Abstract
Key points
Resistance exercise training (RET) is one of the most effective strategies for preventing declines in skeletal muscle mass and strength with age.
Hypertrophic responses to RET with age are diminished compared to younger individuals.
In response to 6 weeks RET, we found blunted hypertrophic responses with age are underpinned by chronic deficits in long‐term muscle protein synthesis.
We show this is likely to be the result of multifactorial deficits in anabolic hormones and blunted translational efficiency and capacity.
These results provide great insight into age‐related exercise adaptations and provide a platform on which to devise appropriate nutritional and exercise interventions on a longer term basis.
Abstract
Ageing is associated with impaired hypertrophic responses to resistance exercise training (RET). Here we investigated the aetiology of ‘anabolic resistance’ in older humans. Twenty healthy male individuals, 10 younger (Y; 23 ± 1 years) and 10 older (O; 69 ± 3 years), performed 6 weeks unilateral RET (6 × 8 repetitions, 75% of one repetition maximum (1‐RM), 3 times per week). After baseline bilateral vastus lateralis (VL) muscle biopsies, subjects consumed 150 ml D2O (70 atom%; thereafter 50 ml week−1), further bilateral VL muscle biopsies were taken at 3 and 6 weeks to quantify muscle protein synthesis (MPS) via gas chromatography–pyrolysis–isotope ratio mass spectrometry. After RET, 1‐RM increased in Y (+35 ± 4%) and O (+25 ± 3%; P < 0.01), while MVC increased in Y (+21 ± 5%; P < 0.01) but not O (+6 ± 3%; not significant (NS)). In comparison to Y, O displayed blunted RET‐induced increases in muscle thickness (at 3 and 6 weeks, respectively, Y: +8 ± 1% and +11 ± 2%, P < 0.01; O: +2.6 ± 1% and +3.5 ± 2%, NS). While ‘basal’ longer term MPS was identical between Y and O (∼1.35 ± 0.1% day−1), MPS increased in response to RET only in Y (3 weeks, Y: 1.61 ± 0.1% day−1; O: 1.49 ± 0.1% day−1). Consistent with this, O exhibited inferior ribosomal biogenesis (RNA:DNA ratio and c‐MYC induction: Y: +4 ± 2 fold change; O: +1.9 ± 1 fold change), translational efficiency (S6K1 phosphorylation, Y: +10 ± 4 fold change; O: +4 ± 2 fold change) and anabolic hormone milieu (testosterone, Y: 367 ± 19; O: 274 ± 19 ng dl−1 (all P < 0.05). Anabolic resistance is thus multifactorial.
Keywords: ageing, exercise, hypertrophy, muscle, protein synthesis, ribosomal biogenesis, signalling, stable isotope
Key points
Resistance exercise training (RET) is one of the most effective strategies for preventing declines in skeletal muscle mass and strength with age.
Hypertrophic responses to RET with age are diminished compared to younger individuals.
In response to 6 weeks RET, we found blunted hypertrophic responses with age are underpinned by chronic deficits in long‐term muscle protein synthesis.
We show this is likely to be the result of multifactorial deficits in anabolic hormones and blunted translational efficiency and capacity.
These results provide great insight into age‐related exercise adaptations and provide a platform on which to devise appropriate nutritional and exercise interventions on a longer term basis.
Abbreviations
- 1‐RM
one repetition maximum
- ABR
absolute breakdown rate
- Akt
protein kinase B
- APE
atom percent excess
- ASP
alkali soluble protein
- ASR
absolute synthetic rate
- BMI
body mass index
- D2O
deuterium oxide
- DXA
dual energy X‐ray absorptiometry
- 4EBP
eukaryotic translation initiation factor 4E‐binding protein 1
- eEF2
eukaryotic elongation factor 2
- ERK
extracellular signal‐regulated kinase
- FBR
fractional breakdown rate
- FFM
fat‐free mass
- FC
fold change
- FGR
fractional growth rate
- FSR
fractional synthetic rate
- GnRH
gonadotropin‐releasing hormone
- IGF‐1
insulin‐like growth factor 1
- Lf
fibre length
- MAFbx
muscle atrophy F‐box
- MET
metabolic equivalent
- MPB
muscle protein breakdown
- MPS
muscle protein synthesis
- MT
muscle thickness
- MuRF1
muscle RING finger 1
- MVC
maximum voluntary contraction
- mTORC1
mechanistic target of rapamycin complex 1
- PCA
perchloric acid
- P70S6K1
ribosomal protein S6 kinase 1
- RB
retinoblastoma
- rDNA
ribosomal DNA
- RE
resistance exercise
- RET
resistance exercise training
- rps6
ribosomal protein S6
- SMI
skeletal muscle index
- T
trained
- TIF1a
transcription initiation factor 1
- TFFM
thigh fat‐free mass
- θ
pennation angle
- UBF
upstream binding factor 1
- UT
untrained
- VL
vastus lateralis
Introduction
Advancing age is associated with incipient declines in skeletal muscle mass, strength and quality, processes termed sarcopenia and dynapenia (Mitchell et al. 2012). Since skeletal muscles are central for locomotion and the performance of everyday habitual activities, declines in muscle mass and function lead to impaired quality of life and independence. Furthermore, as skeletal muscle plays a substantial role in whole body metabolic health, muscle loss results in decreased metabolic rate (Tzankoff & Norris, 1977) and increased risk of morbidity (Luukinen et al. 1997) and mortality (Laukkanen et al. 1995). With increases in life expectancy showing no signs of slowing (Christensen et al. 2009) and those > 60 years predicted to represent 25% of the population by 2050, increased strains on the healthcare system are expected to rise (Janssen et al. 2004).
Muscle mass is controlled by the diurnal balance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB) (Atherton & Smith, 2012). Therefore, declines in mass occur when MPS is chronically lower than MPB. On a diurnal basis, the most significant regulators of MPS and MPB are food intake (in particular protein; Atherton et al. 2010; Bukhari et al. 2015; Wilkinson et al. 2015) and physical activity (Kumar et al. 2009 a, 2012; Breen et al. 2013; Wilkinson et al. 2014). Both feeding and exercise transiently stimulate MPS, and when combined result in sustained positive net balance and eventually protein accretion, i.e. following a resistance exercise training (RET) programme (Phillips et al. 1997; Moore et al. 2009, 2015; Fry et al. 2011; Bukhari et al. 2015). Whilst basal rates of MPS and MPB are not detectably lower in old age (Volpi et al. 2001; Kumar et al. 2009 b), older individuals do exhibit ‘anabolic resistance’, manifesting as a reduced anabolic response to these key growth signals regulating homeostasis (Cuthbertson et al. 2005; Kumar et al. 2009 b; Fry et al. 2011; Wall et al. 2015). Over time, inadequate replenishment of muscle protein lost during fasting or inactivity leads to a progressive loss of muscle with age.
RET arguably offers the most effective countermeasure and lowest risk profile strategy to mitigate age‐related declines in skeletal muscle mass and strength (Fiatarone et al. 1994). However in response to the same RET programme, older individuals generally show attenuated muscle hypertrophy (Welle et al. 1996; Kosek et al. 2006; Greig et al. 2011; Peterson et al. 2011; Mero et al. 2013), although equal adaptations have been reported (Häkkinen et al. 1998 b; Mayhew et al. 2009). The exact mechanisms leading to RET‐induced anabolic resistance are unclear. Ageing can be associated with neuromuscular deficits (e.g. loss of motor units; Campbell et al. 1973), sustained malnutrition (Morley, 2012), physical inactivity (Troiando et al. 2008), suboptimal anabolic hormonal profiles (Feldman et al. 2002) and various co‐morbidities and chronic diseases (Marengoni et al. 2011), all of which theoretically represent unfavourable attributes for hypertrophy. Additionally at the cellular level, muscle signalling responses linked with increased translational efficiency (e.g. mechanistic target of rapamycin complex 1 (mTORC1) signalling) and capacity (ribosomal biogenesis) have been shown to be blunted ‘acutely’ with ageing (Kumar et al. 2009 a; Fry et al. 2011; Stec et al. 2015). Nonetheless, the coordinate mechanisms remain elusive and are likely to be multifactorial in nature.
Current assumptions on the mechanisms of muscle hypertrophy in youth and ageing have been largely derived from ‘acute’ metabolic and molecular studies, i.e. those undertaken after a single bout of resistance exercise (RE)(Miller et al. 2005; Kumar et al. 2009 b; Fry et al. 2011; Bukhari et al. 2015). Nonetheless, due to the disparity in acquisition of these acute response, i.e. nutritional provision, length of measurement and exercise paradigms, the exact relationships between acute responses and blunted adaptations are unclear (Mitchell et al. 2014; Atherton et al. 2015). Similar issues are associated with RET studies where hypertrophic adaptations are assessed with a single beginning and end‐point (Häkkinen et al. 1998 b; Mayhew et al. 2009) in which physiological insight is great but metabolic and molecular insight is limited. To circumvent these limitations, we quantified MPS responses to 6 weeks RET in young and older individuals using novel heavy water, deuterium oxide (D2O) techniques (Robinson et al. 2011; Wilkinson et al. 2014); simultaneously we temporally assessed indices of cellular translational efficiency and capacity. We hypothesized that following 6 weeks of RET, skeletal muscle hypertrophy would be blunted with age, primarily due to long‐term deficits in cumulative MPS responses with the underlying mechanisms multifactorial.
Methods
Subject characteristics and ethics
Ten healthy younger (23 ± 1 years, BMI: 24 ± 1) (Brook et al. 2015) and older (69 ± 1 years, BMI 25.8 ± 1) men were recruited. Volunteers were screened by medical questionnaire, physical examination, and resting electrocardiogram, with exclusions for metabolic, respiratory and cardiovascular disorders or any other symptoms of ill health. Subjects had clinically normal blood chemistry, were normotensive (<140/90), and were not prescribed any medications: all subjects performed activities of daily living and recreation but did not undertake in any RET other than that described in the study and had not participated in any RET within the last 12 months. All subjects provided their written, informed consent to participate after all procedures and risks (in relation to muscle biopsies, blood sampling, etc.) were explained. This study was approved by The University of Nottingham Ethics Committee, with all studies conducted according to the declaration of Helsinki and preregistered (clinicaltrials.gov registration no. NCT02152839).
Conduct of the study
This study involved a bilateral (i.e. two legged) protocol whereby one leg was used as an untrained (UT) internal time control with the other leg acting as the RET (trained; T) leg, with the dominant leg assigned as T. Following inclusion to the study, subjects were studied over a 6‐week period. On the first day of study, subjects arrived at the laboratory at 08.30 h following an overnight fast. Following assessment of vastus lateralis (VL) muscle architecture by ultrasound (Mylab 70; Esaote Biomedica, Italy) and body composition by dual energy X‐ray absorptiometry (DXA; Lunar Prodigy II, GE Medical Systems, Little Chalfont, UK), subjects completed the first session of RET consisting of unilateral knee‐extension exercise (i.e. 6 × 8 repetitions at 75% of one repetition maximum (1‐RM)). Bilateral biopsies were taken from the VL muscle 60–90 min (75 ± 2 min) after unilateral exercise under sterile conditions, using the conchotome biopsy technique (Dietrichson et al. 1987) with 1% lidocaine (B. Braun, Melsungen, Germany) as local anaesthetic. Muscle was rapidly dissected free of fat and connective tissue, washed in ice‐cold phosphate‐buffered saline (PBS), and then frozen in liquid N2 and stored at –80°C until further analysis. Immediately post‐RET, subjects provided a saliva sample (collected in sterile plastic tubes) and consumed a 150 ml bolus of D2O (70 atom%; Sigma‐Aldrich, Poole, UK), with the aim to label the body water pool to ∼0.2% atom percent excess (APE), which was maintained in a pseudo‐steady state with weekly top‐up boluses (50 ml week−1). In addition, venous blood samples were collected into lithium heparin coated tubes, immediately cold centrifuged at 1750 g, with plasma fractions aliquoted and frozen at –80°C until analysis. Thereafter, subjects returned to the lab 3 times per week to undertake supervised unilateral RET with 1‐RM assessments of the T leg every ∼10 days to ensure progressive intensity. Further bilateral muscle biopsies (∼90 min after RET in order to investigate the temporal nature of acute anabolic signalling responses to progressive RET), ultrasound measures of muscle architecture and venous blood samples were taken at 3 and 6 weeks and DXA performed at 6 weeks. For the temporal monitoring of body water enrichment, each participant provided a saliva sample on RET‐visits > 30 min after their last meal or drink, with extra samples taken ∼3 h after weekly 50 ml boluses to ensure body water enrichment was accurately represented. These were collected in sterile plastic tubes and immediately cold‐centrifuged at 16,000 g to remove any debris that might be present; they were then aliquoted into 2 ml glass vials and frozen at –20°C until analysis. To measure levels of physical activity, subjects wore combined heart rate and activity monitors (Actiheart, CamMtech Ltd, Cambridge, UK) for 1 week during the study. Actiheart data were checked for missing or extrapolated values; ≥ 80% of minute‐by‐minute physical activity data had to be available for a 24 h recording to be accepted, 85% of subjects had at least ≥ 4 days of data with the remaining having ≥ 1 day of data that were included in the analysis. Dietary intake was monitored by completion of 4‐day diet diaries during the study and was analysed using Microdiet (Downlee Systems Ltd, Chapel‐en‐le‐Frith, UK). A detailed representative schematic diagram of the study protocol in its entirety is depicted in Fig. 1.
Figure 1.
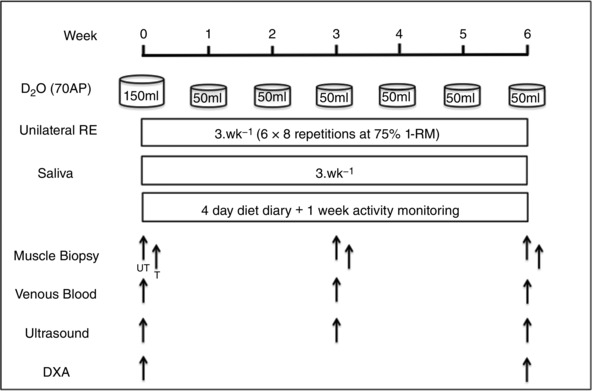
Schematic diagram of study protocol
Muscle functional tests
Basal measures of maximal voluntary contraction (MVC) and 1‐RM were ascertained in the week prior to the first study day. MVC was measured with isometric contractions conducted in a sitting position using an isokinetic dynamometer (Isocom; Isokinetic Technologies, Eurokinetics, UK) over a range of four knee joint angles (90–60 deg), with full extension corresponding to 0 deg. Subjects were seated in the dynamometer chair and secured into position using a chest strap. Contractions lasted 4 s, with a 30 s rest period between contractions and 90 s between knee joint angles. Unilateral leg extension 1‐RM was assessed on the dominant leg (Technogym, Gambettola, Italy). After the procedure was explained, subjects performed a light warm up to avoid injury and ensure familiarity whilst avoiding fatigue; 1‐RM was then achieved (20 on Borg's rating of perceived exertion scale) in as few repetitions as possible with a maximum of five repetitions. The first repetition was estimated at 50% 1‐RM and then increased until subjects could not complete controlled contraction throughout the range of motion, with 3 min in‐between attempts. Functional tests were repeated throughout (about every 10 days), with each set of tests performed on a non‐biopsy day to avoid influencing subsequent measures.
Muscle architecture and DXA derived mass
Muscle architecture was measured as described (Franchi et al. 2014). Briefly, the architectural parameters muscle thickness (MT), fibre length (L f) and pennation angle (θ) were quantified, by the same ‘blinded’ investigator (M.B.), from ultrasound scans using ImageJ 1.42q (National Institutes of Health, Bethesda, MD, USA). The visible portion of the L f was assessed directly using this software with MT measured as the distance between the superficial and deep aponeuroses. θ was measured as the intersection between fascicles and the deep tendon aponeurosis. DXA‐derived thigh fat‐free mass (TFFM) was determined from the lowest point of the ischium to the knee space.
Body water and protein bound alanine muscle fraction enrichment
Body water and muscle protein enrichment were measured as previously described (Wilkinson et al. 2014). Briefly, pure fractions of body water were extracted by heating 100 μl of saliva in an inverted 2 ml autosampler vial for 4 h at 100°C. Vials were then placed upright on ice to condense extracted body water and transferred to a clean autosampler vial ready for injection. Body water (0.1 μl) was injected into a high‐temperature conversion elemental analyser (Thermo Finnigan, Thermo Scientific, Hemel Hempstead, UK) connected to an isotope ratio mass spectrometer (Delta V Advantage, Thermo Scientific). For isolation of myofibrillar protein, 30–50 mg of muscle was homogenized in ice‐cold homogenization buffer (Wilkinson et al. 2014), rotated for 10 min, and the supernatant was collected after centrifugation at 13,000 g for 5 min at 4°C. The myofibrillar pellet was solubilized in 0.3 m NaOH and separated from the insoluble collagen by centrifugation, and the myofibrillar protein was precipitated using 1 m perchloric acid (PCA). Sarcoplasmic proteins were precipitated from the sample homogenate with 1 m PCA and separated by centrifugation. Protein‐bound amino acids were released using acid hydrolysis by incubating at 110°C in 0.1 m HCl in Dowex H+ resin slurry overnight before being eluted from the resin with 2 m NH4OH and evaporated to dryness; amino acids were then derivatized as their n‐methoxycarbonyl methyl esters. Dried samples were suspended in 60 μl distilled water and 32 μl methanol, and following vortex, 10 μl of pyridine and 8 μl of methylchloroformate were added. Samples were vortexed for 30 s and left to react at room temperature for 5 min. The newly formed n‐methoxycarbonyl methyl esters of amino acids were then extracted into 100 μl of chloroform. A molecular sieve was added to each sample for ∼20 s before being transferred to a clean glass Gas Chromatography insert, removing any remaining water by size exclusion absorption. Incorporation of deuterium into protein bound alanine was determined by gas chromatography–pyrolysis–isotope ratio mass spectrometry (Delta V Advantage) alongside a standard curve of known l‐alanine‐2,3,3,3‐d4 enrichment to validate measurement accuracy of the instrument.
Calculation of fractional synthetic rate
Myofibrillar MPS was calculated from incorporation of deuterium‐labelled alanine into protein, using the enrichment of body water (corrected for the mean number of deuterium moieties incorporated per alanine, 3.7, and the dilution from the total number of hydrogens in the derivative, i.e. 11) as the surrogate precursor labelling between subsequent biopsies. Fractional synthetic rate (FSR) is as follows:
where APEAla is deuterium enrichment of protein‐bound alanine, APEP is mean precursor enrichment over the time period, and t is the time between biopsies. Thigh absolute synthetic rate was calculated as:
where TFFM is thigh fat‐free mass derived by DXA and alkali soluble protein (ASP) is the average soluble protein over the 6 weeks. Absolute protein breakdown rate (ABR) was calculated as:
where fractional breakdown rate (FBR) is calculated as , with the fractional growth rate (FGR) assumed to be linear over 6 weeks. Using a linear growth rate takes into account the average adaptation in growth and turnover rates over the 6 weeks; however, this limits the ability to investigate temporal changes in absolute rates.
Muscle protein, DNA and RNA concentrations (translational efficiency/capacity)
To determine muscle alkaline soluble protein, DNA and RNA concentrations (per mg dry weight), ∼15 mg muscle tissue was freeze dried. Dry tissue was homogenized in 0.2 m PCA. Following centrifugation at 11,680 g and washing with 0.2 m PCA, the resulting pellet was resuspended in 0.3 m NaOH and alkali soluble proteins were quantified by spectrophotometry (NanoDrop Lite, Thermo Scientific). Thereafter proteins were precipitated with 1 m PCA before centrifugation and removal of the supernatant for RNA quantification by UV spectrophotometry. The remaining pellet was resuspended in 2 m PCA and incubated at 70°C for 1 h before centrifugation and removal of the supernatant for quantification of DNA by spectrophotometry (Laurent et al. 1978; Smith et al. 2011).
Immunoblotting for Akt–mTORC1 signalling components and ELISA
Immunoblotting was performed on VL muscle sarcoplasmic fractions with protein concentrations determined by spectrophotometry (NanoDrop Lite); samples were standardized to 1 mg ml−1 by dilution with 3× Laemmli loading buffer and heated at 95°C for 5 min. Precisely 15 μg of proteins were loaded onto Criterion XT Bis–Tris–12% SDS‐PAGE gels (Bio‐Rad) for electrophoresis at 200 V for 1 h. As previously described (Atherton et al. 2009; Crossland et al. 2013) samples were transferred to polyvinylidene difluoride membranes for 45 min at 100 V. Membranes were subsequently blocked in 2.5% low‐fat milk (diluted in Tris‐buffered saline and 0.1% Tween‐20 (TBS‐T)) for 1 h at ambient temperature and then incubated rocking overnight at 4°C in the presence of the following primary antibodies: ∼30 mg primary antibodies (1:2000 dilution in 2.5% BSA in TBS‐T) against mechanistic target of rapamycin (mTOR)Ser2448, total mTOR, ribosomal protein S6 kinase 1 (P70S6K1)Thr389, total P70S6K1, protein kinase B (Akt)Ser473, total Akt, eukaryotic translation initiation factor 4E‐binding protein 1 (4EBP1)Thr37/46, total 4EBP1, eukaryotic elongation factor 2 (eEF2)Thr56, total eEF2, ribosomal protein S6 (rps6)ser240/244, total rps6, Beclin‐1, ERK 1/2Thr202/Tyr204 (New England Biolabs, Hitchin, UK), Cathepsin L and calpain 1 (Abcam, Cambridge, UK). For nuclear proteins, immunoblotting was performed as above with muscle initially homogenized in RIPA buffer (Thermo Scientific) with 1 mm EDTA, 1 mm activated Na3VO4 (Sigma‐Aldrich) and a complete protease inhibitor cocktail tablet (Roche, Burgess Hill, UK). Primary antibody concentrations were 1:2000 for retinoblastoma (pRB)S780 (New England Biolabs), upstream binding factor 1 (UBF1)S484, total upstream binding factor 1 (UBF1), total transcription initiation factor 1 (TIF1a) (Abcam, Cambridge, UK) and 1:1000 for C‐Myc (New England Biolabs) and transcription initiation factor 1 (RRN3)S649 (Abcam). Membranes were washed 3 × 5 min in TBS‐T, incubated in horseradish peroxidase (HRP)‐conjugated secondary antibody (New England Biolabs; 1:2000 in 2.5% BSA in TBS‐T) at room temperature for 1 h, before the last 3 × 5 min washes in TBS‐T. Membranes were exposed to Chemiluminescent HRP Substrate (Millipore Corp., Billerica, MA, USA) for 5 min and bands quantified by Chemidoc MP (Bio‐Rad, Hemel Hempstead, UK). Software measures were taken to prevent pixel saturation. Protein‐loading anomalies were corrected to Coomassie protein (Welinder & Ekblad, 2011); relative arbitrary units (RAU) were normalized to Coomassie‐stained membranes and subsequently normalized to the rest leg at that specific time point. Plasma levels of testosterone, IGF1 and myostatin were analysed in all O subjects and nine Y subjects using enzyme‐linked immunosobent assays (ELISA) according to the manufacturer's protocol with the following commercially available kits: GDF‐8/Myostatin Quanitkine ELISA Kit (DGDF80, R&D Systems, Abingdon, UK), Testosterone ELISA Kit (Abcam ab108666) and IGF1Quantikine Elisa Kit (R&D DG100).
Gene expression analysis
Total RNA was isolated by homogenizing 5–10 mg of muscle in 200 μl of TRizol (Life Technologies/Thermo Fisher Scientific) using two stainless steel beads (Tissue Lyser II, Qiagen, UK) for 1 min at 30 s–1. Samples were placed at ambient temperature for 10 min before 80 μl of chloroform was added and samples vortexed and incubated at ambient temperature for 10 min. After centrifugation at 12,000 g for 15 min at 4°C the upper aqueous layer was removed and RNA precipitated with an equal volume of isopropanol, incubation at room temperature for 10 min and subsequent centrifugation at 7000 g for 10 min at 4 °C. The pellet was washed twice with 1 ml of 80% ethanol, dissolved in 22 μl of RNA‐free water and quantified by spectrophotometry (NanoDrop Lite). For RT‐qPCR 500 ng of total RNA was reversed‐transcribed with the high‐capacity cDNA reverse transcription kit (Life Technologies) according to the manufacturer's protocol. Resulting cDNA was diluted 1:5 and 1 μl was added per well of 384‐optical well plates (Life Technologies). Exon specific primers were mixed with SYBR Select Master Mix (Life Technologies) and 11 μl of master‐mix was added to each well, with samples run in triplicate. Thermal cycling conditions were 2 min at 50°C followed by 2 min at 95°C and 40 cycles of 15 s at 95°C and 60 s at 60°C on a ViiATM 7 Real‐Time PCR System (Life Technologies). To control for RNA input, peptidylprolyl isomerase A levels were measured and target mRNA expression was quantified using the ΔΔC t method (Schmittgen & Livak, 2008).
Statistical analyses
Descriptive statistics were produced for all data sets to check for normal distribution (accepted if P > 0.05) using a Kolmogorov–Smirnov test. All data are presented as means ± SEM. Subject physical characteristics were measured via Student's t‐test and all other data sets were analysed by repeated measures two‐way ANOVA with a Bonferroni correction using Prism v. 5 (GraphPad Software, La Jolla, CA, USA). Correlations were assessed using Pearson's product moment correlation coefficient. The α level of significance was set at P < 0.05.
Results
Subject characteristics
Subject baseline characteristics are shown in Table 1. The significantly different physical characteristics were skeletal muscle index (SMI), TFFM, 1‐RM and MVC, which were lower in O vs. Y subjects. Additionally O subjects had lower circulating concentrations of testosterone and insulin‐like growth factor 1 (IGF‐1), with no difference in myostatin levels. Only young subjects significantly increased testosterone levels after the first bout of RET. Monitoring of habitual dietary and activity behaviours revealed that during the study older subjects registered on average less activity counts per day and tended to have a decreased protein intake (g (kg fat free mass (FFM))–1 day−1).
Table 1.
Subject characteristics
| Young | Old | |
|---|---|---|
| Age (years) | 23 ± 1 | 69 ± 1* |
| Height (m) | 1.80 ± 0.02 | 1.75 ± 0.02 |
| Weight (kg) | 76 ± 3 | 80 ± 3 |
| BMI (kg m−2) | 23.6 ± 1 | 25.8 ± 1 |
| FFM (kg) | 62 ± 2 | 59 ± 2 |
| ALM (kg) | 27.7 ± 1 | 25.2 ± 1 |
| ALM/h2 (ALM kg m−2) | 8.5 ± 0.2 | 8.1 ± 0.1 |
| SMI | 33 ± 1 | 29 ± 1* |
| TFFM (kg) | 6.0 ± 0.2 | 5.2 ± 2* |
| 1‐RM (N) | 546 ± 31 | 352 ± 31* |
| MVC (N m) | 232 ± 20 | 172 ± 15* |
| Activity counts | 72,584 ± 5746 | 52,445 ± 7462* |
| MET (min day−1) | ||
| METs < 1.5 | 932 ± 65 | 932 ± 18 |
| 1.5 ≤ METs < 3 | 231 ± 32 | 314 ± 94 |
| 3 ≤ METs < 6 | 150 ± 30 | 149 ± 32 |
| 6 ≤ METs < 10.2 | 3 ± 1 | 4 ± 1 |
| 10.2 ≤ METs | 1.3 ± 0.4 | 0.9 ± 0.4 |
| Protein (g (kg FFM)−1 day−1) | 1.73 ± 0.19 | 1.37 ± 0.09 |
| Hormones | Rest | Acute RET | Rest | Acute RET |
|---|---|---|---|---|
| Testosterone (ng dl−1) | 367 ± 19* | 394 ± 20*† | 274 ± 19 | 276 ± 18 |
| Myostatin (pg ml−1) | 4563 ± 403 | 4859 ± 450 | 3781 ± 373 | 3798 ± 424 |
| IGF1 (ng ml−1) | 155 ± 16* | 158 ± 16* | 84 ± 9 | 80 ± 9 |
Values are means ± SEM. *Significantly different between young and old subjects P < 0.05, †significantly different frm rest P < 0.05. 1‐RM, one repetition maximum; ALM, appendicular lean mass; FFM, fat‐free mass; MET, metabolic equivalent; MVC, maximum voluntary contraction; SMI, skeletal muscle index; TFFM, thigh fat free mass.
Muscle strength, mass and architecture
Over the training programme (Fig. 1) Y and O subjects both increased 1‐RM, Y achieving 35.0 ± 4%, P < 0.01 and O 25.3 ± 3%, P < 0.01 after 6 weeks with no difference in the increase between groups (Fig. 2 A). In contrast, measures of MVC showed increases in Y only, with the average change across all joint angles at 6 weeks of 21 ± 5% (P < 0.01) in Y and 6.3 ± 3% in O (NS) (Fig. 2 B). However, taking the peak performance at any given angle for each individual (since optimal performance may vary by angle within individual subjects), both Y and O showed increased MVC (Y, 36.2 ± 5%, P < 0.001; O 16.4 ± 4%, P < 0.05). TFFM measured by DXA showed that in addition to having greater mass before and after RET, only Y increased TFFM after the 6‐week training programme, from 6023 ± 284 to 6249 ± 315 g (P < 0.05) vs. O, 5156 ± 198 to 5218 ± 194 g (Fig. 2 C). Temporal changes in VL architecture measured via ultrasound revealed by 3 weeks, RET induced increases in Y muscle thickness (MT, from 2.46 ± 0.1 to 2.66 ± 0.1 cm, P < 0.01; Fig. 2 D), pennation angle (θ, from 18.6 ± 1 to 19.7 ± 1 deg, P < 0.01; Fig. 2 E) and fibre length (L f, from 7.8 ± 0.4 to 8.2 ± 9 0.4 cm, P < 0.01; Fig. 2 F). By 6 weeks of training there was no further increase in any of these variables (2.72 ± 0.1 cm, 20.2 ± 1 deg and 8.3 ± 0.4 cm, MT, θ and L f, respectively). In contrast, O did not significantly increase any of these measures over the 6 weeks: MT, 2.0 ± 0.1 to 2.1 ± 0.1 and 2.1 ± 0.1 cm; θ, 15.6 ± 0.6 to 16.3 ± 0.8 and 16.4 ± 0.8 deg; and L f, 7.3 ± 0.1 to 7.33 ± 0.1 and 7.36 ± 0.1 cm at 0, 3 and 6 weeks, respectively. The untrained (UT) legs showed no changes in muscle architecture from baseline over the 6 weeks.
Figure 2. Muscle strength, mass and architecture.
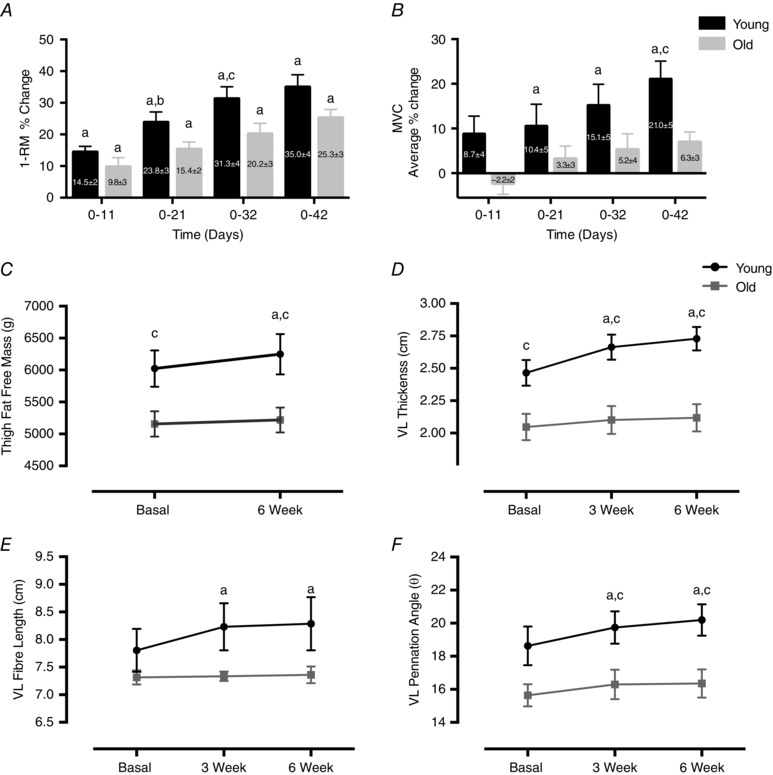
Time course of changes in T legs as percentage change in 1‐RM from baseline (A), average percentage MVC from baseline (B), thigh fat free mass 0–6 weeks (C), VL MT (D), L f (E) and θ (F). Values are means ± SEM. aSignificantly different from baseline, P < 0.05; bsignificantly different from previous time point, P < 0.05; csignificantly different from old, P < 0.05. 1‐RM, one repetition maximum; L f, fibre length; MT, muscle thickness; T, trained; VL, vastus lateralis; θ, pennation angle.
Muscle RNA, DNA and protein concentrations
There were no changes in the alkali soluble protein (ASP) per wet weight muscle (μg mg−1) in trained (T) legs of either group throughout the 6‐week RET period, although Y had greater ASP at 3 weeks than O (613.5 ± 25 vs. 480.5 ± 33, P < 0.05; Fig. 3 A). The ratio of ASP:DNA (μg μg−1), a measure of cell size, displayed a trend to increase by 6 weeks (88 ± 8 to 103 ± 5, P = 0.1), whilst O showed no change at any time point (Fig. 3 D). RNA content (μg mg−1) increased in Y only at 3 weeks (2.75 ± 0.2 to 3.91 ± 0.2, P < 0.01) with a trend to increase at 6 weeks (3.38 ± 0.1, P = 0.1), whilst there was no change in RNA content in O (Fig. 3 B). The DNA content (μg (mg wet weight of muscle)−1; Fig. 3 C) did not change in either group over the training period; however, the ratio of μg RNA:μg DNA a reflection of ribosomal capacity per unit DNA, increased in Y at 3 weeks (0.47 ± 0.05 to 0.62 ± 0.05, P < 0.05) and remained increased at 6 weeks (0.64 ± 0.03, P < 0.01, Fig. 3 E). In contrast to Y, there was no change in RNA:DNA in O (0.53 ± 0.05 to 0.57 ± 0.05 and 0.58 ± 0.05, NS, at 0, 3 and 6 weeks, respectively). Finally the ratio of RNA:ASP (μg mg−1), primarily a measure of ribosomal capacity (Laurent et al. 1978; Waterlow et al. 1978), was elevated at 3 weeks (5.0 ± 0.3 to 6.0 ± 0.3, P = 0.06) and increased at 6 weeks (6.1 ± 0.3, P < 0.05) with no change in O (4.9 ± 0.5 to 5.7 ± 0.4 and 5.6 ± 0.5, NS) at 0, 3 and 6 weeks, respectively (Fig. 3 F).
Figure 3. Muscle protein, RNA and DNA concentrations.
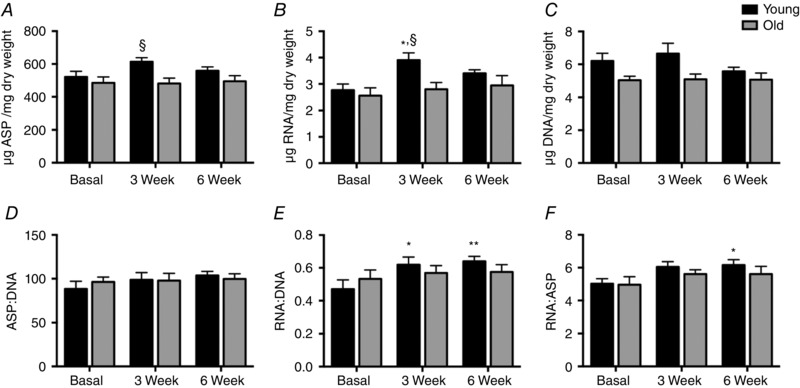
Time course of the changes in μg (mg dry weight muscle)−1: ASP (A), RNA (B) and DNA (C); and ratios of ASP:DNA (D), RNA:DNA (E) and RNA:ASP (F). Values are means ± SEM. Significantly different from baseline: * P < 0.05, ** P < 0.01; significantly different from old: § P < 0.05. ASP, alkali soluble protein.
Muscle protein synthesis, absolute and fractional growth rates
There was no difference in muscle protein synthesis (MPS) in the UT leg between Y and O; 1.35 ± 0.08% day−1 and 1.34 ± 0.09% day−1, respectively. With the onset of RET, only Y increased MPS from 0 to 3 weeks (1.6 ± 0.01% day−1, P < 0.05 vs. O, 1.49 ± 0.08% day−1, NS); however, by 6 weeks MPS was no longer increased in Y (1.45 ± 0.05% day−1) and remained unchanged in O (1.39 ± 0.1% day−1; Fig. 4 A). Absolute synthetic rate (ASR; g d−1) was increased in the T leg only in the Y group over the 6 weeks (8.1 ± 0.1 to 9.3 ± 0.7; P < 0.05), with similar findings of increased fractional growth rate (FGR; % day−1) in Y only (–0.002 ± 0.016 to 0.086 ± 0.02, P < 0.001; Fig. 4 B and C). In contrast there was no change in absolute breakdown rate (ABR; g d−1) observed with either group over the 6‐week RET period (Y, 8.5 ± 0.6 to 8.1 ± 0.8; O, 6.6 ± 0.5 to 7.3 ± 0.7 g; both NS; Fig. 4 D).
Figure 4. Muscle protein synthesis, absolute synthetic rate, fractional growth rate and absolute breakdown rate.
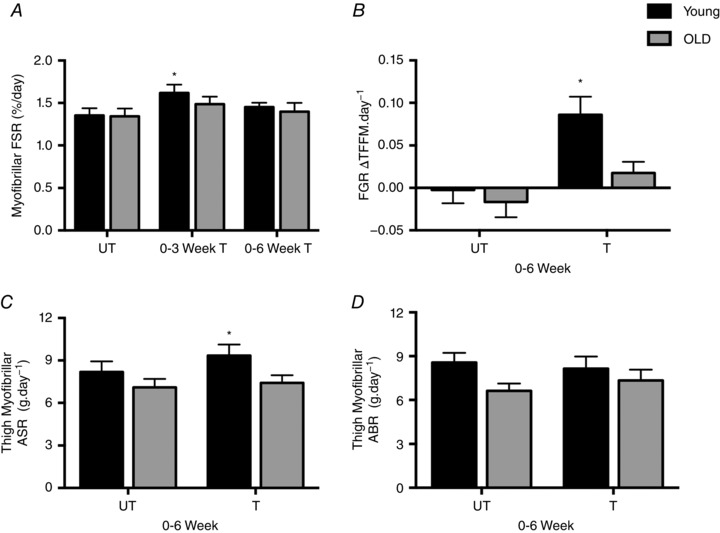
A, muscle protein synthesis (MPS) rates in UT and T legs in Y and O individuals. B, fractional growth rate (FGR). C, absolute synthetic rate (ASR). D, absolute breakdown rate (ABR). Values are means ± SEM. *Significantly different from UT, P < 0.05. T, trained; UT, untrained.
Intramuscular mRNA expression and signalling
There were no differences between Y and O in the baseline expression of any of the genes measured (Table 2). However, 60–90 min after the first exercise bout Y showed increases in c‐MYC expression (P < 0.05), indicating activation of ribosomal transcription (P < 0.05). Additionally, only O increased MAFBx and RPS26 60–90 min after the first exercise bout in T legs compared to UT (P < 0.05) and showed a trend for increase with MuRF‐1 (P = 0.06). Muscle anabolic signalling 60–90 min (Fig. 5) after the first exercise bout showed there were significant increases compared to UT in phosphorylation of P70SK1Thr389, RPS6ser240/244 and ERKThr202/Tyr204. Further, only Y increased total levels of c‐MYC and TIF1a after the first exercise bout, whilst O increased phosphorylation of RPS6ser240/244 after the first exercise bout and MuRF‐1 60–90 min after RE at 3 weeks.
Table 2.
Muscle mRNA expression
| Basal | 3 weeks | 6 weeks | |||||
|---|---|---|---|---|---|---|---|
| Rest | Acute RET | Rest | Acute RET | Rest | Acute RET | ||
| C‐MYC | |||||||
| 5’‐GGTAGTGGAAAACCAGCAGCC‐3’ | Y | 1.00 ± 0.4 | 4.01 ± 1.6* | 1.52 ± 0.6 | 2.45 ± 0.6 | 1.16 ± 0.3 | 2.49 ± 0.5 |
| 5’‐TCTCCTCCTCGTCGCAGTA‐3’ | O | 0.75 ± 0.3 | 1.89 ± 0.8 | 0.66 ± 0.2 | 2.95 ± 0.9 | 0.73 ± 0.2 | 2.73 ± 0.7 |
| POL1RA | |||||||
| 5’‐CCTCAAGGTATCGCCCAGTC‐3’ | Y | 1.00 ± 0.1 | 1.41 ± 0.2 | 1.25 ± 0.2 | 1.49 ± 0.2 | 1.41 ± 0.2 | 1.03 ± 0.2 |
| 5’‐GGCAACTTCTGTTCTTGGGC‐3’ | O | 1.21 ± 0.2 | 1.36 ± 0.2 | 0.95 ± 0.1 | 0.97 ± 0.2 | 0.81 ± 0.1 | 1.41 ± 0.3 |
| POL1RB | |||||||
| 5’‐TACTGTGCAACTTGGGGGTC‐3’ | Y | 1.00 ± 0.2 | 1.62 ± 0.4 | 1.93 ± 0.4 | 1.39 ± 0.3 | 1.71 ± 0.3 | 1.39 ± 0.2 |
| 5’‐GAGAATCTGCGATGCCTGGA‐3’ | O | 1.24 ± 0.4 | 1.22 ± 0.3 | 1.47 ± 0.5 | 1.69 ± 0.5 | 1.33 ± 0.3 | 2.60 ± 0.6 |
| TAF1A | |||||||
| 5’‐AGGTTTAGCGCCTGCTCATA‐3’ | Y | 1.00 ± 0.3 | 1.62 ± 0.4 | 1.47 ± 0.3 | 0.84 ± 0.2 | 1.21 ± 0.1 | 0.69 ± 0.1 |
| 5’‐CTGAAATCACTCATACCCGCCT‐3’ | O | 1.14 ± 0.3 | 1.28 ± 0.4 | 1.27 ± 0.3 | 1.44 ± 0.3 | 1.55 ± 0.6 | 2.01 ± 0.6 |
| TIF1A | |||||||
| 5’‐CATTTTGTGCCTCCCCGAGT | Y | 1.00 ± 0.3 | 1.43 ± 0.5 | 1.61 ± 0.5 | 0.87 ± 0.3 | 1.61 ± 0.3 | 0.98 ± 0.3 |
| 5’‐GTATTGGCATGAGAAACCACGG‐3’ | O | 1.29 ± 0.4 | 1.25 ± 0.5 | 1.04 ± 0.2 | 1.18 ± 0.3 | 1.49 ± 0.4 | 1.57 ± 0.4 |
| UBF | |||||||
| 5’‐AAGAAGCCTCCCATGAACGG‐3’ | Y | 1.00 ± 0.2 | 1.59 ± 0.4 | 1.34 ± 0.2 | 1.77 ± 0.4 | 1.51 ± 0.2 | 1.79 ± 0.6 |
| 5’‐CGGCCAGCTTTTTGTAGTGC‐3’ | O | 1.42 ± 0.4 | 2.00 ± 0.6 | 1.98 ± 0.6 | 0.69 ± 0.2 | 1.58 ± 0.3 | 3.03 ± 0.6 |
| MuRF1 | |||||||
| 5’‐GTGTTTGGGGCTCACCAGGC‐3’ | Y | 1.00 ± 0.2 | 2.86 ± 0.8 | 0.67 ± 0.1 | 1.61 ± 0.4 | 1.22 ± 0.5 | 1.01 ± 0.4 |
| 5’‐ACCTGGTGGCTATTCTCCTTGGT‐3’ | O | 1.41 ± 0.3 | 3.86 ± 1.5 | 1.01 ± 0.3 | 1.81 ± 0.5 | 1.74 ± 0.9 | 2.43 ± 0.8 |
| MAFbx | |||||||
| 5’‐CTTTCAACAGACTGGACTTCTCGA‐3’ | Y | 1.00 ± 0.2 | 2.05 ± 0.4 | 1.19 ± 0.3 | 2.07 ± 0.3 | 1.47 ± 0.2 | 1.26 ± 0.2 |
| 5’‐CAGCTCCAACAGCCTTACTACGT‐3’ | O | 1.26 ± 0.2 | 3.12 ± 0.9* | 1.20 ± 0.2 | 2.23 ± 0.7 | 1.38 ± 0.4 | 2.12 ± 0.6 |
| 20S proteasome | |||||||
| 5’‐CGTTTTCAACGGAGGTACTA‐3’ | Y | 1.00 ± 0.31 | 1.31 ± 0.4 | 1.36 ± 0.3 | 1.10 ± 0.3 | 1.41 ± 0.3 | 1.13 ± 0.2 |
| 5’‐TCAGCGTAAGACAGTCTCCA‐3’ | O | 1.21 ± 0.22 | 1.47 ± 0.5 | 1.18 ± 0.3 | 1.27 ± 0.3 | 1.69 ± 0.5 | 1.94 ± 0.6 |
| Caspase 3 | |||||||
| 5’‐TCCACAGCACCTGGTTATTATTC‐3’ | Y | 1.00 ± 0.3 | 1.45 ± 0.4 | 1.38 ± 0.3 | 1.00 ± 0.2 | 1.73 ± 0.4 | 1.52 ± 0.4 |
| 5’‐GCGTCAAAGGAAAAGGACTC‐3’ | O | 0.79 ± 0.2 | 0.91 ± 0.3 | 1.09 ± 0.3 | 1.27 ± 0.3 | 1.27 ± 0.4 | 1.85 ± 0.6 |
| Calpain L | |||||||
| 5’‐GCCAAGCAGGTGAAC‐3’ | Y | 1.00 ± 0.3 | 1.81 ± 0.8 | 1.53 ± 0.4 | 1.01 ± 0.2 | 2.35 ± 0.9 | 0.80 ± 0.2 |
| 5’‐TGAAGTCTCGGAATGACATC‐3’ | O | 1.39 ± 0.3 | 1.95 ± 0.7 | 1.82 ± 0.5 | 2.01 ± 0.6 | 2.11 ± 0.7 | 2.55 ± 0.7 |
| RPS5 | |||||||
| 5’‐ATCATCAACAGTGGTCCCCG‐3’ | Y | 1.00 ± 0.3 | 1.52 ± 0.7 | 1.24 ± 0.3 | 1.13 ± 0.3 | 1.22 ± 0.2 | 1.18 ± 0.2 |
| 5’‐AGATGGCCTGGTTCACACG‐3’ | O | 1.42 ± 0.4 | 1.24 ± 0.4 | 1.80 ± 1.0 | 1.05 ± 0.3 | 1.32 ± 0.4 | 1.68 ± 0.3 |
| RPSA | |||||||
| 5’‐CCCTACTGAAGACTGGAGCG‐3’ | Y | 1.00 ± 0.2 | 1.07 ± 0.2 | 0.96 ± 0.2 | 0.78 ± 0.2 | 0.78 ± 0.1 | 0.81 ± 0.2 |
| 5’‐AGAGCCTATGCAAGAACAGCTT‐3’ | O | 0.77 ± 10.1 | 1.19 ± 0.4 | 0.75 ± 0.3 | 0.45 ± 0.1 | 0.65 ± 0.1 | 0.76 ± 0.1 |
| RPS26 | |||||||
| 5’‐CGAGCGTCTTCGATGCCTAT‐3’ | Y | 1.00 ± 0.1 | 0.97 ± 0.1 | 1.11 ± 0.1 | 0.97 ± 0.1 | 1.01 ± 0.1 | 0.87 ± 0.1 |
| 5’‐CAGGTCTAAATCGGGGTGGG‐3’ | O | 1.00 ± 0.1 | 1.48 ± 0.2* | 1.16 ± 0.1 | 1.32 ± 0.2 | 1.04 ± 0.1 | 1.17 ± 0.2 |
| RPS3 | |||||||
| 5’‐GGAAGTTTGTCGCTGATGGC‐3’ | Y | 1.00 ± 0.1 | 1.23 ± 0.2 | 1.19 ± 0.2 | 1.27 ± 0.2 | 1.17 ± 0.1 | 0.92 ± 0.1 |
| 5’‐ACATTCTGTGTTCTGGTGGCT‐3’ | O | 1.22 ± 0.3 | 1.49 ± 0.3 | 1.26 ± 0.2 | 1.35 ± 0.2 | 1.21 ± 0.2 | 1.82 ± 0.3 |
| RPL13A | |||||||
| 5’‐TAAACAGGTACTGCTGGGCCG | Y | 1.00 ± 0.2 | 1.31 ± 0.2 | 1.52 ± 0.2 | 1.19 ± 0.2 | 1.37 ± 0.1 | 1.11 ± 0.2 |
| 5’‐CTCGGGAAGGGTTGGTGTTC | O | 1.44 ± 0.4 | 1.31 ± 0.3 | 1.19 ± 0.3 | 1.31 ± 0.4 | 1.11 ± 0.3 | 1.53 ± 0.3 |
| RPL11 | |||||||
| 5’‐AGGGTCTAAAGGTGCGGGA‐3’ | Y | 1.00 ± 0.2 | 1.91 ± 0.5 | 2.19 ± 0.4 | 1.12 ± 0.3 | 1.73 ± 0.2 | 1.09 ± 0.4 |
| 5’‐AGTCCAGGCCGTAGATACCA‐3’ | O | 1.33 ± 0.4 | 1.55 ± 0.5 | 1.05 ± 0.3 | 1.31 ± 0.4 | 1.15 ± 0.3 | 1.95 ± 0.4 |
| Androgen receptor | |||||||
| 5’‐TCAGCATTATTCCAGTGGATG‐3’ | Y | 1.00 ± 0.2 | 0.99 ± 0.2 | 0.99 ± 0.3 | 1.04 ± 0.2 | 1.82 ± 0.4 | 0.83 ± 0.1 |
| 5’‐GGAGCTTGGTGAGCTGGTAG‐3’ | O | 0.88 ± 0.2 | 1.15 ± 0.3 | 1.06 ± 0.3 | 1.45 ± 0.3 | 0.90 ± 0.2 | 1.21 ± 0.3 |
| Myostatin | |||||||
| 5’‐GCTGCGCCTGGAAACAGCTC‐3’ | Y | 1.00 ± 0.2 | 1.42 ± 0.4 | 2.08 ± 0.5 | 1.17 ± 0.4 | 2.74 ± 1.1 | 1.39 ± 0.3 |
| 5’‐ATCAGTTCCCGGAGTGGAGGC‐3’ | O | 2.32 ± 0.5 | 1.96 ± 0.6 | 2.78 ± 0.6 | 2.01 ± 0.3 | 2.58 ± 0.9 | 1.72 ± 0.3 |
Data expressed as fold change from young (means ± SEM). Forward primer is the upper sequence and reverse primer lower sequence. *Significantly different from UT leg at that time point. MAFbx, muscle atrophy F‐box; MuRF1, muscle RING finger 1; RPL, ribosomal protein large; RPS, ribosomal protein small; TAF1A, TBP‐associated factor 1A; TIF1a, transcription initiation factor 1; UBF, upstream binding factor 1. POL1RA, RNA polymerase 1 subunit A; POL1RB, RNA polymerase 1 subunit B.
Figure 5. Intramuscular signalling.
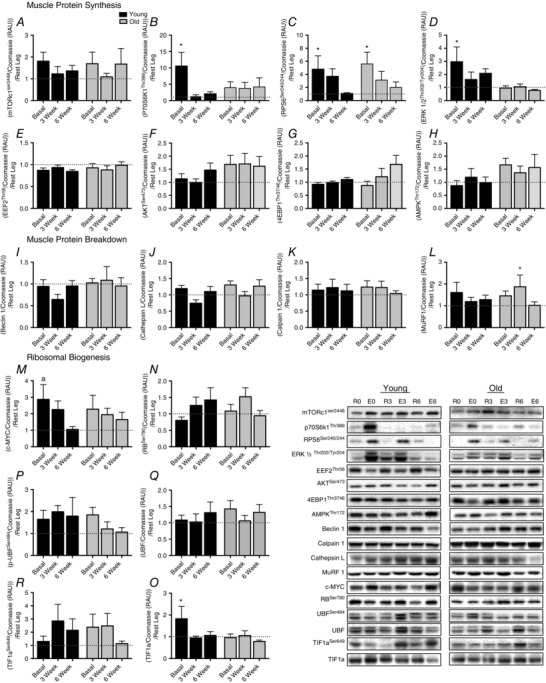
Relative change compared with UT in mTORc1Ser2448 (A), p70S6K1Thr389 (B), rps6ser240/244 (C), ERK1/2Thr202Tyr204 (D), eEF2Thr56 (E), AktSer473 (F), 4EBP1Thr37/46 (G), AMPKThr172 (H), Beclin‐1 (I), Cathepsin L (J), calpain 1 (K), MuRF1 (L), c‐MYC (M), pRBSer780 (N), TIF1a (O), UBF1Ser484 (P), UBF1 (Q), TIF1aSer649 (R). Values are means ± SEM. *Significantly different from UT, P < 0.05. UT, untrained.
Correlations
RNA:DNA was not correlated with MT in the UT state; however, at 3 weeks MT was correlated with the RNA:DNA content being driven primarily by changes in the young subjects (Fig. 6 A), although RNA:DNA was not correlated with TFFM at 6 weeks (Fig. 6 B). The change in P70S6K1 phosphorylation showed a trend for association with the change in TFFM across all subjects (P = 0.07) (Fig. 6 D), although it was not correlated with the change in MT at 3 weeks (Fig. 6 C). The acute RE‐induced levels of testosterone were correlated with the change in MT at 3 weeks (Fig. 6 E) and this change in acute testosterone levels was correlated with the change in TFFM at 6 weeks (Fig. 6 F). Protein intake across all subjects showed a trend for correlation with the early changes in MT at 3 weeks (Fig. 6 G, P = 0.07), although it was not correlated with the change in TFFM at 6 weeks (Fig. 6 H).
Figure 6. Correlations.
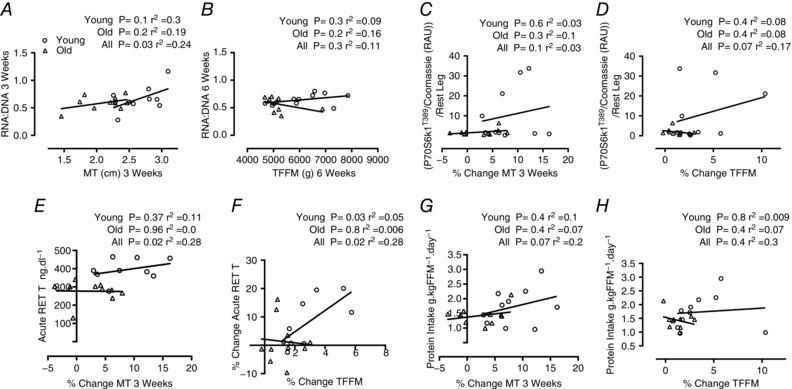
Correlations between RNA:DNA at 3 weeks and MT at 3 weeks (A), RNA:DNA at 6 weeks and TFFM at 6 weeks (B), the increase in P70S6K1T389 after first RET bout and the percentage change in MT at 3 weeks (C), the increase in P70S6K1T389 after first RET bout and the percentage change in TFFM at 6 weeks (D), testosterone after first RET bout and the percentage change in MT at 3 weeks (E), percentage change in testosterone after first RET bout and the percentage change in TFFM at 6 weeks (F), protein intake (g (kg FFM)−1 day−1) and percentage change in MT at 3 weeks (G), and protein intake (g (kg FFM)−1 day−1) and percentage change in TFFM at 6 weeks (H). MT, muscle thickness; RET, resistance exercise trained; TFFM, thigh fat free mass.
Discussion
There has been a great deal of interest in recent years in uncovering the mechanisms underlying hypertrophic adaptations to exercise, and how these adaptations are influenced by age. Yet much of this information has been largely derived from ‘acute’ metabolic and molecular studies, which may not accurately reflect the true chronic adaptation. Therefore this study used novel D2O tracer techniques to investigate the temporal effects of RET on longer term MPS, hypertrophy and strength adaptations in groups of young and older individuals. This provides the first evidence that blunted hypertrophic responses in older individuals are underpinned by chronic deficits in MPS reflecting blunted ribosomal biogenesis and translational efficiency and lower anabolic hormone profiles, thus highlighting the multifactorial nature of the blunted hypertrophic responses.
Whether ageing impairs hypertrophic responses to RET remains contentious. For instance, RET‐induced hypertrophy of thigh muscles has been shown to be equal (Häkkinen et al. 1998 b; Mayhew et al. 2009) or impaired (Kosek et al. 2006; Greig et al. 2011; Mero et al. 2013) with age. In the present study, we utilized a 6‐week‐long progressive RET training programme during which we detected no significant increases in either TFFM (DXA) or hypertrophy‐related alterations in architecture (ultrasound) in old individuals indicative of blunted RET‐induced hypertrophy in thigh muscles, which are among the muscle groups more profoundly affected by ageing (Janssen et al. 2000) and most important for stability and preventing falls. Similarly, studies of an equal time period in older males have reported no increases in total lean body mass (Fragala et al. 2014) and similarly no change in VL muscle architecture or TFFM after 6 weeks RET (Scanlon et al. 2014). Indeed, even with long‐term (∼20 weeks) progressive RET, muscle gains are subtle, e.g. ∼1 kg in lean mass (Peterson et al. 2011), and diminished compared to younger counterparts (Phillips et al. 2012). Intriguingly, these data reflect the notion that RET‐induced hypertrophy occurs rapidly (first 3–4 weeks), diminishing thereafter (Brook et al. 2015). As such, long‐term stimuli are neither a prerequisite for muscle growth nor likely to significantly alter age‐related blunted responses. Thus, our present data support the prevailing notion of impaired hypertrophic responses to RET with ageing, although, with time, old individuals will see benefits in whole body strength (Peterson et al. 2010), and noticeable increases in 1‐RM and MVC were seen here after 6 weeks. Improvements in strength, whether dynamic or static, with little or no increases in muscle mass essentially derive from improvements in ‘muscle quality’. Whilst improvements in muscle quality are generally attributed to increased neural activity (Häkkinen et al. 1998 a; Tracy et al. 1999), improved strength may occur by many other modifiable aspects of muscle contraction (Fragala et al. 2015) improving physical function (Fiatarone et al. 1994; Fragala et al. 2014) and ultimately quality of life (Masel et al. 2009).
Skeletal muscle mass is controlled by the balance between MPS and MPB. Acute amino acid stable isotope tracer studies have shown no difference in fasting or resting MPS or MPB as a result of ageing (Volpi et al. 2001). The present study provides the first report of longer term MPS (over weeks) in young and older individuals in the same study under ‘basal’, conditions. The longer term D2O‐derived measures represent free‐living rates of MPS incorporating integrated responses to habitual nutritional intake and physical activity behaviours in young and older recruits. Since loss of muscle mass beyond ∼70 years of age occurs at a rate of 0.5–1% year−1 (and assuming no changes in MPB), daily MPS would only need to be decreased 0.0015–0.003% day−1 to account for such a loss. Thus, it is unsurprising that cumulative MPS (even over weeks) was not detectably modified by age. Instead, older muscle demonstrates blunted responses to anabolic stimuli key to regulating muscle homeostasis, i.e. nutrition and physical activity (Cuthbertson et al. 2005; Kumar et al. 2009 b). For example, the RET‐induced stimulation of MPS is attenuated (< 6 h) after a bout of RE in older vs. young subjects (Kumar et al. 2009 b). A similar result has been reported for responses to RET at 3, 6 and 24 h (Fry et al. 2011) with others also showing reduced rates in older individuals being sustained 24 h after a bout of RET (Mayhew et al. 2009). This study shows that findings from the acute setting translate into longer term deficits in MPS in the elderly, a notion that would not necessarily be obvious given that acute bouts of exercise (on which the foundation of anabolic resistance has been built) do not correlate to longer term hypertrophy (Mitchell et al. 2014). Finally, it is noteworthy that measures of MPS using D2O incorporate behavioural activities outside of study control, i.e. habitual physical activity and dietary behaviours. Although the older subjects registered fewer activity counts in this study, they were more active than an average population of their age group (Schrack et al. 2014) and additionally spent equal time in physical activities requiring the same level of metabolic equivalent (MET) as younger recruits. Studies where drastic decrements in activity are enforced have negative effects on muscle (Olsen et al. 2008; Krogh‐Madsen et al. 2010; Breen et al. 2013); in contrast, our old subjects are likely to maintain lower levels of activity (Dipietro, 2001) in which we are unaware of any evidence that habitual ‘inactivity’ has deleterious consequences upon RET‐induced muscle hypertrophy. Habitual behaviours may differ both among individuals and across age. It cannot therefore be entirely ruled out that slight age‐related behavioural differences could contribute to anabolic resistance, in both acute experimental settings and the chronic accumulation of these over time. However, intervention studies would be needed to resolve these interesting concepts and may warrant further study.
One of the key facets regulating MPS is ribosomal activity (so called ‘translational efficiency’). The acute simulation of MPS by RE relies upon increased translational efficiency (Chesley et al. 1992; Drummond et al. 2009). Acute RE (and nutrient interactions) converge on the pleiotropic protein kinase mTORc1, with subsequent downstream signalling enhancing translation initiation and elongation, a pathway essential to acute RE‐induced stimulation of MPS (Drummond et al. 2009). We and others have previously shown that P70S6K1 signalling after RE is blunted in older individuals (Kumar et al. 2009 b; Fry et al. 2011). In the present study we were able to look at the temporal activation of mTORc1 signalling in young and older individuals. In doing so, we found that P70S6K1 increased only after the first bout of exercise and that this was only observed in the younger group, while RPS6 phosphorylation was increased in both young and older individuals, again only after the first bout. The increase in P70S6K1 activation displayed a trend to be correlated with the increase in TFFM, adding to previous associations between P70S6K1 activation and hypertrophy (Baar & Esser, 1999) and dysregulation in MPS responses with age (Kumar et al. 2009 a; Fry et al. 2011). Additionally, these findings are in agreement with the notion that hypertrophy occurs early and also that RE‐trained individuals display attenuations in P70S6K1 (Coffey et al. 2006; Gonzalez et al. 2015). The absolute onset of attenuated signalling responses is unclear, and acute stimulation of anabolic signalling responses may remain up until before the 3‐week time point, with the duration and pattern of these responses unknown. However, early transcriptional responses are quickly modifiable (Murton et al. 2014) and activation of key signalling proteins is attenuated with repeated exercise stimulus (Ogasawara et al. 2013). This suggests that with prolonged training these signalling pathways become refractory to loading accounting for reduced rates of hypertrophy with training progression. Nonetheless, while age‐related blunting was seen in P70S6K1, this was not a uniform response across signals following the first exercise bout, suggesting that blunted translational efficiency is one aspect of, but perhaps not the entire explanation for, blunted muscle hypertrophy that is seen in older individuals.
In addition to increasing translational efficiency, prolonged muscle overload produces increases in RNA content and therefore capacity for protein synthesis (Laurent & Sparrow, 1977; Kirby et al. 2015), a subject matter which has been the of interest in renewed research (Nader et al. 2014; Figueiredo et al. 2015, 2016; Kirby et al. 2015; Stec et al. 2015; West et al. 2016). Indices of synthetic capacity (RNA:protein and RNA:DNA ratios; Laurent et al. 1978; Smith et al. 2011) increased early into RET in young individuals, plateauing by 3 weeks RET, and furthermore these responses were blunted with age. Additionally, with 3 weeks RET, MT was correlated with indices of synthetic capacity (RNA:DNA), which was not evident in the UT state, suggesting early hypertrophy is supported by early increases in synthetic capacity. To gain insight into the molecular mechanism regulating ribosomal biogenesis, upstream signalling pathways controlling ribosomal DNA (rDNA) transcription such as c‐MYC, which controls many aspects of cell growth and ribosomal biogenesis, were investigated. c‐MYC gene expression and protein abundance were increased in young individuals but were blunted with age, which is in line with previous reports showing that markers of ribosomal biogenesis are reduced in older adults 24 h after a bout of RET and suggesting rDNA transcription is compromised with age (Stec et al. 2015). Successful rDNA transcription requires multiple transcription factors, including UBF and TIF1a, with their activities controlled by many phosphorylation sites and upstream signalling pathways (reviewed in Kusnadi et al. 2015). However, similar to previous studies, TIF1a increased only in young individuals ostensibly driving rDNA transcription (Stec et al. 2015 a). Together, our data point to impairments in the regulation of translational capacity in addition to the aforementioned translational efficiency.
Beyond mechano‐sensitive pathways, muscle mass and RET‐induced muscle hypertrophy are also regulated by hormones, e.g. testosterone, IGF‐1, myostatin, etc. (Bhasin et al. 1996; Barton‐Davis et al. 1998; Whittemore et al. 2003; Kvorning et al. 2006). Ageing negatively regulates the ‘anabolic’ environment with declining endocrine function resulting in chronic low levels of many hormones such testosterone and IGF‐1 (Leifke et al. 2000). Whilst myostatin negatively regulates muscle mass, growth hormone, IGF‐1 and testosterone, whose levels when enhanced, or restored in older age, act as powerful anabolic agents and can substantially increase muscle mass (Bhasin et al. 2001; Sattler et al. 2009; Neto et al. 2015). Compromised hormonal balances with age are therefore likely to impair exercise adaptations, yet the precise influence in early exercise adaptations is unclear. Upon examination of these, we found no difference in myostatin between young and old individuals, and this was not acutely affected by RE. Similarly, sensitive liquid chromatography tandem mass spectrometry methods have demonstrated that in males, serum myostatin acts as a homeostatic regulator of muscle mass rather than a cause of sarcopenia (Bergen et al. 2015), further adding to oppose evidence that myostatin is implicated in the age relate muscle mass loss (Yarasheski et al. 2002). Circulating levels of many anabolic hormones decrease with age, and we found significantly lower circulating levels of testosterone and IGF‐1; moreover only younger individuals increased testosterone after the first bout of RE. Prevention of testosterone production in young males through provision of a GnRH inhibitor has been shown to prevent RET‐induced hypertrophy (Kvorning et al. 2006), whilst blunted hypertrophy has previously been reported in older men displaying reduced RE‐induced increases in testosterone (Kraemer et al. 1999). This highlights impaired endocrine response in older age, yet whether a lack of acute hormonal responses contributes to diminished hypertrophy is unclear. Acute RE‐induced increase in testosterone was correlated with the increase in TFFM, and this was strongest in Y where testosterone had increased. Further, whole body RET in older subjects with low testosterone shows minimal (∼+0.6%) changes in fat‐free mass (FFM) after 12 months RET (Hildreth et al. 2013) while no change in FFM occurred in elderly males with low normal testosterone after 24 weeks RET (Kvorning et al. 2013). Both studies showed greater improvements in FFM when testosterone supplementation and RET were combined. Together with our data this demonstrates that chronic low levels of testosterone and possibly a lack of acute increases may play a significant role in attenuated hypertrophy with age.
In addition to transient increases in MPS with RE, MPB is also increased (Phillips et al. 1997), probably to remove old or damaged proteins. The protein ligases muscle RING finger 1 (MuRF1) and muscle atrophy F‐box (MAFbx) are markers of ubiquitin proteasome pathway activity, which is thought to be predominant in acute RE responses (Fry et al. 2013). However, after a single bout of exercise, expression of MuRF‐1 and or MAFbx have shown no change (Greig et al. 2011; Fry et al. 2013), equal increases (Fry et al. 2013; Stefanetti et al. 2014) or a greater increase with age (Raue et al. 2007) and therefore much uncertainty exists within the literature. We found an initial increases in MAFbx expression and a tendency towards greater MuRf1 expression that only occurred in our older volunteers. Our measures were made 60–90 min after RET, in comparison to ≥ 2 h in these other studies and so our results may highlight key early differences in MPB. Measures of the FBR 24 h after RET have shown no difference between young and old; however, whether there are any earlier differences is unknown (Fry et al. 2013). Nonetheless, despite greater initial increases in MPB markers with age, there was no increase in ABR, highlighting that lack of muscle hypertrophy in older age is likely to be predominantly driven by blunted MPS responses rather than increases in MPB.
We also need to acknowledge limitations within our study. The data are limited to changes between three time points and each individual's response may temporally differ across the study period. Further, biopsy timings within this study reflect acute responses to RE and how these adapt over time, and do not demonstrate how resting levels of gene and protein expression may change with training. Additionally, although D2O permits long‐term measures of MPS, these are highly integrated, incorporating everyday habitual regimes with RE responses. In this study, subjects followed their usual habitual physical activity (potential impacts as previously discussed) and diet, which we recorded. In this study, older subjects tended, i.e. non‐significantly, to consume lower protein levels than younger subjects, which could theoretically impact our findings since acute protein feeding coupled to RET enhances MPS (Bukhari et al. 2015). That said, results of protein supplementation with RET in ageing have been mixed, i.e. shown to enhance (Esmarck et al. 2001) or not affect (Verdijk et al. 2009) muscle hypertrophy, while the effects of habitual protein feeding behaviours in this context of RET, ageing and hypertrophy remain poorly defined. That said, since protein intake was not correlated with muscle mass changes in young, old or all subjects combined in our study, any slight differences in protein intake are highly unlikely to be responsible for the observed anabolic resistance. In conclusion we have shown that age‐related deficits in anabolic responses to RET are multifactorial, a product of attenuations in translational efficacy and capacity and unfavourable hormone profiles, culminating in reduced MPS and compromised overall hypertrophy.
Additional information
Competing interests
No conflicting interests.
Author contributions
All experiments were performed at the Clinical, Metabolic and Molecular Physiology laboratories, Royal Derby Hospital, University of Nottingham. D.J.W., K.S., P.L.G., N.J.S. and P.J.A. carried out the conception and design; M.S.B., W.K.M., B.E.P. and D.J.W. performed the experiments; M.S.B., B.E.P., D.J.W., K.S. and P.J.A. analysed the data; M.S.B., B.E.P., D.J.W., K.S. and P.J.A. interpreted the results; M.S.B., B.E.P., D.J.W., K.S. and P.J.A. drafted the manuscript; M.S.B., B.E.P., W.K.M., D.J.W., K.S., N.J.S., J.N.L., P.L.G. and P.J.A. edited and revised the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by a grant from The Physiological Society (to P.J.A. and K.S.), a project grant from the Dunhill Medical Trust (R264/1112) (to K.S., P.J.A. and D.J.W.), and a Medical Research Council Confidence in Concept award (CIC12019; to P.J.A., P.L.G., N.J.S. and K.S.) D.J.W. is a Medical Research Council‐Arthritis Research United Kingdom (MRC‐ARUK) Centre‐funded postdoctoral research fellow, and M.S.B. is supported by a University of Nottingham and BBSRC DTP award. Equipment was funded through monies provided from an award by the MRC‐ARUK Centre to the Universities of Nottingham and Birmingham.
Acknowledgements
The authors are greateful for the clinical, technical and administrative support of Margaret Baker, Amanda Gates and Tanya Fletcher, and to Professor Marco Narici for his guidance in obtaining ultrasound measures.
References
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K & Rennie MJ (2010). Muscle full effect after oral protein: Time‐dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92, 1080–1088. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Miller BF, Burd NA, et al (2015). Commentaries on Viewpoint: What is the relationship between acute measure of muscle protein synthesis and changes in muscle mass? J Appl Physiol 118, 498–503. [Google Scholar]
- Atherton PJ & Smith K (2012). Muscle protein synthesis in response to nutrition and exercise. J Physiol 590, 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton PJ, Szewczyk NJ, Selby A, Rankin D, Hillier K, Smith K, Rennie MJ & Loughna PT (2009). Cyclic stretch reduces myofibrillar protein synthesis despite increases in FAK and anabolic signalling in L6 cells. J Physiol 587, 3719–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K & Esser K (1999). Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise.Am J Physiol Cell Physiol 276, C120–C127. [DOI] [PubMed] [Google Scholar]
- Barton‐Davis ER, Shoturma DI, Musaro A, Rosenthal N & Sweeney HL (1998). Viral mediated expression of insulin‐like growth factor I blocks the aging‐related loss of skeletal muscle function. Proc Natl Acad Sci USA 95, 15603–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen HR, Farr JN, Vanderboom PM, Atkinson EJ, White TA, Singh RJ, Khosla S & LeBrasseur NK (2015). Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry‐based assay. Skelet Muscle 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A & Casaburi R (1996). The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335, 1–7. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha‐hikim I, Shen R & Storer TW (2001). Testosterone dose‐response relationships in healthy young men. Am J Physiol Endocrinol Metab 281, E1172–E1181. [DOI] [PubMed] [Google Scholar]
- Breen L, Stokes KA, Churchward‐Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ & Phillips SM (2013). Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98, 2604–2612. [DOI] [PubMed] [Google Scholar]
- Brook MS, Wilkinson DJ, Mitchell WEEK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K & Atherton PJ (2015). Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide‐derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29, 4485–4496. [DOI] [PubMed] [Google Scholar]
- Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WEEK, Koboyashi H, Greenhaff PL, Smith K & Atherton PJ (2015). Intake of low‐dose leucine‐rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women, at rest and after exercise. Am J Physiol Endocrinol Metab 308, E1056–E1065. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, McComas AJ & Petito F (1973). Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA & Smith K (1992). Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 73, 1383–1388. [DOI] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R & Vaupel JW (2009). Ageing populations: the challenges ahead. Lancet 374, 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR & Hawley JA (2006). Early signaling responses to divergent exercise stimuli in skeletal muscle from well‐trained humans. FASEB J 20, 190–192. [DOI] [PubMed] [Google Scholar]
- Crossland H, Kazi AA, Lang CH, Timmons JA, Pierre P, Wilkinson DJ, Smith K, Szewczyk NJ & Atherton PJ (2013). Focal adhesion kinase is required for IGF‐I‐mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1‐associated pathway. Am J Physiol Endocrinol Metab 305, E183–E193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM & Rennie MJ (2005). Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19, 422–424. [DOI] [PubMed] [Google Scholar]
- Dietrichson P, Coakley J, Smith PE, Griffiths RD, Helliwell TR & Edwards RH (1987). Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry 50, 1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro L (2001). Physical activity in aging: changes in patterns and their relationship to health and function. J Gerontol A Biol Sci Med Sci 56, 13–22. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E & Rasmussen BB (2009). Rapamycin administration in humans blocks the contraction‐induced increase in skeletal muscle protein synthesis. J Physiol 587, 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M & Kjær M (2001). Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol 535, 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ & McKinlay JB (2002). Age trends in the level of serum testosterone and other hormones in middle‐aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87, 589–598. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA & Evans WJ (1994). Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330, 1769–1775. [DOI] [PubMed] [Google Scholar]
- Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron‐Smith D & Blazevich AJ (2015). Ribosome biogenesis adaptation in resistance training‐induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309, E72–E83. [DOI] [PubMed] [Google Scholar]
- Figueiredo VC, Roberts LA, Markworth JF, Barnett MPG, Coombes JS, Raastad T, Peake JM & Cameron‐Smith D (2016). Impact of resistance exercise on ribosome biogenesis is acutely regulated by post‐exercise recovery strategies. Physiol Rep 4, e12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragala MS, Fukuda DH, Stout JR, Townsend JR, Emerson NS, Boone CH, Beyer KS, Oliveira LP & Hoffman JR (2014). Muscle quality index improves with resistance exercise training in older adults. Exp Gerontol 53, 1–6. [DOI] [PubMed] [Google Scholar]
- Fragala MS, Kenny AM & Kuchel GA (2015). Muscle quality in aging: a multi‐dimensional approach to muscle functioning with applications for treatment. Sport Med 45, 641–658. [DOI] [PubMed] [Google Scholar]
- Franchi MV, Atherton PJ, Reeves ND, Flück M, Williams J, Mitchell WEEK, Selby A, Beltran Valls RM & Narici MV (2014). Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol (Oxf) 210, 642–654. [DOI] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E & Rasmussen BB (2011). Aging impairs contraction‐induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E & Rasmussen BB (2013). Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 68, 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Hoffman JR, Jajtner AR, Townsend JR, Boone CH, Beyer KS, Baker KM, Wells AJ, Church DD, Mangine GT, Oliveira LP, Moon JR, Fukuda DH & Stout JR (2015). Protein supplementation does not alter intramuscular anabolic signaling or endocrine response after resistance exercise in trained men. Nutr Res 35, 990–1000. [DOI] [PubMed] [Google Scholar]
- Greig CA, Gray C, Rankin D, Young A, Mann V, Noble B & Atherton PJ (2011). Blunting of adaptive responses to resistance exercise training in women over 75y. Exp Gerontol 46, 884–890. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, Kraemer WJ, Newton RU & Alen M (1998. a). Changes in agonist‐antagonist EMG, muscle CSA, and force during strength training in middle‐aged and older people. J Appl Physiol 84, 1341–1349. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, Gotshalk LA, Campbell WW, Evans WJ, Häkkinen A, Humphries BJ & Kraemer WJ (1998. b). Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 53, B415–B423. [DOI] [PubMed] [Google Scholar]
- Hildreth KL, Barry DW, Moreau KL, Vande Griend J, Meacham RB, Nakamura T, Wolfe P, Kohrt WM, Ruscin JM, Kittelson J, Cress ME, Ballard R & Schwartz RS (2013). Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low‐normal testosterone levels. J Clin Endocrinol Metab 98, 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM & Ross R (2000). Skeletal muscle mass and distribution in 468 men and women aged 18‐88 yr. J Appl Physiol 89, 81–88. [DOI] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT & Roubenoff R (2004). The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52, 80–85. [DOI] [PubMed] [Google Scholar]
- Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA & McCarthy JJ (2015). Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol 119, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek DJ, Kim J, Petrella JK, Cross JM, Bamman MM, David J & James M (2006). Efficacy of 3 days/week resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101, 531–544. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Häkkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M & Evans WJ (1999). Effects of heavy‐resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol 87, 982–992. [DOI] [PubMed] [Google Scholar]
- Krogh‐Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW & Pedersen BK (2010). A 2 week reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol 108, 1034–1040. [DOI] [PubMed] [Google Scholar]
- Kumar V, Atherton PJ, Selby A, Rankin D, Williams J, Smith K, Hiscock N & Rennie MJ (2012). Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci 67, 1170–1177. [DOI] [PubMed] [Google Scholar]
- Kumar V, Atherton P, Smith K & Rennie MJ (2009. a). Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 106, 2026–2039. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N & Rennie MJ (2009. b). Age‐related differences in the dose‐response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnadi EP, Hannan KM, Hicks RJ, Hannan RD, Pearson RB & Kang J (2015). Regulation of rDNA transcription in response to growth factors, nutrients and energy. Gene 556, 27–34. [DOI] [PubMed] [Google Scholar]
- Kvorning T, Andersen M, Brixen K & Madsen K (2006). Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo‐controlled, and blinded intervention study. Am J Physiol Endocrinol Metab 291, E1325–E1332. [DOI] [PubMed] [Google Scholar]
- Kvorning T, Christensen LL, Madsen K, Nielsen JL, Gejl KD, Brixen K & Andersen M (2013). Mechanical muscle function and lean body mass during supervised strength training and testosterone therapy in aging men with low‐normal testosterone levels. J Am Geriatr Soc 61, 957–962. [DOI] [PubMed] [Google Scholar]
- Laukkanen PIA, Heikkinen E & Kauppinen M (1995). Muscle strength and mobility as predictors of survival in 75‐84‐year‐old people. Age Ageing 24, 468–473. [DOI] [PubMed] [Google Scholar]
- Laurent GJ & Sparrow MP (1977). Changes in RNA, DNA and protein content and the rates of protein synthesis and degradation during hypertrophy of the anterior latissimus dorsi muscle of the adult fowl (Gallus domesticus). Growth 41, 249–262. [PubMed] [Google Scholar]
- Laurent GJ, Sparrow MP, Bates PC & Millward DJ (1978). Turnover of muscle protein in the fowl (Gallus domesticus). Rates of protein synthesis in fast and slow skeletal, cardiac and smooth muscle of the adult fowl. Biochem J 176, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifke E, Gorenoi V, Wichers C & Von Mu A (2000). Age‐related changes of serum sex hormones, insulin‐like growth factor‐1 and sex‐hormone binding globulin levels in men: cross‐sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 53, 689–695. [DOI] [PubMed] [Google Scholar]
- Luukinen H, Koski K & Laippala P (1997). Factors predicting fractures during falling impacts among home‐dwelling older adults. J Am Geriatr Soc 45, 1302–1309. [DOI] [PubMed] [Google Scholar]
- Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Meinow B & Fratiglioni L (2011). Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev 10, 430–439. [DOI] [PubMed] [Google Scholar]
- Masel MC, Graham JE, Reistetter TA, Markides KS & Ottenbacher KJ (2009). Frailty and health related quality of life in older Mexican Americans. Health Qual Life Outcomes 7, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew DL, Kim J, Cross JM, Ferrando AA & Bamman MM (2009). Translational signaling responses preceding resistance training‐mediated myofiber hypertrophy in young and old humans. J Appl Physiol 107, 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, Ridanpää T, Häkkinen K, Kovanen V, Ahtiainen JP & Selänne H (2013). Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol 113, 641–650. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K & Rennie MJ (2005). Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567, 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, Churchward‐Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ & Phillips SM (2014). Acute post‐exercise myofibrillar protein synthesis is not correlated with resistance training‐induced muscle hypertrophy in young men. PLoS One 9, e89431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WEEK, Williams J, Atherton P, Larvin M, Lund J & Narici M (2012). Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Churchward‐Venne TA, Witard O, Breen L, Burd NA, Tipton KD & Phillips SM (2015). Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70, 57–62. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA & Phillips SM (2009). Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE (2012). Undernutrition in older adults. Fam Pract; 29 (Suppl 1), i89–i93. [DOI] [PubMed] [Google Scholar]
- Murton AJ, Billeter R, Stephens FB, Des Etages SG, Graber F, Hill RJ, Marimuthu K & Greenhaff PL (2014). Transient transcriptional events in human skeletal muscle at the outset of concentric resistance exercise training. J Appl Physiol 116, 113–125. [DOI] [PubMed] [Google Scholar]
- Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE & Gordon PM (2014). Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol 116, 693–702. [DOI] [PubMed] [Google Scholar]
- Neto WEEK, Gama EF, Rocha LY, Ramos CC, Taets W, Scapini KB, Ferreira JB, Rodrigues B & Caperuto É (2015). Effects of testosterone on lean mass gain in elderly men: systematic review with meta‐analysis of controlled and randomized studies. Age (Dordr) 37, 9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara R, Kobayashi K, Tsutaki A, Lee K, Abe T, Fujita S, Nakazato K & Ishii N (2013). mTOR signaling response to resistance exercise is altered by chronic resistance training and detraining in skeletal muscle. J Appl Physiol 114, 934–940. [DOI] [PubMed] [Google Scholar]
- Olsen RH, Krogh‐Madsen R, Thomsen C, Booth FW & Pedersen BK (2008). Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299, 1261–1263. [DOI] [PubMed] [Google Scholar]
- Peterson M, Rhea M, Sen A & Gordon P (2010). Resistance exercise for muscular strength in older adults: a meta‐analysis mark. Ageing Res Rev 9, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M, Sen A & Gordon P (2011). Influence of resistance exercise on lean body mass in ageing adults: A meta‐analysis. Sci Sport Exerc 43, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B, Williams J, Atherton P, Smith K, Hildebrandt W, Rankin D, Greenhaff P, Macdonald I & Rennie MJ (2012). Resistance exercise training improves age‐related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J Appl Physiol 112, 347–353. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR & Wolf E (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273, E99–E107. [DOI] [PubMed] [Google Scholar]
- Raue U, Slivka D, Jemiolo B, Hollon C & Trappe S (2007). Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62, 1407–1412. [DOI] [PubMed] [Google Scholar]
- Robinson MM, Turner SM, Hellerstein MK, Hamilton KL & Miller BF (2011). Long‐term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25, 3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler FR, Castaneda‐Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R & Azen SP (2009). Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94, 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon TC, Fragala MS, Stout JR, Emerson NS, Beyer KS, Oliveira LP & Hoffman JR (2014). Muscle architecture and strength: adaptations to short‐term resistance training in older adults. Muscle Nerve 49, 584–592. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD & Livak KJ (2008). Analyzing real‐time PCR data by the comparative CT method. Nat Protoc 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schrack JA, Zipunnikov V, Goldsmith J, Bai J, Simonsick EM, Crainiceanu C & Ferrucci L (2014). Assessing the “physical cliff”: detailed quantification of age‐related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci 69, 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ & Mittendorfer B (2011). Omega‐3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia‐hyperaminoacidaemia in healthy young and middle‐aged men and women. Clin Sci (Lond) 121, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec MJ, Mayhew DL & Bamman MM (2015). The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol 119, 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanetti RJ, Zacharewicz E, Della Gatta P, Garnham A, Russell AP & Lamon S (2014). Ageing has no effect on the regulation of the ubiquitin proteasome‐related genes and proteins following resistance exercise. Front Physiol 5, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy BL, Ivey FM, Hurlbut D, Martel GF, Lemmer JT, Siegel EL, Metter EJ, Fozard JL, Fleg JL & Hurley BF (1999). Muscle quality. II. Effects of strength training in 65‐ to 75‐yr‐old men and women. J Appl Physiol 86, 195–201. [DOI] [PubMed] [Google Scholar]
- Troiando RP, Berrigan D, Dodd KW, Mâsse LC, Ti Tilert & Mcdoweell M (2008). Physical activity in the United States measured by accelerometer. Med Sci Sport Exerc 40, 181–188. [DOI] [PubMed] [Google Scholar]
- Tzankoff SP & Norris AH (1977). Effect of muscle mass decrease on age‐related BMR changes. J Appl Physiol 43, 1001–1006. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WEEK, Dendale P & van Loon LJ (2009). Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 89, 608–616. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield‐Moore M, Rasmussen BB & Wolfe RR (2001). Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286, 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BBL, Verdijk LB & Van Loon LJC (2015). Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterlow JC, Garlick PJ & Millward DJ (1978). Protein Turnover in Mammalian Tissues and in the Whole Body. Elsevier/North Holland, Amsterdam. [Google Scholar]
- Welinder C & Ekblad L (2011). Coomassie staining as loading control in Western blot analysis. J Proteome Res 10, 1416–1419. [DOI] [PubMed] [Google Scholar]
- Welle S, Totterman S & Thornton C (1996). Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci 51, M270–M275. [DOI] [PubMed] [Google Scholar]
- West DWD, Baehr LM, Marcotte GR, Chason CM, Tolento L, Gomes AV, Bodine SC & Baar K (2016). Acute resistance exercise activates rapamycin‐sensitive and insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol 594, 453–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L & Wolfman NM (2003). Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300, 965–971. [DOI] [PubMed] [Google Scholar]
- Wilkinson DJ, Cegielski J, Phillips BE, Boereboom C, Lund JN, Atherton PJ & Smith K (2015). Internal comparison between deuterium oxide (D2O) and L‐[ring‐13C6] phenylalanine for acute measurement of muscle protein synthesis in humans. Physiol Rep 3, e12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WEEK, Szewczyk NJ, Greenhaff PL, Atherton PJ & Smith K (2014). A validation of the application of D2O stable isotope tracer techniques for monitoring day‐to‐day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab 306, E571–E579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Bhasin S, Sinha‐Hikim I, Pak‐Loduca J & Gonzalez‐Cadavid NF (2002). Serum myostatin‐immunoreactive protein is increased in 60‐92 year old women and men with muscle wasting. J Nutr Health Aging 6, 343–348. [PubMed] [Google Scholar]


