Abstract
The conventional approach to developing disease-modifying treatments for neurodegenerative conditions has been to identify drivers of pathology and inhibit such pathways. Here we discuss the possibility that the efficacy of such approaches may be increasingly attenuated as disease progresses. This is based on experiments using a mouse models of spinocerebellar ataxia type 1 and Huntington’s disease, where expression of the dominantly acting mutations could be switched off, as well as studies in human Huntington’s disease, which suggest that the primary genetic driver of age-of-onset of disease is a much weaker determinant of disease progression in affected individuals. The idea that one may approach a point in the disease course where such rational therapeutic strategies based on targets which determine onset of disease have minimal efficacy, suggests that one needs to consider other approaches to therapies and clinical trial design, including initiation of therapies in presymptomatic individuals.
Keywords: Huntington’s disease, neurodegeneration, point of no return, spinocerebellar ataxia type 1, therapy
Introduction
The increasing human life expectancies coupled with the age-dependent risk for most neurodegenerative diseases has focussed attention on developing therapies for common polygenic conditions like Alzheimer’s and Parkinson’s disease, as well as monogenic conditions like Huntington’s disease and certain spinocerebellar ataxias. The strategy that has been considered most widely has been to identify mechanistic drivers of pathogenesis and develop agents that interfere with these putative disease-causing pathways. Such strategies require that the disease processes do not reach a stage where such mechanistic intervention strategies are ineffective. In some other disease scenarios, this consideration is relevant. For example, if a physician in the emergency room is confronted with a patient in cardiac failure due to severe coronary artery disease caused by raised cholesterol levels, then the priority is not to reduce the cholesterol levels, but to treat the cardiac failure and the coronary artery infarction. While the initial and critical treatment plan of the symptomatic patient is not directed primarily at the primary cause of disease, it is likely that long term-treatment with cholesterol lowering drugs, like statins, for years prior to the predicted age of disease onset, would have been effective in reducing the risk of disease and/or delaying its onset. In various autoimmune diseases, there have been suggestions that there are important genetic determinants of disease course that are distinct from the loci that influence risk of disease manifestation, reinforcing the concept that the biology of disease progression may be different to that of disease initiation [1]. Thus, in such diseases, the optimal targets for therapy may be the determinants of disease progression, rather than the causes of disease presentation.
Approaching points of no return in a neurodegenerative disease mouse model
In neurodegenerative disease, a point of no return is reached once a substantial number of neurons have died. However, the possibility that this scenario may exist in neurodegenerative diseases before neuronal death is important to consider, as it may have major ramifications for treatment strategies, as well as for clinical trials, which are expensive and time consuming. Our concerns are stimulated by a number of studies. Perhaps the most powerful strategy to address this question in the preclinical setting is to switch on and off a genetic factor that causes disease in a model organism. This experiment has been done in a mouse model of spinocerebellar ataxia type 1, an autosomal dominant neurodegenerative disease, which is caused by a polyglutamine expansion mutation in the ataxin 1 gene. It is one of the nine known diseases caused by such mutations, the most common which is Huntington’s disease. Overexpression of mutant ataxin 1 in the cerebellar Purkinje cells (which are a prominent target of the human disease) is sufficient to cause abnormalities in the dendritic arborisation and spine densities in these cells and abnormalities in the Purkinje cell-parallel fibre synapses, which are associated with loss of coordination/motor abnormalities that result from such dysfunction of these cerebellar cells [2]. The mouse coordination and motor abnormalities were assessed using a rotarod test, which places the mice on a rotating cylinder and measures how long the mice stay on this apparatus before falling off. Thus, in these mice, the disease is caused by the presence of the transgenic protein. To address whether mice could recover after the initiation of disease, a model was generated where the transgene could be conditionally switched off using a tetracycline-regulatable system. This was done in early, mid and late disease in the mice – in all cases prior to Purkinje cell loss. In the early disease, switching off the transgene essentially led to a recovery of both the motor and Purkinje cell morphological abnormalities. In contrast to the complete recovery assessed by these measures in early disease, the improvement in mid-disease after switching off the transgene was partial. However, in advanced disease, the mice failed to show a significant improvement on the rotarod test and while there was some improvement in Purkinje cell morphology, this was still abnormal after switch-off. Thus, as disease progresses in this model, the ability to return to normal is severely attenuated after removal of the primary causative agent, mutant ataxin 1. This could not be attributed to failure to switch off the transgene or remove the mutant protein [2] (Figure 1).
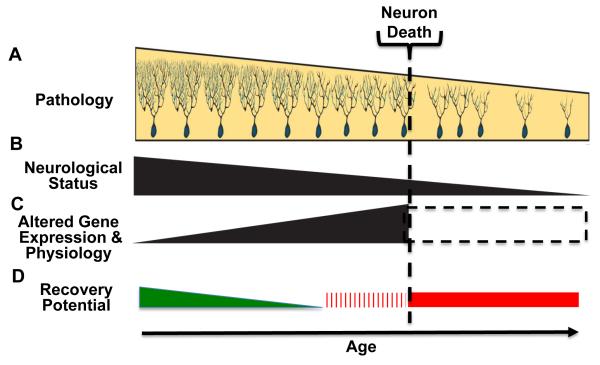
Figure 1.
Points of no-recovery in neurodegenerative disease. With increasing age and disease duration neuronal pathology progressively worsens, reaching a point where neuronal death ensues (A). During this period there is a decline in neurological status (B) and alterations in gene expression and physiology (C). Changes in gene expression typically fall into two categories, those associated with disease progression – solid triangle – and those largely reflecting neuronal loss – hatched box. Changes in A-C impact ability of therapeutic approaches to impact disease progression and induce recovery (D). During early phase of disease there is a period when mechanistic therapeutic approaches are effective, but with diminishing effect with age – green triangle. With neuronal death a point of no-return is reached – red bar. It is proposed that an initial point of no-recovery occurs prior to neuronal death – hatched red bar
Another approach to switching off the transgene is to use single stranded antisense oligonucleotides. In an Huntington’s disease model, initiation of such treatment at three months of age led to a better phenotypic improvement than when treatment was started at six months of age [3]. It will be interesting to study this phenomenon in other disease models in mice which may be amenable to such manipulations. However, there may be clues that suggest a point-of no return in human neurodegenerative diseases. The most obvious suggestion comes from diseases like Parkinson’s disease, where there can be major loss of the most vulnerable neuronal populations prior to disease onset [4]. Significant neuronal loss also occurs in forms of Alzheimer’s disease and Huntington’s disease prior to clinical manifestation [5]. Again, if one accepts that it may be impossible to recover such neuronal loss, then this will set a baseline beyond which further improvement may not be achievable. However, in many of these diseases, the clinical signs and symptoms are also likely due to neuronal dysfunction, which may be rescuable to some extent, and may vary from neuron to neuron. Furthermore, there may be non-cell-autonomous factors that contribute to disease, some of which may be more reversible than others.
Genetic studies in Huntington’s disease uncouple polyglutamine length from disease duration
Genetic studies have also informed this question. In Huntington disease (HD), as in the other polyglutamine diseases, the number of glutamine residues in the polyglutamine tract is inversely correlated with age-at-onset. The polyglutamines are encoded by a CAG triplet repeat expansion in the gene. In Huntington disease, the CAG number accounts for about 70% of the variance in age-at-onset [6]. Thus, in HD, one has a paradigm where one can examine the “severity” of the disease–causing monogenic mutation in relation to disease progression. For example, if one were to assume that the CAG were as strong a determinant of disease progression as for onset, then one idealised therapy for such diseases would be to reduce the CAG repeat length via some type of genetic editing strategy. Even if one could not reduce the repeat length to below the disease threshold, a modest reduction in CAG repeat length would be predicated to have a significant impact.
Surprisingly, in HD, the duration of disease, i.e. time from onset to death, is not correlated with polyglutamine length [7]. This led the authors to two possible models, which they articulated elegantly, as follows. Either “(1) HD pathogenesis is driven by mutant huntingtin, but before or near motor onset, sufficient CAG-driven damage occurs to permit CAG-independent processes and then lead to eventual death. In this scenario, some pathological changes and their clinical correlates could still worsen in a CAG-driven manner after disease onset, but these CAG-related progressive changes do not themselves determine duration. Alternatively, (2) HD pathogenesis is driven by mutant huntingtin acting in a CAG-dependent manner with different time courses in multiple cell types, and the cellular targets that lead to motor onset and death are different and independent. In this scenario, processes driven by HTT CAG length lead directly to death but not via the striatal pathology associated with motor manifestations” [7]. Interestingly, this same group has identified a few loci distinct from the CAG repeats in huntingtin that contribute to some of the residual variability in age-at-onset that is not accounted for by the repeats. They analysed the locus with strongest effect, which also did not affect disease duration, again suggesting a critical dissociation [6]. But does the CAG/polyglutamine repeat number determine disease course, which may be distinct from disease duration (although this would not be the most parsimonious model)? This is critical to answer in terms of the therapeutic strategy.
This issue has been previously addressed in HD. As clinical features of progression may be difficult to assess, this was done with standardised clinical scales [8]. While one may argue that such scales may lack sensitivity and be prone to noise, it is important to point out that the definition of a precise age-at-onset for a disease with an insidious initiation may be similarly noisy. It appears that CAG repeat number only affects disease progression when one corrects for age-at-onset – an older age of onset appears to increase the rate of progression. Indeed, CAG number appears to explain about 20% of the variation of disease duration at the time of institutionalisation (and CAG repeat length correlated inversely with this duration). However, this was only apparent if one corrected for age at onset [9]. Since the CAG repeat number accounts for approximately 70% of the variance of age-at-onset, this would be compatible with the concept that the severity of the primary mutation in terms of its effect on onset is much greater than its effect on disease course.
One may also be able to learn from large prospective studies that have examined both neuroimaging and clinical parameters. In the TRACK-HD study, the CAG repeat length had less predictive power for such measures in early HD patients than in presymptomatic HD mutation carriers for the net changes assessed over a 36 month period [10]. While such differences may be due to ceiling and floor effects of the measures used, these factors cannot explain why the CAG repeats in early HD cases have less predictive value for changes in the neuroimaging measures when compared to the presymptomatic cases. For all parameters and in all groups, the correlation coefficients versus CAG length were much less than 0.7, again consistent with the idea that disease progression correlates less with repeat length than disease onset.
While these data and the other papers discussed above do not demonstrate that there is a point of no return for therapy of such diseases, they are consistent with the concept that the ability to treat such diseases by modulating the primary drivers of onset will wane as disease progresses. This is not unexpected, due to the increased cell loss and pathway perturbation. This may vary from disease to disease. Ultimately, the question can only be tested definitively in humans with strategies like antisense reduction of mutant proteins in autosomal dominant diseases like Huntington’s disease, assuming one can achieve sufficient knockdown across all the relevant areas in human brains.
Human trial in a neurodegenerative protein-misfolding disease reveals greater efficacy when treatment is initiated early
Important lessons about the timing of such clinical trials may be suggested by studies in the neurodegenerative protein misfolding disease, transthyretin familial amyloid polyneuropathy (TTR-FAP). TTR-FAP is caused by autosomal dominant TTR gene mutations, which destabilise the tetramers of the transthyretin (TTR) protein. This ultimately leads to misfolding of the monomers and aggregation/amyloidogenesis [11]. TTR is a plasma protein mainly derived from the liver and its aggregated forms in TTR-FAP are associated with axonal degeneration, which causes progressive sensorimotor and autonomic neuropathy [11]. Tafamidis has been shown to stabilise the mutant TTR tetramers and has been tested in patients. In an interesting study which followed a double-blind trial of this drug with an open label study in both the previously-treated and placebo groups, the authors found that earlier treatment enabled more effective responses than delayed treatment as determined by a number of neurological measures [12]. While this disease is not the same as the common CNS-focussed protein-misfolding diseases, its similarities hint at the likelihood that earlier initiation of therapies may have better outcomes even in such human conditions.
We believe that the likelihood of diminishing return with disease progression for mechanistic therapeutic strategies should stimulate alternative strategies. As with certain autoimmune diseases, there may be targets for disease progression that differ from those causing onset [13]. It is difficult to know what these targets may be. However, these could include neuronal network disturbances caused by cell death, and glial abnormalities. Some of these targets may be identifiable by genetic approaches in large scale cohorts. A better understanding of these processes will be critical for developing disease-modifying strategies for patients with established disease.
The ideal scenario to work towards is to delay disease onset. In monogenic diseases like HD, most individuals at risk will have a family history and one could start a preventive drug/agent many years prior to the anticipated age-at-onset with the aim of delaying disease eventually beyond the normal human lifespan. For complex diseases like Alzheimer and Parkinson disease, one may strive towards identifying a protective pathway that can have modest effects over many years. For example, if one were to identify a drug analogous to statins for heart disease that delayed the onset of Alzheimer’s disease by even only one year, then the impact on individuals, families and health services would be huge, given the prevalence of the condition.
Conclusions
For both complex and monogenic diseases, we believe that strategies that delay the onset of disease should be considered – thus, one will need to identify biomarkers of pathological progression prior to onset and test these. This challenging task may be simplified in some cases by studying Mendelian forms of disease. For example, the Dominantly Inherited Alzheimer Network (DIAN), an international registry of individuals at risk for developing autosomal dominant Alzheimer’s disease (ADAD), has embarked on an ambitious plan to longitudinally assess asymptomatic and symptomatic ADAD mutation carriers and their non-carrier siblings (genetically similar controls) for clinical, cognitive, and imaging and fluid biomarkers. This has the prospect of identifying biomarkers that precede disease and the ability to perform “preventive” trials in such cases [14]. Data from such studies will likely inform “sporadic” Alzheimer’s disease. Similar studies are underway in Huntington’s disease (TRACK-HD), where recent imaging studies in presymptomatic HD gene carriers suggest that this may be an amenable strategy conditions [10]. One needs to acknowledge that the biological power to detect the impact of a mechanistic therapeutic intervention is likely to diminish with disease course. The corollary of this is that such strategies may be missed when symptomatic individuals are tested and discarded, while they may be powerful when initiated prior to disease onset. Finally, strategies with modest defects may have big impacts, if they can be administered over many years prior to the expected onset of disease. We appreciate that such proposed studies in asymptomatic patients using surrogate biomarkers as endpoints will raise issues from a regulatory perspective. However, the potential benefits of such a strategy may heavily outweigh the risks.
Acknowledgements
DCR is grateful for funding from the Tau consortium, Alzheimer’s Research UK (DCR) Wellcome Trust (Principal Research Fellowship to 095317/Z/11/Z), a Wellcome Trust Strategic Grant to Cambridge Institute for Medical Research (100140/Z/12/Z), NIHR Biomedical Research Unit in Dementia at Addenbrooke’s Hospital and the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 2012-305121 “Integrated European –omics research project for diagnosis and therapy in rare neuromuscular and neurodegenerative diseases (NEUROMICS). HTO is appreciative for research support from the NINDS/NIH, grants R37NS022920 and RO1NS045667 and grants from the National Ataxia Foundation and the Bob Allison Ataxia Research Center. We are grateful to Anne Jackson for help with the graphical abstract.
Abbreviation
- HD
Huntington disease
References
- 1.Lee JC, Smith KG. Prognosis in autoimmune and infectious disease: new insights from genetics. Clin Transl Immunol. 2014;3:e15. doi: 10.1038/cti.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zu T, Duvick LA, Kaytor MD, Berlinger MS, et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J Neurosci. 2004;24:8853–61. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, et al. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–44. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jankovic J. Progression of Parkinson disease: are we making progress in charting the course? Arch Neurol. 2005;62:351–2. doi: 10.1001/archneur.62.3.351. [DOI] [PubMed] [Google Scholar]
- 5.Aylward EH, Sparks BF, Field KM, Yallapragada V, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 6.Imarisio S, Carmichael J, Korolchuk V, Chen CW, et al. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- 7.Keum JW, Shin A, Gillis T, Mysore JS, et al. The HTT CAG-Expansion Mutation Determines Age at Death but Not Disease Duration in Huntington Disease. Am J Hum Genet. 2016;98:287–98. doi: 10.1016/j.ajhg.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenblatt A, Kumar BV, Mo A, Welsh CS, et al. Age, CAG repeat length, and clinical progression in Huntington's disease. Mov Disord. 2012;27:272–6. doi: 10.1002/mds.24024. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblatt A, Kumar BV, Margolis RL, Welsh CS, et al. Factors contributing to institutionalization in patients with Huntington's disease. Mov Disord. 2011;26:1711–6. doi: 10.1002/mds.23716. [DOI] [PubMed] [Google Scholar]
- 10.Tabrizi SJ, Scahill RI, Owen G, Durr A, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–49. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 11.Ando Y, Nakamura M, Araki S. Transthyretin-related familial amyloidotic polyneuropathy. Arch Neurol. 2005;62:1057–62. doi: 10.1001/archneur.62.7.1057. [DOI] [PubMed] [Google Scholar]
- 12.Coelho T, Maia LF, da Silva AM, Cruz MW, et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260:2802–14. doi: 10.1007/s00415-013-7051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JC, Espéli M, Anderson CA, et al. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell. 2013;155:57–69. doi: 10.1016/j.cell.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulder KL, Snider BJ, Mills SL, et al. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimers Res Ther. 2013;5:48. doi: 10.1186/alzrt213. [DOI] [PMC free article] [PubMed] [Google Scholar]