopterin (BH4), an essential cofactor for NO synthesis, resulting in uncoupling of the eNOS dimer (Eskurza et al. 2005). Once uncoupled, eNOS produces less NO and instead becomes a superoxide generator itself, further perpetuating oxidative stress and contributing to endothelial dysfunction (Seals et al. 2011). The superoxide generating activity of uncoupled eNOS can also be triggered by low substrate availability, such as that which occurs during ageing due to increased expression and/or activity of the enzyme arginase, which consumes intracellular l‐arginine (Berkowitz et al. 2003).
Tightly coupled to oxidative stress, ageing is also associated with a state of chronic, low‐grade inflammation that stems from the production and release of a wide array of pro‐inflammatory cytokines, chemokines and other factors that interact with the endothelium and contribute to endothelial dysfunction (Donato et al. 2015). Within endothelial cells, activation of the pro‐inflammatory transcription factor nuclear factor kappa B (NFκB) with ageing initiates the transcription of several downstream mediators that induce or sustain endothelial oxidative stress and promote further inflammation (Walker et al. 2014 b). The combined accumulation of excess free radicals and pro‐inflammatory cytokines with ageing contributes to a state of stress that prematurely drives endothelial cells into cellular senescence – the irreversible arrest of cell proliferation – which has been implicated as an important contributor to endothelial dysfunction and age‐related CVD (Donato et al. 2015). Senescent cells not only demonstrate increased oxidative stress and inflammation, but also a senescence‐associated secretory phenotype (SASP), characterized by the secretion of damaging free radicals and inflammatory cytokines that act upon neighbouring non‐senescent cells to spread dysfunction. Collectively, these mechanisms perpetuate a positive feedback cycle that promotes a dysfunctional and pro‐atherogenic endothelium during ageing and greatly increases the risk for developing CVD.
Large elastic artery stiffening
In addition to endothelial dysfunction, stiffening of the large elastic arteries occurs with ageing, in large part as a result of structural changes within the arterial wall (Fig. 1; bottom panel) (Fleenor, 2013). The key structural components of arteries include the extracellular matrix proteins collagen and elastin. Type I collagen fibres confer tensile strength to arteries, allowing them to withstand large increases in pressure such as those that are produced by the systolic ejection of blood during each cardiac cycle. In contrast, elastin fibres provide arteries with the ability to distend and recoil in response to an increase in pressure, ensuring a continuous flow of blood to organs and tissues and protecting more sensitive downstream arterioles and capillaries from large pulse pressures. A critical balance between collagen and elastin is necessary to maintain proper structure and function of the large elastic arteries. During ageing, the composition of these arteries shifts towards an increase in collagen deposition and fragmentation of elastin fibres resulting in the loss of distensibility and a net increase in arterial stiffness (Fleenor, 2013). In humans, large elastic artery stiffness is assessed in vivo by measuring carotid–femoral aortic pulse wave velocity in which a faster traveling pressure wave is indicative of a stiffer aorta, or by assessing the compliance (change in diameter) of the carotid arteries with respect to a change in arterial pressure (pulse pressure).
In the carotid arteries, age‐related collagen deposition is reversed following short‐term treatment with the superoxide dismutase mimetic TEMPOL, whereas cultured aortic fibroblasts treated with the superoxide generator pyrogallol demonstrate increased collagen abundance, suggesting that excess collagen deposition is related to oxidative stress (Fleenor et al. 2010, 2012). Additionally, increases in the cytokine transforming growth factor β (TGFβ) have been implicated in the regulation of collagen deposition through a mechanism involving NADPH oxidase‐derived superoxide (Fleenor et al. 2010). Interestingly, TEMPOL supplementation does not reverse age‐related elastin degradation (Fleenor et al. 2012; Fleenor, 2013); however, elastin abundance is significantly attenuated in SOD‐deficient mice and is associated with increased matrix metalloproteinase (MMP) expression (Zhou et al. 2012). Thus, these findings suggest that ROS may be important signalling molecules for the regulation of arterial elastin.
Ageing is also associated with the formation of advanced glycation end products (AGEs) within the arterial wall, a byproduct of the non‐enzymatic glycation of proteins, lipids and nucleic acids. In addition to forming cross‐links between collagen and elastin fibres, AGEs interact with the receptor for AGEs (RAGE), which activates a pro‐inflammatory signalling cascade via the stimulation of NFκB that induces further inflammation and oxidative stress, and perpetuates arterial stiffness, in part, by promoting the further production of AGEs (Lin et al. 2009). Finally, in addition to the structural changes that contribute to arterial stiffness, decreases in autocrine/paracrine signalling molecules such as NO lead to an increase in arterial tone and impart a ‘functional stiffness’ to arteries during ageing.
Energy sensing pathways and vascular ageing
Considerable overlap exists in the mechanisms that mediate endothelial dysfunction and large artery stiffening with ageing, both of which are largely attributable to oxidative stress and inflammation. Although many factors contribute, the increases in oxidative stress and inflammation appear to be strongly modulated by age‐related reductions in cellular energy homeostasis. The maintenance of cellular energy balance occurs through several key ‘energy‐ and nutrient‐sensing’ pathways that become less efficient during ageing and, as such, have been implicated in the development of age‐related physiological dysfunction, including vascular ageing (Fig. 2). The exact role of these energy and nutrient sensors in the maintenance of vascular homeostasis has not been completely established, with very few studies conducted in humans. It is likely, however, that these pathways represent an evolutionarily conserved mechanism for ensuring that blood flow is appropriately matched to the metabolic demand of tissues. These energy‐ and nutrient‐sensing pathways and their role in mechanisms of vascular ageing are summarized below.
Figure 2. Effect of energy‐ and nutrient‐sensing pathways on vascular function during ageing and calorie restriction.
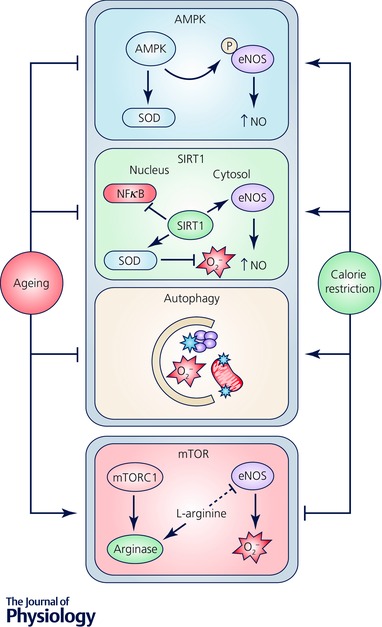
Vascular function is responsive to changes in cellular energy status through interaction with evolutionarily conserved energy‐ and nutrient‐sensing pathways. For example, AMP‐activated protein kinase (AMPK) phosphorylates eNOS resulting in increased NO synthesis and activates superoxide dismutase (SOD) leading to reduced oxidative stress. Sirtuin‐1 (SIRT‐1) directly activates eNOS in the cytosol and regulates gene expression in the nucleus that reduces oxidative stress and inflammation. Autophagy removes damaged cellular constituents that promote oxidative stress and inflammation. mTOR activation decreases NO production and triggers O2 − generation by eNOS through an upregulation of arginase. Ageing and calorie restriction have opposite effects on these cellular processes and modulate vascular function by regulating oxidative stress and inflammation, thereby influencing all of the mechanisms of vascular dysfunction depicted in Fig. 1.
AMP‐activated protein kinase
The primary source of energy in eukaryotic cells comes from the breakdown of adenosine triphosphate (ATP) into adenosine diphosphate (ADP) and inorganic phosphate in the mitochondria. The rate of mitochondrial ATP synthesis is tightly matched to ATP consumption over the long term in order to maintain energy balance and sustain normal cellular processes; however, during periods of low energy availability, cells replenish ATP stores more rapidly via the enzymatic coupling of two ADP molecules in order to preserve the activity of processes that are critical for cell survival. Adenosine monophosphate (AMP) is the key byproduct of this reaction and serves as an important signalling molecule for detecting low cellular energy status via its activation of AMP‐activated protein kinase (AMPK). Often described as a master regulator of metabolism, AMPK acts by phosphorylating downstream protein targets that restore cellular energy balance (Canto & Auwerx, 2011). In general, AMPK preserves cellular energy homeostasis by activating catabolic pathways such as glucose transport, glycolysis and fatty acid oxidation, and by suppressing anabolic pathways such as fatty acid synthesis, cholesterol formation and protein synthesis.
Despite its critical role in regulating energy homeostasis during ageing, relatively little is known about the role of AMPK in arterial ageing. The available literature, primarily from animal studies, suggests that AMPK plays a protective role in the vasculature by maintaining eNOS activation via phosphorylation of eNOS at serine‐1177, and triggering the expression of endogenous antioxidants that limit the NO‐reducing effects of superoxide (Fig. 2) (Fisslthaler & Fleming, 2009). The activation of AMPK is reduced in the vasculature with ageing, suggesting that loss of AMPK signalling is a potential contributor to age‐related vascular dysfunction (Lesniewski et al. 2012). However, more translational work is necessary to determine the specific role of impaired AMPK signalling in vascular ageing.
Sirtuins
The mammalian sirtuins are a family of nicotinamide adenine dinucleotide (NAD+)‐dependent deacetylases and ADP‐ribosyltransferases that respond to a decrease in the cellular NAD:NADH ratio by activating energy preserving and stress resistance pathways that protect cells from energetic failure. Of the seven mammalian sirtuin enzymes that have been identified, sirtuin‐1 (SIRT‐1) is widely recognized to be the most important with regard to vascular ageing. SIRT‐1 is capable of translocating between the cytosol and nucleus of endothelial cells, thus acting through genomic and non‐genomic mechanisms to regulate vascular function. In the cytosol, SIRT‐1 directly deacetylates eNOS, leading to an increase in NO synthesis (Mattagajasingh et al. 2007), whereas inhibition of SIRT‐1 reduces NO‐mediated vasodilatation in conduit arteries (Mattagajasingh et al. 2007; Donato et al. 2011) and impairs cerebral resistance artery function (Tajbakhsh & Sokoya, 2012). These findings suggest that SIRT‐1‐mediated control of NO synthesis plays an important role in the regulation of vascular function (Fig. 2). Interestingly, eNOS itself directly increases SIRT‐1 activity, resulting in a positive feedback loop that improves vascular function (Potente & Dimmeler, 2008; Ota et al. 2008).
In the nucleus, activation of SIRT‐1 indirectly increases NO bioavailability by modulating the gene expression of proteins responsible for the maintenance of NO synthesis and scavenging of NO‐degrading free radicals. One key example of this is deacetylation of the forkhead box O (FOXO) transcription factors FOXO1 and FOXO3a, which leads to increased eNOS mRNA expression (Xia et al. 2013) and up‐regulation of several antioxidant enzymes including manganese superoxide dismutase (MnSOD), catalase, peroxiredoxins, thioredoxins and uncoupling protein 2 (UCP‐2) that protect endothelial cells from oxidative stress‐mediated reductions in NO bioavailability (Olmos et al. 2013). Additionally, SIRT‐1 activation reduces the transcription of genes that contribute to oxidative stress and inflammation such as the pro‐inflammatory transcription factor NFκB.
Arterial SIRT‐1 abundance and activity typically decline with ageing and contribute to vascular dysfunction through a decrease in eNOS activity and increased endothelial cell senescence (Donato et al. 2011; Bai et al. 2014). Accumulation of acetylated eNOS occurs in the arteries of old mice and is associated with a decline in eNOS phosphorylation, a key marker of eNOS activation, and reduced NO bioavailability (Donato et al. 2011). Likewise, translational evidence in brachial artery endothelial cells biopsied from older humans confirms the loss of SIRT‐1 with ageing and demonstrates a positive association between SIRT‐1 levels and endothelium‐dependent dilatation of the forearm microvasculature (Donato et al. 2011). Taken together, these findings suggest that SIRT‐1 is an important regulator of vascular function in animal models and its impairment with ageing contributes to the vascular ageing phenotype.
Mammalian target of rapamycin
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that integrates multiple signals related to cellular energy and nutrient availability to regulate cell growth and metabolism (Ming et al. 2012). The discovery that inhibition of mTOR leads to increased lifespan in model organisms such as yeast, flies and C. elegans has sparked interest in the role of mTOR signalling in the regulation of ageing and age‐related diseases. In this regard, increased mTOR signalling has been implicated in the pathogenesis of several age‐related diseases such as type II diabetes mellitus and cancer (Zoncu et al. 2011); however, its potential role in mediating age‐related vascular dysfunction and CVD risk is less established. Two structurally and functionally distinct complexes of mTOR have been identified, mTORC1 and mTORC2. While considerably less is known about the role of mTORC2 in the context of vascular ageing, the age‐related activation of mTORC1 appears to contribute to decreased NO synthesis with ageing (Rajapakse et al. 2011).
mTORC1 activity is predominantly regulated by the availability of nutrients, particularly amino acids; however, it remains unclear whether mTORC1 itself is a direct nutrient sensor, as the presence of insulin and insulin‐like growth factor (IGF‐1) appear to be necessary for mTORC1 activation in higher organisms (Xu et al. 2014). In contrast to the AMPK and SIRT‐1 energy sensing systems, mTOR activity is inhibited by decreases in nutrient availability in order to conserve energy reserves by preventing protein synthesis and cellular growth. Conversely, a nutrient abundance triggers an increase in cell growth and protein synthesis through the activation of mTORC1 and its downstream effector, ribosomal S6 protein kinase (S6K1) (Ming et al. 2012). Although the total abundance of arterial S6K1 does not appear to change with ageing in mice, its phosphorylation status (an indicator of mTOR/S6K1 activation) is increased in old mice that exhibit age‐related vascular dysfunction (Lesniewski et al. 2016). The mechanisms by which increases in mTOR/S6K1 activity contribute to arterial ageing are incompletely understood; however, the mTOR‐dependent increase in arginase activity has been shown to play an important role (Fig. 2). Consumption of l‐arginine by arginase decreases the substrate availability for NO synthesis and results in increased superoxide generation through eNOS uncoupling (Yepuri et al. 2012). This mechanism may contribute to the vascular ageing phenotype as indicated by increases in the abundance and activity of both mTORC1 and arginase II in senescent endothelial cells and aortas of aged mice and rats, along with increased superoxide generation and reduced NO production (Rajapakse et al. 2011; Yepuri et al. 2012; Donato et al. 2013). Future work is necessary to confirm the role of the mTORC1 complex in mediating vascular ageing in both animal models and humans.
Cellular homeostasis, stress resistance and autophagy
The cellular energy‐ and nutrient‐sensing pathways discussed above are not mutually exclusive but rather work synergistically to detect and respond to fluctuations in energy status and thereby maintain cellular homeostasis. One important mechanism by which energy‐sensing pathways converge is through the process of autophagy, an internal cellular recycling system that triggers the lysosomal removal of damaged organelles and other cellular constituents, and mobilizes available energy stores to preserve critical cellular functions (Rubinsztein et al. 2011). Like energy sensing pathways themselves, the process of autophagy is evolutionarily conserved across multiple organisms and as such, there is considerable cross‐talk among energy sensing pathways and autophagic signalling. Indeed, decreased macronutrient availability is one of the most common triggers for autophagy activation, in part through the activation of AMPK and SIRT‐1, and inhibition of mTOR signalling (Rubinsztein et al. 2011). Thus, modulation of these pathways may protect against endothelial dysfunction and arterial stiffening by increasing autophagy and reducing oxidative stress and inflammation (Fig. 2). Likewise, direct activation of autophagy may compensate for impaired signalling of the energy and nutrient sensors and lead to improvements in vascular function.
Preservation of vascular function by calorie restriction
Favourable modulation of these cellular energy‐ and nutrient‐sensing pathways and activation of autophagy can be achieved through regular calorie restriction, which has often been referred to as the ‘gold‐standard’ intervention for slowing the rate of biological ageing due to its strong association with lifespan extension in model organisms (Chung et al. 2013). Both lifelong and shorter duration CR have been linked to decreased CVD risk factors with ageing, including markers of glucose/insulin sensitivity, circulating cholesterol and triglycerides, and central body fat and blood pressure (Ungvari et al. 2008; Cruzen & Colman, 2009). However, many of the reductions in CVD risk associated with CR are likely to stem from improvements in vascular function that occur secondary to the modulation of energy‐ and nutrient‐sensing pathways. A summary of the beneficial effects of lifelong and shorter duration CR on these mechanisms and their impact on vascular function during ageing is presented below.
Lifelong calorie restriction and vascular ageing
Forty per cent CR (compared with ad libitum‐fed control animals) over the entire lifespan prevents age‐related changes in endothelial function in the conduit arteries of rodents through a mechanism involving the maintenance of NO bioavailability (Donato et al. 2013) secondary to the prevention of arterial oxidative stress and inflammation (Csiszar et al. 2009; Donato et al. 2013). In contrast with age‐matched ad libitum‐fed control animals, lifelong CR prevents the age‐associated increase in arterial oxidative stress in mice by reducing the activity of NADPH oxidases and increasing endogenous antioxidant defences. Additionally, lifelong CR increases the protein expression of eNOS within arteries to levels that exceed young control animals, suggesting that CR not only preserves, but also enhances arterial eNOS when sustained throughout the lifespan (Donato et al. 2013). Increased NO bioavailability with CR is thought to be mediated, at least in part, by increased abundance and activity of arterial SIRT‐1, which improves NO production by deacetylating eNOS and increasing the expression of several endogenous antioxidants. Lifelong CR may also influence endothelial function by preventing the age‐related increase in arterial mTOR activity in the absence of any obvious effect on total mTOR protein expression (Donato et al. 2013).
It is important to note that the preservation of NO‐mediated dilatation by CR may be more beneficial in specific vascular beds as NO‐mediated vasodilatation of the cerebral vasculature is only modestly improved by lifelong CR (Walker et al. 2014 a). Nevertheless, lifelong CR improves cognitive function in mice and in humans (Witte et al. 2009; Kuhla et al. 2013) and may be linked to improvements in cerebrovascular function via other mechanisms, such as an increase in endothelium‐derived hyperpolarizing factors (EDHFs) within the cerebral arteries, or reductions in arterial stiffness as these have been linked to both cerebrovascular function and cognitive function (Wahl & Schilling, 1993; Singer et al. 2014). Interestingly, the effects of lifelong CR on endothelial function appear to extend beyond changes that arise within the vasculature itself. For example, incubating endothelial cells with serum from rats that undergo lifelong CR decreases markers of oxidative stress and inflammation and increases SIRT‐1 activity (Csiszar et al. 2009), suggesting the possible involvement of circulating neuroendocrine factors that help protect the endothelium (and presumably other tissues) from age‐related dysfunction in order to preserve blood flow metabolic coupling.
Lifelong CR also prevents stiffening of the large elastic arteries in rodents, primarily by preventing age‐related increases in collagen deposition (Ahmet et al. 2005; Donato et al. 2013) and elastin degradation (Donato et al. 2013). In mice, lifelong CR prevents the increase in expression of MMP‐9, a key enzyme responsible for elastin degradation (Donato et al. 2013). This has also been attributed to SIRT‐1 activity as acetylation of the MMP‐9 gene promoter region at the NFκB binding site enhances MMP‐9 transcription while direct acetylation of NFκB at lysine residue 310 increases its affinity for the MMP‐9 promoter, resulting in increased MMP‐9 expression and resultant adverse changes to the arterial extracellular matrix (Nakamaru et al. 2009). The direct and indirect mechanisms by which acetylation augments NFκB activity and MMP‐9 expression are inhibited by the overexpression of the SIRT‐1 deacetylase enzyme in isolated macrophages, a major source of MMP‐9 secretion into vascular tissue (Yabluchanskiy et al. 2013). Thus, increased SIRT‐1 deacetylase activity, such as that which occurs during lifelong CR, is associated with a reduction in NFκB acetylation and corresponding deactivation of MMP‐9, likely contributing to the prevention of adverse changes to the extracellular matrix and consequent reduced arterial stiffening induced by lifelong CR (Donato et al. 2013). Finally, the observed benefits of lifelong CR on NO bioavailability in animal models may also contribute to reductions in arterial stiffness by reducing vascular tone and decreasing the ‘functional stiffness’ of arteries (Safar et al. 2003).
Short‐term calorie restriction and vascular ageing
Although studies of lifelong CR have been useful in shaping our understanding of the physiological mechanisms by which CR prevents vascular ageing, very few community‐dwelling humans are capable of sustaining lifelong energy restriction. Fortunately, similar beneficial effects on vascular function have been shown to occur with shorter duration CR. In this regard, late‐life initiation of ∼30% CR for 3 or 8 weeks completely normalized endothelial function in rats and mice, respectively (Zanetti et al. 2010; Rippe et al. 2010). However, whereas 8 weeks of CR resulted in the activation of SIRT‐1 and improved eNOS protein abundance in mouse aortas (Rippe et al. 2010), these mechanisms did not appear to be altered in rats after only 3 weeks of CR, despite similar reductions in oxidative stress and improvements in endothelial function (Zanetti et al. 2010). Although species‐related differences and other methodological influences cannot be ruled out, it is possible that a reduction in oxidative stress by short‐term CR may precede alterations in energy sensing pathways (e.g. SIRT‐1) and NO synthesis that lead to sustained improvements in endothelial function that are more reminiscent of the effects of lifelong CR. Thus, more work is needed to identify the most efficacious duration and magnitude of CR in order to maximize the benefits of shorter‐term CR.
Limited translational evidence exists to support the efficacy of short term CR during healthy ageing in humans; however, some insight can be gained from intentional weight loss studies. In this regard, a ∼30% reduction in energy intake over 12 weeks, without any increase in physical activity, improved conduit and resistance artery endothelium‐dependent dilatation (endothelial function) (Pierce et al. 2008) and reversed vascular stiffness (Dengo et al. 2010) in overweight or obese middle‐aged and older adults. Whether similar improvements could be achieved in normal weight older adults remains unclear as improvements in endothelial function in these weight loss studies appeared to occur independent of age (Pierce et al. 2008) and were related to the degree of reduction in abdominal adiposity (Dengo et al. 2010) suggesting that different factors may contribute to improvements in vascular function in the setting of weight loss compared with healthy ageing.
Practical alternatives to caloric restriction
Despite the potential benefits of CR for improving vascular function with ageing, the majority of older adults are not willing to practice sustained CR. Aside from poor adherence, sustained CR poses a potential risk to normal weight older adults who are already prone to age‐associated loss of bone density and lean muscle mass. Indeed, recent findings from the NIH‐sponsored Comprehensive Assessment of Long‐term Effects of Reducing Intake of Energy (CALERIE) trial suggest that 2 years of sustained reductions in energy intake by 25% leads to a decline in bone mineral density at clinically relevant sites of osteoporotic fractures (Villareal et al. 2016). Likewise, it has been suggested that reducing energy intake may exacerbate the age‐associated loss of skeletal muscle mass in older adults (Miller & Wolfe, 2008). Thus, the vascular benefits of CR may be outweighed by these potentially harmful side effects in normal weight older adults and as such, more practical alternative strategies are necessary to reduce vascular dysfunction and CVD risk with ageing. To capitalize on the positive effects of CR while limiting the risk of adverse side effects, considerable attention has been devoted over the last decade to the development of ‘CR‐mimicking’ strategies including both pharmacological/nutraceutical (Fig. 3) and lifestyle based approaches (Fig. 4) (Ingram et al. 2006; Mattson et al. 2014). A summary of the most promising strategies for delaying or reversing vascular ageing are presented below.
Figure 3. Pharmacological and nutraceutical strategies (interventions) for mimicking regular calorie restriction and preventing vascular ageing.
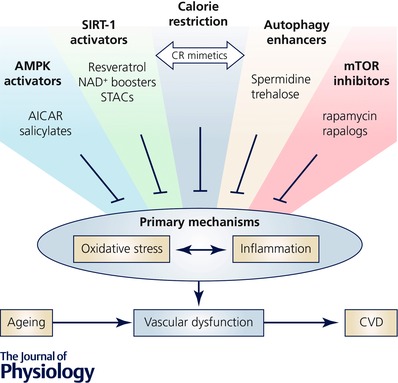
Vascular ageing is an important intermediary event linking ageing to increased risk of cardiovascular diseases (CVD). The primary mechanisms of vascular ageing include oxidative stress and chronic low‐grade inflammation. Regular calorie restriction (CR) prevents or reverses vascular ageing, primarily by inhibiting oxidative stress and inflammation; however, sustained CR is not suitable for normal weight middle‐aged and older adults. As such, a number of pharmacological and nutraceutical strategies have emerged for preventing or reversing vascular ageing by mimicking the beneficial effects of CR, primarily through the modulation of cellular energy‐ and nutrient‐sensing pathways. Examples of such strategies include: (1) activation of adenosine monophosphate (AMP)‐activated protein kinase (AMPK) with aminoimidazole carboxamide ribonucleotide or salicylates; (2) activation of the deacetylase sirtuin‐1 (SIRT‐1) with resveratrol, nicotinamide adenine dinucleotide (NAD+) boosting compounds (e.g. nicotinamide mononucleotide and nicotinamide riboside) and small molecule sirtuin activators (STACs); (3) supplementation with enhancers of cellular autophagy (e.g. spermidine and trehalose); and (4) inhibition of the mammalian target of rapamycin (mTOR) using rapamycin or its derivatives (i.e. ‘rapalogs’). These compounds target key energy‐ and nutrient‐sensing pathways involved in the maintenance of vascular homeostasis and therefore represent possible alternative strategies to conventional CR for lowering CVD risk in middle‐aged and older adults.
Figure 4. Overview of popular intermittent fasting paradigms.
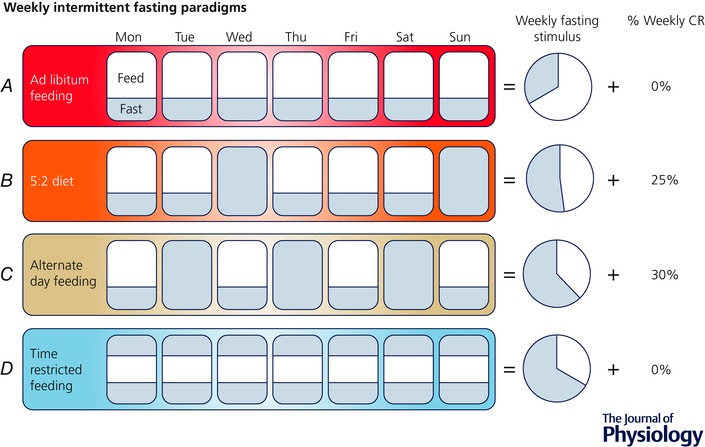
Compared with daily ad libitum feeding (A), the 5:2 diet (B) involves eating ad libitum 5 days of the week and fasting or restricting food intake for two days; this results in an increased fasting stimulus vs. ad libitum intake, but a net reduction in caloric intake over the long‐term. Alternate day feeding (C) involves alternating between days of normal feeding and days of either complete dietary restriction or minimal food intake; this paradigm results in an even greater fasting stimulus, but, as with the 5:2 approach, is associated with long‐term CR that may be particularly detrimental to normal weight older adults. Time restricted feeding (D) may represent the most practical strategy for normal weight older adults in that it allows one to consume all of the required calories within a condensed daily feeding window (e.g. 8 h), resulting in the greatest fasting stimulus without a net reduction in calorie intake.
CR‐mimicking compounds
AMPK activators
Despite the well‐characterized role of AMPK as a central regulator of metabolism and energy balance during CR, much less is known about the efficacy of pharmacological AMPK activators as CR‐mimetics for improving vascular function during ageing. Short‐term treatment with daily, subcutaneous injections of the AMPK agonist aminoimidazole carboxamide ribonucleotide (AICAR), decreases oxidative stress and restores endothelium‐dependent dilatation in old mice; however, in contrast to lifelong and shorter duration CR, the improvement in endothelial function does not appear to be dependent upon an increase in NO synthesis (Lesniewski et al. 2012). Alternatively, the anti‐inflammatory drug salicylate also enhances AMPK activity (McCarty, 2014) and has been linked to improved endothelial function with ageing through a mechanism involving decreased inflammation and enhanced NO bioavailability (Lesniewski et al. 2011). Although AMPK expression and activity were not measured in this study, treatment with salicylate enhanced the phosphorylation of eNOS at ser‐1177, the same serine residue that is phosphorylated by AMPK during CR (Chen et al. 1999; Lesniewski et al. 2011). These preclinical findings are supported by translational evidence from both overweight/obese and normal weight older adults in which treatment with the anti‐inflammatory drug salsalate improved endothelial function (Pierce et al. 2009; Walker et al. 2014 b). Taken together, these results suggest that AICAR and salicylates may be effective pharmacological strategies for improving vascular function during ageing; however, it remains unclear whether the direct stimulation of AMPK alone is enough to target the same underlying mechanisms of vascular ageing as CR, as these drugs affect other pathways such as inhibition of the inflammatory transcription factor NFκB (Pierce et al. 2009; Walker et al. 2014 b). Interestingly, AMPK activation appears to be at least partly mediated by SIRT‐1 activation, suggesting possible synergy amongst the two energy‐sensing systems.
Sirtuin activators
One of the most well studied compounds with regard to its putative role as a CR mimetic is the naturally occurring polyphenol resveratrol (3,5,4′‐trihydroxystilbene). Present in high abundance in red wine, grapes and other berries, considerable interest in the CR‐mimicking properties of resveratrol was generated after it was found to lower the amount of NAD+ required for Sir2 activity (the yeast homologue of SIRT‐1) and extend lifespan in Saccharomyces cerevisiae (Howitz et al. 2003). Supplementation with resveratrol has since been linked to a wide variety of physiological benefits in mice that are characteristic of CR such as improved insulin sensitivity, mitochondrial biogenesis and AMPK activation (Baur, 2010). Likewise, resveratrol treatment appears to induce a similar gene expression profile in mice to conventional CR (Pearson et al. 2008).
Resveratrol has been suggested to be a potential mediator of the ‘French Paradox’, an unexplained phenomenon in which individuals living in a particular region of France who consume a moderate amount of red wine with meals have a lower incidence of CVD, despite leading an otherwise unhealthy lifestyle (Wu et al. 2001). While this hypothesis has not been conclusively tested, resveratrol treatment increases the expression and activity of eNOS in cultured endothelial cells through a mechanism involving SIRT‐1 (Wallerath et al. 2002; Xia et al. 2013) and may therefore lower CVD risk in part by preserving NO‐associated endothelial health with ageing. In this regard, supplementation with either red wine or resveratrol improved endothelium‐dependent relaxation in aortic rings of middle‐aged (da Luz et al. 2012) and older (Pearson et al. 2008) rodents fed a normal chow diet. These findings were associated with enhanced eNOS gene expression and reduced superoxide generation by NADPH oxidase (Pearson et al. 2008) suggesting that resveratrol targets the same mechanisms of age‐related endothelial dysfunction as lifelong and shorter duration CR. The exact mechanisms by which resveratrol reduces NADPH oxidase activity in the vasculature remain to be fully elucidated; however, SIRT‐1‐mediated suppression of NFκB and activation of PGC‐1α in vascular endothelial cells are likely to play an important role as activation of these proteins has been shown to activate or suppress NADPH oxidase expression, respectively (Csiszar et al. 2006; Kim et al. 2007).
Translational evidence exists in humans with hypertension and dyslipidaemia, in which treatment of arterial segments with resveratrol improved vascular function through a mechanism involving AMPK‐mediated activation of eNOS, increased bioavailability of BH4 and the lowering of oxidative stress via the induction of the superoxide scavenging antioxidant manganese (Mn) SOD (Carrizzo et al. 2013). Likewise, acute and chronic administration of resveratrol enhanced endothelial function in overweight or obese middle‐aged and older adults with or without hypertension (Wong et al. 2011, 2013), respectively. Supplementation with resveratrol also prevented the increase in aortic stiffness caused by a high‐fat and high‐sucrose diet in non‐human primates through a mechanism involving decreased NFκB‐mediated oxidative stress and inflammation (Mattison et al. 2014). Limited evidence exists regarding the efficacy of resveratrol supplementation for improving vascular function in older adults. In this regard, resveratrol improved endothelial function in overweight and obese older adults with impaired glucose tolerance (Crandall et al. 2012); however, the efficacy of resveratrol supplementation for treating age‐related vascular dysfunction in healthy, normal weight adults remains uncertain.
Careful consideration must be taken with regard to the effects of resveratrol and similar polyphenol compounds in combination with other healthy lifestyle practices in humans. Interestingly, resveratrol supplementation appears to blunt several of the positive adaptations to aerobic exercise training in older adults, including the exercise‐induced upregulation of endogenous antioxidant enzymes (Gliemann et al. 2013). Likewise, resveratrol supplementation increased oxidative stress and decreased aortic distensibility (indicative of an increase in arterial stiffness) in older mice fed a high protein diet designed to mimic that prescribed to elderly adults to prevent age‐related malnutrition (Baron et al. 2014). Thus, more research is needed to understand the role of resveratrol and other polyphenol compounds for ameliorating vascular ageing.
Many of the potentially adverse consequences of resveratrol supplementation may be associated with off‐target effects, including the ability of resveratrol to act as a direct antioxidant and therefore blunt the hormetic response of aerobic exercise to stimulate the expression of endogenous antioxidants (Santos‐Parker & Kaplon, 2014). As such, there has been considerable interest in the development of more specific small molecule SIRT‐1 activating compounds (STACs) for treating age‐related diseases (Hubbard & Sinclair, 2014). In this regard, 4 weeks of treatment with SRT1720 (Sitris Pharmaceuticals/GlaxoSmithKline, Cambridge, MA, USA) by oral gavage, ameliorated endothelial dysfunction in old mice through a mechanism involving increased SIRT‐1 expression and activity and normalization of oxidative stress and inflammation (Gano et al. 2014). Interestingly, the improvements in endothelial function were independent of changes in NO bioavailability but rather were associated with enhanced cyclooxygenase (COX)‐2 mediated vasodilatation. More work is needed to determine the efficacy of SR1720 and other STACs for ameliorating vascular ageing in humans.
NAD+ boosting compounds
One of the key drivers of decreased SIRT‐1 activity with ageing is a decline in the bioavailability of NAD+, the essential co‐substrate for SIRT‐1 activation (Imai, 2009). Although NAD+ has been classically described as a coenzyme involved in oxidative phosphorylation, the utilization of NAD+ by SIRT‐1 and other NAD+‐consuming enzymes involves the complete breakdown of NAD+ into nicotinamide and ADP ribose, thus requiring a continuous supply of NAD+ in order to sustain SIRT‐1 activity. To compensate for this irreversible destruction of NAD+, a salvage pathway has evolved by which nicotinamide is reconverted into NAD+ through a process involving the intermediate compounds nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) (Bogan & Brenner, 2008). Several studies have demonstrated that supplementation with NMN or NR raises tissue levels of NAD+ and extends lifespan and stress responses in preclinical models of ageing or age‐related diseases (Imai, 2010; Canto et al. 2012; Gomes et al. 2013; Gong et al. 2013). In this regard, de Picciotto et al. (2016) has recently demonstrated that supplementation with NMN in the drinking water for 8 weeks restores endothelial function and decreases arterial stiffness in old mice through a mechanism involving increased NO bioavailability and decreased oxidative stress (de Picciotto et al. 2016). Importantly, the improvements in vascular function were accompanied by an increase in SIRT‐1 activity (assessed by the acetylation status of NFκB), suggesting that NMN may be a suitable CR‐mimetic for improving vascular function with ageing. Several clinical trials investigating the effects of the alternative NAD+ boosting compound, nicotinamide riboside (NR), for improving physiological function are currently underway (www.clinicaltrials.gov) and will undoubtedly provide important insight into the feasibility and safety of these compounds for use as CR mimetics in humans.
mTOR inhibitors
The mammalian target of rapamycin (mTOR) was named for its high affinity for and inhibitory action by the anti‐fungal agent rapamycin. The discovery that rapamycin increases the lifespan of model organisms has generated considerable interest in mTOR as an ‘anti‐ageing’ target (Johnson et al. 2013), and evidence related to its role in reversing vascular ageing is beginning to emerge. In this regard, ex vivo incubation of aortic tissue from aged rats with rapamycin decreases the activity of the S6K1 complex and superoxide production, and restores NO bioavailability, supporting the role of mTOR inhibition for ameliorating age‐related endothelial dysfunction (Rajapakse et al. 2011). Recent findings in mice using an in vivo model of vascular ageing demonstrate that 6–8 weeks of dietary rapamycin supplementation lowers S6K1 activity and reverses endothelial dysfunction through a mechanism involving reduced oxidative stress, decreased arterial senescence and increased AMPK activity (Lesniewski et al. 2016). Supplementation with rapamycin also ameliorated age‐related arterial stiffening and reduced collagen deposition (Lesniewski et al. 2016). Together, these observations suggest that mTOR inhibition with rapamycin may represent a broad therapeutic strategy for mitigating vascular ageing.
Despite these promising benefits, exogenous application of rapamycin has also been associated with decreased eNOS activation and worsened endothelium‐dependent dilatation in human internal thoracic arteries obtained from middle‐aged and older adults undergoing bypass surgery (Reineke et al. 2015). Thus, it remains unclear whether mTOR inhibition with rapamycin is a viable CR‐mimetic for reversing vascular ageing in humans. Adding to this concern is a growing number of adverse side effects associated with rapamycin treatment, leading to advancements in the discovery of safer ‘rapalogs’ as anti‐ageing therapies (Lamming et al. 2013). Whether such compounds will prove effective for treating vascular ageing remains to be determined. Interestingly, resveratrol also inactivates the mTOR/S6K1 pathway (Liu et al. 2010) and appears to have similar effects as rapamycin with regard to the amelioration of age‐related endothelial dysfunction (Rajapakse et al. 2011) and thus may represent a safer strategy than mTOR inhibition for mimicking the beneficial effects of CR.
Autophagy activators
The activation of cellular autophagy may represent a critical component in transducing the beneficial effects of CR into improved physiological function. Indeed, the lifespan extending effect of CR is dependent upon the activation of several autophagy genes in C. elegans (Jia & Levine 2007) and at least part of the CR‐mimicking benefits of resveratrol are the result of enhanced autophagy (Morselli et al. 2010). With respect to vascular ageing, the protein abundance of a key autophagy marker, beclin‐1, is reduced in primary arterial endothelial cells obtained from middle‐aged and older adults compared with younger adults, and was associated with a reduction in forearm microvascular endothelial function (LaRocca et al. 2012). Evidence in aged mice suggests that oral supplementation with the autophagy‐stimulating disaccharide trehalose restores vascular autophagy and ameliorates age‐related endothelial dysfunction (LaRocca et al. 2012). Supplementation with another autophagy enhancing compound, spermidine, reduces age‐related vascular stiffness by lowering collagen deposition and reducing the formation of AGEs, and increases endothelial function by reducing oxidative stress and increasing NO bioavailability (LaRocca et al. 2013). These studies suggest that the enhancement of autophagy has a similar effect as CR on reversing vascular ageing and may be a novel therapeutic target for enhancing or bypassing aberrant energy‐sensing signalling. Future studies are warranted in order to determine the efficacy of these and other autophagy enhancing compounds for improving vascular function with ageing in humans. In this regard, initial translational insight in humans suggests that trehalose supplementation improves forearm microvascular NO bioavailability and endothelial function in older adults (Kaplon et al. 2016); however, it is unclear if this effect was mediated by activation of autophagy or some other mechanism.
CR‐mimicking lifestyle strategies
All of the energy‐ and nutrient‐sensing pathways discussed above are tightly linked to fluctuations in the circadian rhythm, presumably as a mechanism to increase cell growth and differentiation during daytime feeding periods (e.g. mTOR activation) while conserving energy and activating cellular repair pathways during night‐time fasting (e.g. AMPK, SIRT‐1 and autophagy) (Longo & Panda, 2016). Although sustained CR appears to have a tonic modulatory effect on energy‐ and nutrient‐sensing pathways, the ability of these pathways to respond rapidly to fluctuations in energy/nutrient status suggests that similar benefits may be achievable through shorter periods of energy or nutrient restriction than required by conventional CR. In this regard, more feasible lifestyle strategies that mimic the beneficial effects of CR (e.g. by acutely modulating these pathways) have emerged as potential strategies for improving physiological function. Although little is known about the effect of these strategies on vascular ageing per se, a summary of the most promising CR‐mimicking approaches and their putative mechanisms of action is given below.
Nutrient restriction
Similar benefits to conventional CR with regard to lifespan extension have been observed in model organisms in which specific macro‐ or micro‐nutrients were restricted (e.g. dietary protein or methionine restriction), as opposed to total calorie intake. The mechanism by which decreased total protein intake or dietary restriction of the amino acid methionine is linked to extension of lifespan in model organisms is thought to be mediated by the suppression of the mTOR pathway (Santos et al. 2016). In humans, reduced dietary intake of protein is associated with a reduction in all‐cause mortality in younger adults, whereas higher protein intake appears to be protective in older individuals (Levine et al. 2014). Although the specific effects of dietary protein or methionine restriction have not yet been tested with regard to vascular ageing, total protein restriction may not be suitable for older adults due to concerns of inducing malnutrition and exacerbating lean tissue loss. Interestingly, the association between protein intake and mortality is abolished when controlling for whether dietary protein is derived from plant or animal sources (Levine et al. 2014). Thus, more work is necessary to identify the exact mechanisms by which restriction of protein and or micronutrients (e.g. methionine) regulate ageing and influence arterial function in order to design more safe and effective strategies for older adults.
Periodic and intermittent fasting
A number of alternative strategies to conventional CR involving periodic or intermittent fasting have emerged for improving physiological function with ageing (Longo & Mattson, 2014).
Periodic fasting
Periodic fasting, consisting of water‐only fasting for two or more consecutive days, with fasting bouts separated by at least a week, has been shown to extend lifespan and induce stress resistance in model organisms. Although the mechanisms have not been fully elucidated in mammals, evidence from lower organisms (e.g. yeast) suggests that periodic fasting may act by suppressing the activity of the mTOR signalling pathway (Longo & Mattson, 2014). However, several studies of periodic fasting in mice have demonstrated declines in body weight that may not be suitable for normal weight older adults, and adherence to periodic fasting diets may be particularly difficult and possibly dangerous in older adults at risk of malnutrition (Wasselin et al. 2014; Brandhorst et al. 2015). As such, alternative ‘fasting mimicking diets’ designed to induce fasting‐like effects while providing adequate micronutrient nourishment are presently being developed for commercial use, and have been shown to have beneficial effects on biomarkers associated with CVD risk in healthy adults, including reductions in the inflammatory marker C‐reactive protein (Brandhorst et al. 2015). Although initial pilot studies in humans using these diets have demonstrated considerable weight loss compared with ad libitum feeding, the effect of the fasting mimicking diet on body weight did not appear to affect lean mass and may thus be suitable for middle‐aged and older adults (Brandhorst et al. 2015). Future, larger scale clinical trials are needed to determine the efficacy of fasting mimicking diets for improving vascular function in middle‐aged and older groups.
Intermittent fasting
Intermittent fasting (IF) paradigms, consisting of shorter, but more frequent fasting periods than periodic fasting, have also gained popularity as alternative solutions for improving insulin sensitivity and reducing multiple CVD risk factors and may therefore be a feasible therapy for improving endothelial function and reducing arterial stiffness in older adults. Several IF paradigms have been proposed and involve alternating between periods of unrestricted feeding and periods of dietary restriction (Fig. 4) (Mattson & Wan, 2005; Mattson et al. 2014). Alternate day fasting, as the name implies, involves alternating between unrestricted food intake on one day followed by complete fasting or minimal (∼500 kcal) food intake the next day (Varady & Hellerstein, 2007; Varady et al. 2013). Another popular approach referred to as the ‘5:2‐diet’ involves consuming food ad libitum for 5 days during the week, and fasting or significantly reducing food intake for two days (Harvie et al. 2011). Both alternate day fasting and the 5:2‐diet involve the restriction of total energy intake on at least some days of the week and therefore have been used primarily as more feasible strategies for weight loss in overweight and obese individuals. Initial insight from preclinical experiments in young and middle‐aged animals suggests that these and other similar IF paradigms lead to improved CVD risk profiles including reductions in blood pressure and sympathetic nerve activity (Mager et al. 2006), decreased LDL cholesterol and circulating inflammatory adipokines (Kroeger et al. 2012), and improved endothelium‐dependent dilatation (Razzak et al. 2011). As such, IF may represent a novel approach for improving vascular function with ageing; however, it remains unclear whether these strategies are suitable for normal weight older adults as they are likely to be associated with a similar degree of weight loss to conventional CR.
An alternative form of IF that may maximize the benefits of CR, while minimizing the risks associated with weight loss in middle‐aged and older adults is called ‘time‐restricted feeding’ (TRF). TRF involves consuming one's normal calorie intake within a 6–12 h time window, and fasting for the remaining hours of the day (Rothschild et al. 2014). In this regard, mice restricted to an 8‐h nocturnal feeding window resisted weight gain and metabolic consequences associated with a high fat diet, without decreasing total caloric intake (Hatori et al. 2012; Chaix et al. 2014), suggesting that this strategy is likely to be the most feasible IF paradigm for normal‐weight middle‐aged and older adults as it reduces the risk of weight‐loss while allowing older individuals to achieve the positive benefits of fasting. Although the effects of TRF on the vasculature are unknown, mice consuming a high fat diet that were restricted to an 8‐h feeding window exhibited preserved hepatic AMPK activity compared with their ad libitum fed counterparts, suggesting the involvement of energy sensing pathways in the beneficial effects of TRF under these conditions. (Hatori et al. 2012).
Despite the promising benefits of TRF for preventing weight gain and improving metabolic health in mice, the efficacy of TRF for improving physiological function in humans has not been established. The limited evidence in humans for improving cardiovascular risk factors has primarily come from observational studies during the Islamic month of Ramadan (Mazidi et al. 2015). Ramadan fasting consists of eating only during the hours between sunset and sunrise (approximately 10–12 h) over the course of 1 month. The majority of these studies suggest that Ramadan fasting reduces circulating markers of oxidative stress and inflammation in adults between the ages of 18 and 51 years (Faris et al. 2012 a, b ), and improves plasma cholesterol, lipid profiles and blood pressure in adults between the ages of 29 and 70 years with at least one risk factor for CVD (Nematy et al. 2012). Although these results provide preliminary insight into the effects of a ∼10‐h feeding window, they are confounded by the fact that the feeding window occurs during a period of the day when people are also sleeping. Thus, the exact fasting duration is difficult to interpret, and most participants are not capable of maintaining their normal caloric intake during the few hours that they are awake, usually resulting in weight loss. As such, more carefully controlled studies of the benefits of TRF in the absence of CR and/or weight loss are necessary to tease out the effects of fasting on physiological function. If successful, such evidence would provide compelling support for the benefits of TRF for improving vascular health in the context of ageing.
Exercise
Favourable adaptations to cellular energy homeostasis can be achieved through aerobic exercise, which raises energy expenditure and results in a similar energy deficit to conventional CR. As such, aerobic exercise may be viewed as a CR‐mimicking lifestyle strategy; however, similar concerns to conventional CR exist with regard to adherence among older adults that may limit the effectiveness of exercise on a broad population basis.
The effects of regular exercise on arterial ageing have been reviewed elsewhere (Seals et al. 2008; Seals, 2014). Briefly, even moderate (achievable) levels of regular aerobic exercise (e.g. brisk walking) enhance endothelial function in middle‐aged and older adults (DeSouza et al. 2000), and aerobic exercise training is associated with suppression of large elastic artery stiffening with ageing (Tanaka et al. 1998, 2000; Moreau et al. 2003). Although evidence in normal weight older adults is lacking, results from weight loss studies in overweight and obese individuals that employ moderate energy restriction combined with aerobic exercise training demonstrate improvements in vascular endothelial function (Brook, 2006). As such, this combined intervention may represent an effective strategy for preserving vascular function with ageing, particularly if weight loss is desirable.
As emphasized earlier, a major concern regarding the application of conventional CR, particularly in normal weight older adults, is the potentially deleterious loss of lean mass that may exacerbate age‐associated atrophy of skeletal muscle (Waters et al. 2013; Normandin et al. 2015). Although the effects of conventional CR on normal weight older adults are not known, studies performed in overweight and obese individuals suggest that the combination of energy restriction with resistance exercise training may reduce the loss of lean mass and help maintain a desirable body composition (Normandin et al. 2015). Likewise, recent evidence suggests that resistance training combined with CR improves large artery elasticity in overweight and obese older adults (Jefferson et al. 2016). Thus, lifestyle interventions that are associated with any degree of energy restriction (e.g. conventional CR and select intermittent fasting paradigms) may benefit from the addition of resistance exercise. However, the effect of such combined approaches on vascular ageing in normal weight individuals has not been investigated.
Summary and future directions
Vascular dysfunction occurs with advancing age and increases the risk of developing clinical CVD and other age‐related chronic diseases and disabilities (Fig. 5). Regular caloric restriction can prevent or reverse vascular dysfunction with ageing, but this strategy is limited in humans due to poor adherence and may pose a risk to normal weight older adults. As such, the identification of several key molecular mechanisms responsible for the beneficial effects of CR on the vasculature has created new opportunities for the development of ‘CR‐mimetic’ strategies to reverse vascular ageing, many of which have been explored in the context of vascular ageing and others that are deserving of future research. Preclinical research to date has identified a number of promising pharmacological and behavioural strategies for modulating the energy‐ and nutrient‐sensing pathways that underlie the beneficial effects of CR. However, the feasibility, safety and efficacy of these approaches for mitigating vascular ageing remain to be established, particularly in humans. While no direct comparisons have been made between these strategies and chronic CR in terms of their practicality (i.e. feasibility based on adherence), we suspect that CR‐mimicking lifestyle strategies (e.g. intermittent fasting) may prove to be more challenging than chronic pharmacological/nutraceutical treatments; however, we believe that both options are likely to represent more practical alternatives to conventional CR. Additionally, although there is accumulating evidence that modulation of energy‐ and nutrient‐sensing pathways, individually, may improve vascular function with ageing, it is possible that the greatest benefits may be obtained from combining different CR‐mimicking approaches (to more fully recapitulate the molecular profile of CR). Indeed, recent evidence suggests that SIRT‐1 overexpression and intermittent fasting induce distinct gene expression patterns (Boutant et al. 2016) suggesting that SIRT‐1 activation alone may not fully mimic the molecular effects of fasting. Finally, a critical question that remains unanswered is whether or not weight loss is required to induce all of the beneficial effects of CR. Carefully controlled translational studies are needed in order to isolate the primary effect of fasting, while preventing changes in body weight and/or calorie intake in order to determine whether the activation of these energy‐sensing pathways alone are sufficient to mimic the effects of CR on vascular and other important physiological functions with ageing.
Figure 5. Practical alternatives to regular caloric restriction for treating vascular ageing.
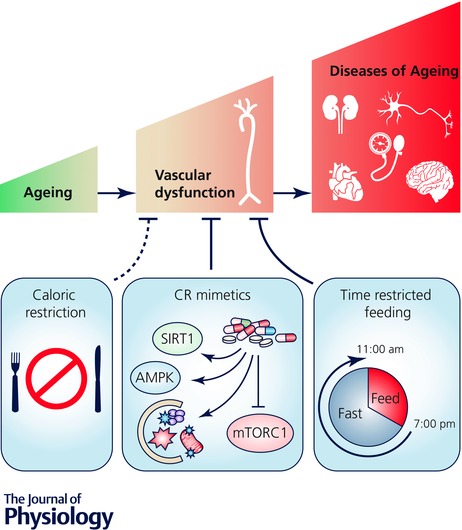
Vascular ageing contributes to the development of cardiovascular, kidney, neurological and brain diseases, cognitive dysfunction and hypertension. Calorie restriction prevents/reverses vascular ageing; however, adherence to sustained CR is not feasible for the majority of humans (dotted line) and may have adverse side effects in older adults. More practical alternative strategies (continuous lines) include: (1) pharmacological (nutraceutical) ‘CR mimetic’ compounds that modulate the energy‐ and nutrient‐sensing pathways sirtuin‐1 (SIRT1), AMP‐activated protein kinase (AMPK), autophagy and/or the mammalian target of rapamycin (mTORC1); and (2) CR‐mimicking lifestyle (behavioural) strategies including time‐restricted feeding, which may simulate the benefits of CR by simultaneously modulating several of these energy‐ and nutrient‐sensing pathways, while allowing older adults to maintain adequate calorie and nutrient intake.
Additional information
Competing interests
The authors declare no conflicts of interest.
Author contributions
C.R.M. and D.R.S. both conceived the idea for the manuscript. C.R.M. wrote the initial draft and both authors reviewed, edited and approved the final manuscript.
Funding
This work was supported by National Institutes of Health awards AG013038, AG006537, AG000279, and TR001082, the Glenn Foundation and the American Federation for Aging Research (AFAR).
Acknowledgements
We thank Melissa Mazzo for her technical assistance with figure development.
Biographies
Christopher Martens earned his PhD from the University of Delaware in Newark, DE, USA and is currently a postdoctoral fellow in the Integrative Physiology of Ageing Laboratory at the University of Colorado Boulder. His research focuses on lifestyle and nutraceutical strategies for improving physiological function in older adults, primarily by mimicking the beneficial effects of regular calorie restriction.

Douglas Seals obtained his PhD from the University of Wisconsin–Madison and performed postdoctoral training at Washington University School of Medicine in St Louis, MO, USA. He is presently a College Professor of Distinction in the Department of Integrative Physiology at the University of Colorado Boulder. His research focuses on the integrative physiology of ageing. For the past decade, his laboratory has used translational experimental approaches to investigate the physiology and pathophysiology of vascular ageing and, more recently, declines in motor function, particularly the underlying mechanisms involved and the efficacy of treatments.
This is an Editor's Choice article from the 15 December 2016 issue.
References
- Ahmet I, Wan RQ, Mattson MP, Lakatta EG & Talan M (2005). Cardioprotection by intermittent fasting in rats. Circulation 112, 3115–3121. [DOI] [PubMed] [Google Scholar]
- Bai B, Vanhoutte PM & Wang Y (2014). Loss‐of‐SIRT1 function during vascular ageing: Hyperphosphorylation mediated by cyclin‐dependent kinase 5. Trends Cardiovasc Med 24, 81–84. [DOI] [PubMed] [Google Scholar]
- Baron S, Bedarida T, Cottart C, Vibert F, Vessieres E, Ayer A, Henrion D, Hommeril B, Paul J, Renault G, Saubamea B, Beaudeux J, Procaccio V & Nivet‐Antoine V (2014). Dual effects of resveratrol on arterial damage induced by insulin resistance in aged mice. J Gerontol A Biol Sci Med Sci 69, 260–269. [DOI] [PubMed] [Google Scholar]
- Baur JA (2010). Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev 131, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz DE, White R, Li DC, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC & Hare JM (2003). Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108, 2000–2006. [DOI] [PubMed] [Google Scholar]
- Bogan KL & Brenner C (2008). Nicotinic acid nicotinamide and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 28, 115–130. [DOI] [PubMed] [Google Scholar]
- Boutant M, Kulkarni SS, Joffraud M, Raymond F, Metairon S, Descombes P & Canto C (2016). SIRT1 gain of function does not mimic or enhance the adaptations to intermittent fasting. Cell Rep 14, 2068–2075. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB & Longo VD (2015). A periodic diet that mimics fasting promotes multi‐system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD (2006). Obesity, weight loss, and vascular function. Endocrine 29, 21–25. [DOI] [PubMed] [Google Scholar]
- Canto C & Auwerx J (2011). Calorie restriction: Is AMPK a key sensor and effector? Physiology 26, 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez‐Marcos PJ, Yamamoto H, Andreux PA, Cettour‐Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA & Auwerx J (2012). The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high‐fat diet‐induced obesity. Cell Metab 15, 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizzo A, Puca A, Damato A, Marino M, Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco V & Vecchione C (2013). Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 62, 359–366. [DOI] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P & Panda S (2014). Time‐restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez‐Crespo I, Witters LA, Power DA, de Montellano PRO & Kemp BE (1999). AMP‐activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443, 285–289. [DOI] [PubMed] [Google Scholar]
- Chung KW, Kim DH, Park MH, Choi YJ, Kim ND, Lee J, Yu BP & Chung HY (2013). Recent advances in calorie restriction research on aging. Exp Gerontol 48, 1049–1053. [DOI] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM & Stern DM (1998). Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91, 3527–3561. [PubMed] [Google Scholar]
- Crandall JP, Oram V, Rescu GT, Reid M, Kishore P, Hawkins M, Cohen HW & Barzilai N (2012). Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 67, 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzen C & Colman RJ (2009). Effects of caloric restriction on cardiovascular aging in non‐human primates and humans. Clin Geriatr Med 25, 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R & Ungvari Z (2009). Anti‐oxidative and anti‐inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech Ageing Dev 130, 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A & Ungvari Z (2006). Resveratrol attenuates TNF‐α‐induced activation of coronary arterial endothelial cells: Role of NF‐κB inhibition. Am J Physiol Heart Circ Physiol 291, H1694–H1699. [DOI] [PubMed] [Google Scholar]
- da Luz PL, Tanaka L, Brum PC, Martins Dourado PM, Favarato D, Krieger JE & Laurindo FRM (2012). Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis 224, 136–142. [DOI] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S & Seals DR (2016). Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM & Davy KP (2010). Arterial destiffening with weight loss in overweight and obese middle‐aged and older adults. Hypertension 55, 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H & Seals DR (2000). Regular aerobic exercise prevents and restores age‐related declines in endothelium‐dependent vasodilation in healthy men. Circulation 102, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA & Seals DR (2011). SIRT‐1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589, 4545–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Morgan RG, Walker AE & Lesniewski LA (2015). Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol 89, 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA & Seals DR (2013). Life‐long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD & Seals DR (2005). Tetrahydrobiopterin augments endothelium‐dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris MAE, Hussein RN, Al‐Kurd RA, Al‐Fararjeh MA, Bustanji YK & Mohammad MK (2012. b). Impact of Ramadan intermittent fasting on oxidative stress measured by urinary 15‐f(2t)‐isoprostane. J Nutr Metab 2012, 802924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris ME, Kacimi S, Al‐Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK & Salem ML (2012. a). Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res 32, 947–955. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B & Fleming I (2009). Activation and signalling by the AMP‐activated protein kinase in endothelial cells. Circ Res 105, 114–127. [DOI] [PubMed] [Google Scholar]
- Fleenor BS (2013). Large elastic artery stiffness with aging: Novel translational mechanisms and interventions. Aging Dis 4, 76–83. [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA & Seals DR (2010). Arterial stiffening with ageing is associated with transforming growth factor‐beta 1‐related changes in adventitial collagen: Reversal by aerobic exercise. J Physiol 588, 3971–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML & Sindler AL (2012). Superoxide‐lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano LB, Donato AJ, Pasha HM, Hearon CM Jr, Sindler AL & Seals DR (2014). The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307, H1754–H1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H & Hellsten Y (2013). Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591, 5047–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodor JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL & Sinclair DA (2013). Declining NAD+ induces a pseudohypoxic state disrupting nuclear‐mitochondrial communication during aging. Cell 155, 1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA & Pasinetti GM (2013). Nicotinamide riboside restores cognition through an upregulation of proliferator‐activated receptor‐γ coactivator 1α regulated β‐secretase 1 degradation and mitochondrial gene expression in alzheimer's mouse models. Neurobiol Aging 34, 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A & Howell A (2011). The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int J Obes 35, 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH & Panda S (2012). Time‐restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high‐fat diet. Cell Metab 15, 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B & Sinclair DA (2003). Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature 425, 191–196. [DOI] [PubMed] [Google Scholar]
- Hubbard BP & Sinclair DA (2014). Small molecule SIRT1 activators for the treatment of aging and age‐related diseases. Trends Pharmacol Sci 35, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S (2009). The NAD world: A new systemic regulatory network for metabolism and aging‐Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys 53, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S (2010). A possibility of nutriceuticals as an anti‐aging intervention: Activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol Res 62, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou SG, Lane MA, Roth GS & deCabo R (2006). Calorie restriction mimetics: An emerging research field. Aging Cell 5, 97–108. [DOI] [PubMed] [Google Scholar]
- Jefferson ME, Nicklas BJ, Chmelo EA, Crotts CI, Shaltout HA, Diz DI, Marsh AP & Brinkley TE (2016). Effects of resistance training with and without caloric restriction on arterial stiffness in overweight and obese older adults. Am J Hypertens 29, 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K & Levine B (2007). Autophagy is required for dietary restriction‐mediated life span extension in C. elegans. Autophagy 3, 597–599. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS & Kaeberlein M (2013). mTOR is a key modulator of ageing and age‐related disease. Nature 493, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplon RE, Hill SD, Bispham NZ, Santos‐Parker JR, Nowlan MJ, Snyder LL, Chonchol M, LaRocca TJ, McQueen MB & Seals DR (2016). Oral trehalose supplementation improves resistance artery endothelial function in healthy middle‐aged and older adults. Aging 8, 1167–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Park K, Yoo E, Kim Y, Kim Y, Kim H, Kim HT, Park J, Lee K, Jang WG, Kim J, Kim B & Lee I (2007). Effects of PGC‐1α on TNF‐α‐induced MCP‐1 and VCAM‐1 expression and NF‐κB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal 9, 301–307. [DOI] [PubMed] [Google Scholar]
- Kroeger CM, Klempel MC, Bhutani S, Trepanowski JF, Tangney CC & Varady KA (2012). Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations. Nutr Metab 9, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhla A, Lange S, Holzmann C, Maass F, Petersen J, Vollmar B & Wree A (2013). Lifelong caloric restriction increases working memory in mice. Plos One 8, e68778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG & Levy D (2003). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises part I: Aging arteries: A "set up" for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM & Baur JA (2013). Rapalogs and mTOR inhibitors as anti‐aging therapeutics. J Clin Invest 123, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Gioscia‐Ryan RA, Hearon CM Jr & Seals DR (2013). The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134, 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL & Seals DR (2012). Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590, 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ & Seals DR (2011). Salicylate treatment improves age‐associated vascular endothelial dysfunction: Potential role of nuclear factor κB and forkhead box O phosphorylation. J Gerontol A Biol Sci Med Sci 66, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC & Donato AJ (2016). Dietary rapamycin supplementation reverses age‐related vascular dysfunction and oxidative stress, while modulating nutrient sensing, cell cycle and senescence pathways. Aging Cell (in press; doi: 10.1111/acel.12524). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Zigler MC, Durrant JR, Donato AJ & Seals DR (2012). Sustained activation of AMPK ameliorates age‐associated vascular endothelial dysfunction via a nitric oxide‐independent mechanism. Mech Ageing Dev 133, 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng C, Madia F, Fontana L, Mirisola MG, Guevara‐Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM & Longo VD (2014). Low protein intake is associated with a major reduction in IGF‐1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Park S & Lakatta EG (2009). RAGE signaling in inflammation and arterial aging. Front Biosci 14, 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wilk SA, Wang A, Zhou L, Wang R, Ogawa W, Deng C, Dong LQ & Liu F (2010). Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem 285, 36387–36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD & Mattson MP (2014). Fasting: Molecular mechanisms and clinical applications. Cell Metab 19, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD & Panda S (2016). Fasting, circadian rhythms, and time‐restricted feeding in healthy lifespan. Cell Metab 23, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher TF & Barton M (1997). Biology of the endothelium. Clin Cardiol 20 (Suppl II), II‐3‐II‐10. [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR & Mattson MP (2006). Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J 20, 631–637. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim C, Naqvi A, Yamamori T, Hoffman TA, Jung S, DeRicco J, Kasuno K & Irani K (2007). SIRT1 promotes endothelium‐dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104, 14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park S, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG & de Cabo R (2014). Resveratrol prevents high fat/sucrose diet‐induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab 20, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FAJL, Seyfried TN, Varady KA & Panda S (2014). Meal frequency and timing in health and disease. Proc Natl Acad Sci USA 111, 16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP & Wan RQ (2005). Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem 16, 129–137. [DOI] [PubMed] [Google Scholar]
- Mazidi M, Rezaie P, Chaudhri O, Karimi E & Nematy M (2015). The effect of Ramadan fasting on cardiometabolic risk factors and anthropometrics parameters: A systematic review. Pak J Med Sci 31, 1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF (2014). AMPK activation‐protean potential for boosting healthspan. Age 36, 641–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL & Wolfe RR (2008). The danger of weight loss in the elderly. J Nutr Health Aging 12, 487–491. [DOI] [PubMed] [Google Scholar]
- Ming X, Montani J & Yang Z (2012). Perspectives of targeting mTORC1‐S6K1 in cardiovascular aging. Front Physiol 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA & Tanaka H (2003). Regular exercise, hormone replacement therapy and the age‐related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57, 861–868. [DOI] [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N & Kroemer G (2010). Caloric restriction and resveratrol promote longevity through the sirtuin‐1‐dependent induction of autophagy. Cell Death Dis 1, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres J, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee & Stroke Statistics Subcommittee (2016). Heart disease and stroke statistics‐2016 update: A report from the American Heart Association. Circulation 133, e38–e360. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ & Ito K (2009). A protein deacetylase SIRT1 is a negative regulator of metalloproteinase‐9. FASEB J 23, 2810–2819. [DOI] [PubMed] [Google Scholar]
- Nematy M, Alinezhad‐Namaghi M, Rashed MM, Mozhdehifard M, Sajjadi SS, Akhlaghi S, Sabery M, Mohajeri SAR, Shalaey N, Moohebati M & Norouzy A (2012). Effects of Ramadan fasting on cardiovascular risk factors: A prospective observational study. Nutr J 11, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin E, Houston DK & Nicklas BJ (2015). Caloric restriction for treatment of geriatric obesity: Do the benefits outweigh the risks? Curr Nutr Rep 4, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos Y, Sanchez‐Gomez FJ, Wild B, Garcia‐Quintans N, Cabezudo S, Lamas S & Monsalve M (2013). SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC‐1α complex. Antioxid Redox Sig 19, 1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Goldman DP, Zheng Y & Rowe JW (2009). Aging in America in the twenty‐first century: Demographic forecasts from the MacArthur Foundation Research Network on an aging society. Milbank Q 87, 842–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M & Ouchi Y (2008). Cilostazol inhibits oxidative stress‐induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol 28, 1634–1639. [DOI] [PubMed] [Google Scholar]
- Palmer RMJ, Ashton DS & Moncada S (1988). Vascular endothelial‐cells synthesize nitric‐oxide from L‐arginine. Nature 333, 664–666. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA & de Cabo R (2008). Resveratrol delays age‐related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ & Seals DR (2008). Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD & Seals DR (2009). Nuclear factor‐κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle‐aged and older humans. Circulation 119, 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M & Dimmeler S (2008). NO targets SIRT1: A novel signaling network in endothelial senescence. Arterioscler Thromb Vasc Biol 28, 1577–1579. [DOI] [PubMed] [Google Scholar]
- Rajapakse AG, Yepuri G, Carvas JM, Stein S, Matter CM, Scerri I, Ruffieux J, Montani J, Ming X & Yang Z (2011). Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: Inhibition by resveratrol. Plos One 6, e19237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzak RLA, Abu‐Hozaifa BM, Bamosa AO & Ali NM (2011). Assessment of enhanced endothelium‐dependent vasodilation by intermittent fasting in wistar albino rats. Indian J Physiol Pharmacol 55, 336–42. [PubMed] [Google Scholar]
- Reineke DC, Mueller‐Schweinitzer E, Winkler B, Kunz D, Konerding MA, Grussenmeyer T, Carrel TP, Eckstein FS & Grapow MTR (2015). Rapamycin impairs endothelial cell function in human internal thoracic arteries. Eur J Med Res 20, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe C, Lesniewski LA, Connell ML, LaRocca TJ, Donato AJ & Seals DR (2010). Short‐term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild J, Hoddy KK, Jambazian P & Varady KA (2014). Time‐restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr Rev 72, 308–318. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G & Kroemer G (2011). Autophagy and aging. Cell 146, 682–695. [DOI] [PubMed] [Google Scholar]
- Safar ME, Levy BI & Struijker‐Boudier H (2003). Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107, 2864–2869. [DOI] [PubMed] [Google Scholar]
- Santos J, Leitao‐Correia F, Sousa MJ & Leao C (2016). Dietary restriction and nutrient balance in aging. Oxid Med Cell Longev 2016, 4010357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐Parker JR & Kaplon RE (2014). Supplementing exercise: Translational considerations for nutraceutical and lifestyle interventions. J Physiol 592, 427–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR (2014). Edward F. Adolph Distinguished Lecture: The remarkable anti‐aging effects of aerobic exercise on systemic arteries. J Appl Physiol 117, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, DeSouza CA, Donato AJ & Tanaka H (2008). Habitual exercise and arterial aging. J Appl Physiol 105, 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL & Donato AJ (2011). Aging and vascular endothelial function in humans. Clin Sci 120, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR & Melov S (2014). Translational geroscience: Emphasizing function to achieve optimal longevity. Aging 6, 718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J, Trollor JN, Baune BT, Sachdev PS & Smith E (2014). Arterial stiffness, the brain and cognition: A systematic review. Ageing Res Rev 15, 16–27. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh N & Sokoya EM (2012). Regulation of cerebral vascular function by sirtuin 1. Microcirculation 19, 336–342. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA & Seals DR (1998). Absence of age‐related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18, 127–132. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA & Seals DR (2000). Aging, habitual exercise, and dynamic arterial compliance. Circulation 102, 1270–1275. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado‐Fernandez C, Csiszar A & de Cabo R (2008). Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK & Calvo Y (2013). Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr J 12, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA & Hellerstein MK (2007). Alternate‐day fasting and chronic disease prevention: A review of human and animal trials. Am J Clin Nutr 86, 7–13. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, Lewis M, Schwartz AV & CALERIE Study Group (2016). Effect of two‐year caloric restriction on bone metabolism and bone mineral density in non‐obese younger adults: A randomized clinical trial. J Bone Miner Res 31, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M & Schilling L (1993). Regulation of cerebral blood‐flow: a brief review. Acta Neurochir 59, 3–10. [DOI] [PubMed] [Google Scholar]
- Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA & Donato AJ (2014. a). Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age 36, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Kaplon RE, Pierce GL, Nowlan MJ & Seals DR (2014. b). Prevention of age‐related endothelial dysfunction by habitual aerobic exercise in healthy humans: Possible role of nuclear factor κB. Clin Sci 127, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K & Forstermann U (2002). Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106, 1652–1658. [DOI] [PubMed] [Google Scholar]
- Wasselin T, Zahn S, Le Maho Y, Van Dorsselaer A, Raclot T & Bertile F (2014). Exacerbated oxidative stress in the fasting liver according to fuel partitioning. Proteomics 14, 1905–1921. [DOI] [PubMed] [Google Scholar]
- Waters DL, Ward AL & Villareal DT (2013). Weight loss in obese adults 65 years and older: A review of the controversy. Exp Gerontol 48, 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S & Floeel A (2009). Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA 106, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RHX, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I & Howe PRC (2013). Chronic resveratrol consumption improves brachial flow‐mediated dilatation in healthy obese adults. J Hypertens 31, 1819–1827. [DOI] [PubMed] [Google Scholar]
- Wong RHX, Howe PRC, Buckley JD, Coates AM, Kunz I & Berry NM (2011). Acute resveratrol supplementation improves flow‐mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis 21, 851–856. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang Z, Hsieh T, Bruder J, Zou J & Huang Y (2001). Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int J Mol Med 8, 3–17. [DOI] [PubMed] [Google Scholar]
- Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Foerstermann U & Li H (2013). Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide Biol Chem 32, 29–35. [DOI] [PubMed] [Google Scholar]
- Xu S, Cai Y & Wei Y (2014). mTOR signaling from cellular senescence to organismal aging. Aging Dis 5, 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabluchanskiy A, Ma Y, Iyer RP, Hall ME & Lindsey ML (2013). Matrix metalloproteinase‐9: Many shades of function in cardiovascular disease. Physiology 28, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepuri G, Velagapudi S, Xiong Y, Rajapakse AG, Montani J, Ming X & Yang Z (2012). Positive crosstalk between arginase‐II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 11, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Cappellari GG, Burekovic I, Barazzoni R, Stebel M & Guarnieri G (2010). Caloric restriction improves endothelial dysfunction during vascular aging effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp Gerontol 45, 848–855. [DOI] [PubMed] [Google Scholar]
- Zhou R, Vendrov AE, Tchivilev I, Niu X, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR & Runge MS (2012). Mitochondrial oxidative stress in aortic stiffening with age the role of smooth muscle cell function. Arterioscler Thromb Vasc Biol 32, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A & Sabatini DM (2011). mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


