Abstract
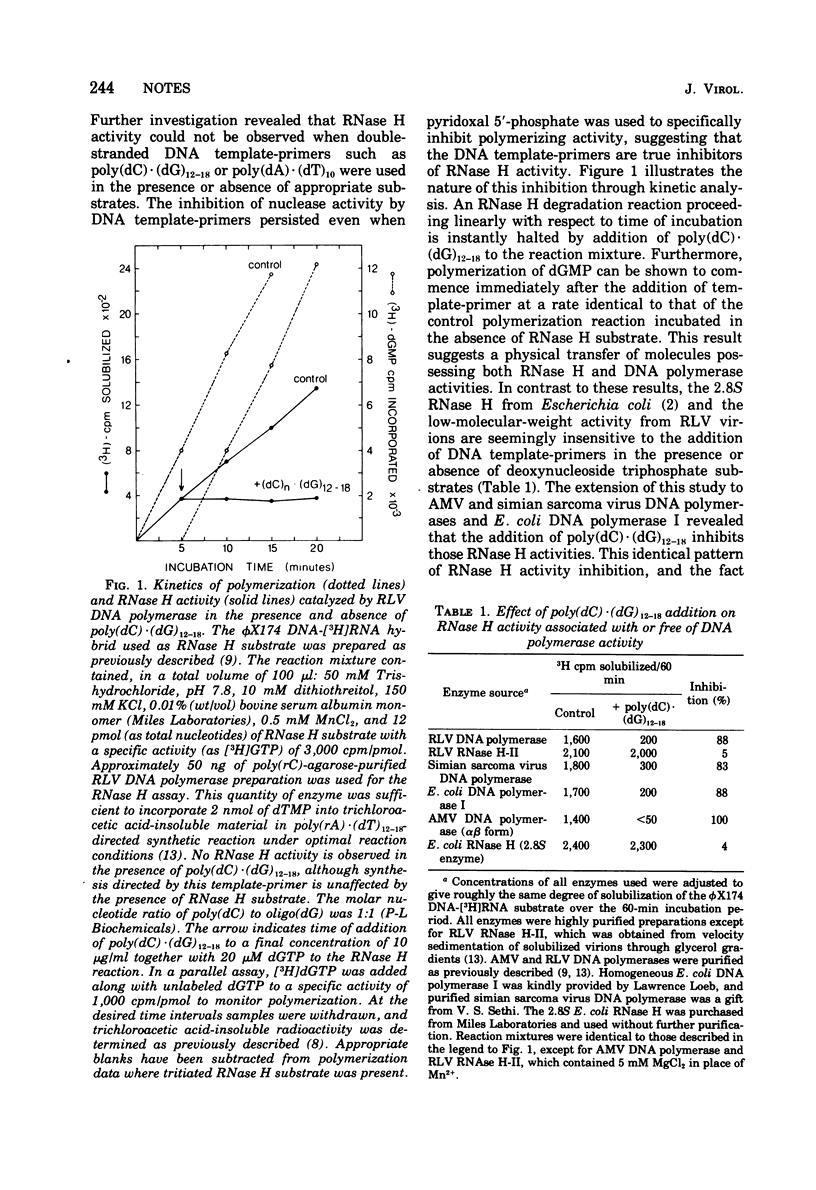
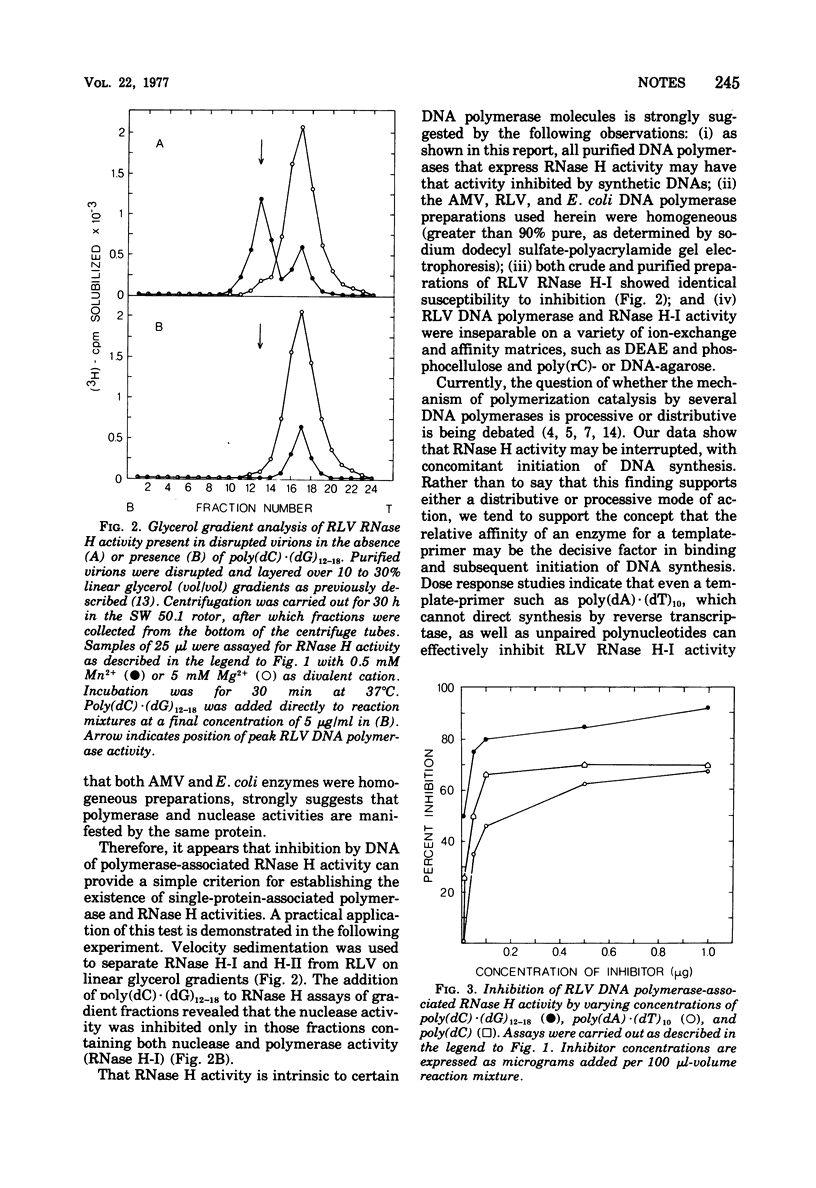
The RNase H activity associated with several RNA-directed DNA polymerases is inhibited by the addition of DNA, in contrast to RNase H activity from enzymes devoid of polymerizing activity. Kinetic investigations of the inhibitory effect of DNA, using purified Rauscher leukemia virus DNA polymerase as a test enzyme, revealed that the addition of DNA to an ongoing RNase H reaction causes an immediate cessation of RNase H activity. Concomitant initiation of DNA synthesis by inhibitory DNA can occur, provided that appropriate substrate and primer is available. Thus, in addition to providing a simple test for the distinction between polymerase-associated and polymerase-independent RNase H activity, this study strongly supports the concepts that (i) RNase H activity expressed by several mammalian oncoviral reverse transcriptases is an integral part of that molecule, and (ii) that the catalytic site of RNase H activity is also involuved in template-primer binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bambara R. A., Uyemura D., Lehman I. R. On the processive mechanism of Escherichia coli DNA polymerase I. Delayed initiation of polymerization. J Biol Chem. 1976 Jul 10;251(13):4090–4094. [PubMed] [Google Scholar]
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Brewer L. C., Wells R. D. Mechanistic independence of avian myeloblastosis virus DNA polymerase and ribonuclease H. J Virol. 1974 Dec;14(6):1494–1502. doi: 10.1128/jvi.14.6.1494-1502.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M. The distributive nature of enzymatic DNA synthesis. J Mol Biol. 1975 Apr 5;93(2):219–235. doi: 10.1016/0022-2836(75)90129-1. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Loeb L. A. On the association of reverse transcriptase with polynucleotide templates during catalysis. Biochemistry. 1976 Aug 10;15(16):3605–3611. doi: 10.1021/bi00661a031. [DOI] [PubMed] [Google Scholar]
- Gerard G. F., Grandgenett D. P. Purification and characterization of the DNA polymerase and RNase H activities in Moloney murine sarcoma-leukemia virus. J Virol. 1975 Apr;15(4):785–797. doi: 10.1128/jvi.15.4.785-797.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor virus. VI. Processive mode of action of avian myeloblastosis virus polymerase. J Virol. 1976 Sep;19(3):932–939. doi: 10.1128/jvi.19.3.932-939.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. L., Modak M. J., Cavalieri L. F. Purification of avian myeloblastosis virus DNA polymerase by affinity chromatography on polycytidylate-agarose. J Virol. 1974 Oct;14(4):853–859. doi: 10.1128/jvi.14.4.853-859.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. L., Modak M. J. Observations on template-specific conditions for DNA synthesis by avian myeloblastosis virus DNA polymerase. Nucleic Acids Res. 1976 Jun;3(6):1473–1486. doi: 10.1093/nar/3.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. L., Sarkar N. H., Modak M. J. Purification and properties of murine mammary tumor virus DNA polymerase. Virology. 1976 May;71(1):242–254. doi: 10.1016/0042-6822(76)90109-4. [DOI] [PubMed] [Google Scholar]
- Modak M. J., Marcus S. L. Purification and properties of Rauscher leukemia virus DNA polymerase and selective inhibition of mammalian viral reverse transcriptase by inorganic phosphate. J Biol Chem. 1977 Jan 10;252(1):11–19. [PubMed] [Google Scholar]
- Modak M. J. Observations on the pyridoxal 5'-phosphate inhibition of DNA polymerases. Biochemistry. 1976 Aug 10;15(16):3620–3626. doi: 10.1021/bi00661a033. [DOI] [PubMed] [Google Scholar]
- Modak M. J. Pyridoxal 5' phosphate: a selective inhibitor of oncornaviral DNA polymerases. Biochem Biophys Res Commun. 1976 Jul 12;71(1):180–187. doi: 10.1016/0006-291x(76)90266-7. [DOI] [PubMed] [Google Scholar]
- Uyemura D., Bambara R., Lehman I. R. On the processive mechanism of Escherichia coli DNA polymerase I. J Biol Chem. 1975 Nov 25;250(22):8577–8584. [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses III. Properties of purified Moloney murine leukemia virus DNA polymerase and associated RNase H. J Virol. 1975 Apr;15(4):843–854. doi: 10.1128/jvi.15.4.843-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses. I. Localization of thermolabile DNA polymerase and RNase H activities on one polypeptide. J Virol. 1975 Jan;15(1):121–126. doi: 10.1128/jvi.15.1.121-126.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



