Abstract
The neuropeptides neurokinin B (NKB) and kisspeptin are potent stimulators of gonadotrophin-releasing hormone (GnRH)/luteinsing hormone (LH) secretion and are essential for human fertility. We have recently demonstrated that selective activation of NKB receptors (NK3R) within the retrochiasmatic area (RCh) and the preoptic area (POA) triggers surge-like LH secretion in ovary-intact ewes, whereas blockade of RCh NK3R suppresses oestradiol-induced LH surges in ovariectomised ewes. Although these data suggest that NKB signalling within these regions of the hypothalamus mediates the positive-feedback effects of oestradiol on LH secretion, the pathway through which it stimulates GnRH/LH secretion remains unclear. We proposed that the action of NKB on RCh neurones drives the LH surge by stimulating kisspeptin-induced GnRH secretion. To test this hypothesis, we quantified the activation of the preoptic/hypothalamic populations of kisspeptin neurones in response to POA or RCh administration of senktide by dual-label immunohistochemical detection of kisspeptin and c-Fos (i.e. marker of neuronal activation). We then administered the NK3R agonist, senktide, into the RCh of ewes in the follicular phase of the oestrous cycle and conducted frequent blood sampling during intracerebroventricular infusion of the kisspeptin receptor antagonist Kp-271 or saline. Our results show that the surge-like secretion of LH induced by RCh senktide administration coincided with a dramatic increase in c-Fos expression within arcuate nucleus (ARC) kisspeptin neurones, and was completely blocked by Kp-271 infusion. We substantiate these data with evidence of direct projections of RCh neurones to ARC kisspeptin neurones. Thus, NKB-responsive neurones in the RCh act to stimulate GnRH secretion by inducing kisspeptin release from KNDy neurones.
Keywords: tachykinins, kisspeptin, KNDy neurones, NK3R, LH surge, retrochiasmatic area
Mutations that inactivate genes encoding the neuropeptides neurokinin B (NKB) and kisspeptin, or their cognate receptors (NK3R and Kiss1r, respectively), are associated with infertility in humans (1–5). NKB and kisspeptin, as well as the endogenous opioid peptide dynorphin A, are coexpressed by a population of neurones, now commonly referred to as KNDy neurones (6,7), within the hypothalamic arcuate nucleus (ARC). Substantial evidence implicates kisspeptin of KNDy origin in mediating episodic gonadotrophin-releasing hormone (GnRH) release (6,8–12), which manifests in the pulsatile secretion of luteinising hormone (LH) (13–16) that is essential for gametogenesis and gonadal steroidogenesis (17–22). Projections from KNDy neurones form direct contacts with GnRH perikarya in the preoptic area (POA) and mediobasal hypothalamus, as well as with axonal termini in the median eminence (6,23–30), and are highly ipsi- and contralaterally interconnected (15,23,24,27,28,31). This is especially noteworthy because GnRH neurones do not express oestrogen receptor a (32,33) and are thus reliant, at least in part, on KNDy neurones that do express this receptor subtype (31,34,35) to mediate the feedback effects of oestradiol (E2). Indeed, levels of kisspeptin and NKB mRNA and peptide expression in the ARC correlate positively with LH levels and negatively with E2 levels, in line with a role for KNDy neurones in mediating the negative-feedback effects of E2 on the reproductive axis (36).
An additional population of kisspeptin neurones exists within the anteroventral periventricular area and the surrounding nuclei of the rostral periventricular region of the third cerebral ventricle (RP3V) in rodents (37), as well as the POA in sheep (38) and humans (39). These rostral kisspeptin neurones also express E2 receptors (34,40,41) and form close contacts with GnRH neurones (28,42,43). However, unlike KNDy neurones, RP3V/POA kisspeptin neurones are positively regulated by E2 (40) and do not coexpress NKB or dynorphin (7,31,39). These features are consistent with their role in mediating the positive-feedback effects of E2 on GnRH secretion that are necessary for the generation of the preovulatory LH surge (36).
In sheep, KNDy neurones express NK3R (44,45) but not Kiss1r (46), whereas GnRH neurones are apparently devoid of NK3R (44,45) but, instead, express abundant Kiss1r (38). We have recently shown that selective activation of ARC NK3R, by means of bilateral microimplants containing the NK3R agonist senktide, elevates LH concentrations commensurate with episodic LH secretion in early follicular phase ewes (47). Indeed, the overwhelming majority of studies on the functions of hypothalamic NKB/NK3R signalling have thus far focused on the regulation of episodic gonadotrophin secretion that is subject to restraint by the negative-feedback effects of E2 (12,48,49). However, recent findings from our laboratory show that i.c.v. administration of senktide (50) or placement of senktide microimplants in the retrochiasmatic area (RCh) (47,50) or the POA (47), both of which express NK3R (44), induces surge-like LH secretion in ewes, indicating that NKB/NK3R signalling could mediate the positive-feedback effects of E2 on LH secretion. We confirmed the notion that NK3R activation in the RCh is necessary for the full LH surge in ewes by demonstrating that RCh microimplantation of SB222200 suppresses the amplitude of E2-induced LH surges by over 40% (47). However, the neural pathway through which activation of NK3R outside of the ARC alters LH secretion remains unknown. Several lines of evidence implicate kisspeptin: (i) kisspeptin/Kiss1r signalling mediates the stimulatory effects of i.c.v. or i.v. administration of senktide on LH secretion in rodents and nonhuman primates (48,49,51,52), although these data may be more relevant to the control of LH pulses than the LH surge; (ii) in ewes, i.c.v. administration of Kp-271 has been shown to cause a decrease in the amplitude of E2-induced LH surges (46) similar to that caused by RCh administration of SB222200 (47); and (iii) ovine kisspeptin neurones in the ARC and POA exhibit increased activation during the preovulatory LH surge (53).
Thus, we hypothesised that activation of RCh NK3R elicits kisspeptinergic stimulation of GnRH neurosecretion as part of the mechanism of LH surge generation. To test this hypothesis, we compared the relative activation of KNDy or POA kisspeptin neurones, as assessed by induction of the immediate-early transcription factor c-Fos (54), in ewes with senktide microimplants placed in the RCh. To address potential connectivity between the RCh and ARC in the ewe, we performed tract-tracing studies involving RCh administration of the predominantly anterograde neuroanatomical tracer, biotinylated dextran amine (BDA). Finally, we investigated the effect of i.c.v. Kp-271 treatment on surge-like LH secretion induced by RCh microimplantation of senktide in follicular phase ewes.
Materials and methods
Animals
Adult blackface ewes of predominantly Suffolk breeding were maintained in an open barn and moved indoors 3–7 days prior to surgeries. Ewes were fed a pelleted alfalfa diet and provided with free access to water and supplemental minerals. Lighting was adjusted every 14 days to mimic the duration of natural lighting. Experiments using anoestrous ewes were performed from May to August, and the experiments performed during the breeding season took place October–February; animals in both seasons were used because the effects of senktide are similar in anoestrus and the breeding season (44). For follicular phase experiments, oestrous cycles were synchronised as reported previously (29). Briefly, two i.m. injections of 5 mg/ml dinoprost tromethamine (Lutalyse, Pharmacia & Upjohn, New York, NY, USA), a synthetic analogue of prostaglandin F-2α, were administered 3 h apart (total dose: 10 mg). At this time, two progesterone-containing controlled internal drug-releasing devices (CIDRs; Eazi-Breed, Pharmacia & Upjohn) were placed intravaginally to produce luteal phase concentrations of progesterone. Seven days later, Lutalyse was again injected and the CIDRs were removed. Experiments commenced 18 h later, during the early follicular phase, when rising E2 levels begin to exert positive-feedback on LH secretion, although an endogenous LH surge is not expected for at least a further 24 h (55). All procedures were approved by the West Virginia University Animal Care and Use Committee and were conducted in accordance with NIH guidelines on the care and use of animals in research.
Surgical procedures
All surgeries were performed under aseptic conditions using 2–4% isofluorane in oxygen for anaesthesia. Bilateral chronic 18-gauge guide cannulae stereotaxically targeted towards either the RCh or the POA were implanted as described previously (47) and were used to administer senktide-filled or empty microimplants for Experiment 1. Injection of anaesthetised ewes with BDA in Experiment 2 was carried out using a similar stereotaxic approach, except that guide cannulae were removed after the administration of the tracer, as described previously (56). For Experiment 3, bilateral guide cannulae targeting the RCh, as well as a 16-gauge cannula placed within the middle of one lateral cerebral ventricle (57), were chronically implanted. All ewes were treated with dexamethasone and penicillin from 1 day before to 5 days after surgery, and with analgesic (125 mg; Banamine, Phoenix Pharmaceutical, St Joseph, MO, USA) at the time of anaesthesia induction and for 5 days after surgery.
Drugs
The agonist of NK3R (senktide) was purchased from Tocris Bioscience (Ellisville, MO, USA) and stored at −20 °C. Microimplants consisting of 22-gauge tubing cut to extend 1 mm beyond the tip of the guide tubes were filled by tamping in crystalline drug at least 60 times (58) under sterile conditions and then cleaning the outside with sterile gauze. For Experiment 2, 10 mg of lysine fixable BDA (molecular weight 10 000; Sigma-Aldrich, St Louis, MO, USA) was dissolved in 100 μl 0.1 M phosphate buffer (PB) the day before injection. The antagonist to Kiss1r, Kp-271 (59), was synthesised by EZ Biolab (Carmel, IN, USA) and solubilised in sterile water to 26.4 mg/ml (0.011 mmol/ml, stored at −20 °C). This stock solution was diluted to 2.5 mg/ml in sterile saline on the day of the experiment.
Experiment 1: Which population of kisspeptin neurones is activated by RCh or POA administration of senktide?
To minimise animal use, tissue was collected shortly after senktide or control treatments from ewes that had been used for studies examining the actions of senktide or SB222200 (47), 10–14 days after the last treatment. On the morning of the experiment, empty or senktide-containing bilateral microimplants were inserted into the RCh of anoestrous ewes (n = 5 and n = 7, respectively) or the POA of follicular phase ewes (n = 5 and n = 6, respectively). Blood samples (3–4 ml) were taken every 12 min from 24 min before to 3 h after insertion. Immediately after the last blood sample was taken, animals were sacrificed and brain tissue collected and processed for immunohistochemistry (see below).
Experiment 2: Do RCh neurones project to ARC kisspeptin neurones?
Unilateral injections (100 nl) of 10% BDA in 0.1 M PB were made into the RCh using a sterile 1-μl Hamilton syringe, the needle of which extended 2 mm beyond the end of the guide tube. Injections were made over 5 min during stereotaxic neurosurgery in three ovary-intact anoestrous ewes and the animals were allowed to recover for 10 days before tissue collection.
Experiment 3: Does endogenous kisspeptin mediate surge-like LH secretion induced by RCh administration of senktide?
The effects of lateral ventricular infusion of saline (vehicle) or Kiss1r antagonist (Kp-271) on LH secretion in ewes treated with senktide-containing microimplants in the RCh were determined using a cross-over design (n = 8) during two successive follicular phases. Blood was collected through jugular catheters that were inserted the day before the experiment. Blood samples were collected into heparinised tubes, centrifuged and plasma was collected and stored at −20 °C until radioimmunoassayed for LH. Starting 18 h after the injection of Lutalyse and CIDR removal, 3–4 ml blood samples were collected every 12 min for 2 h before to 4 h after the insertion of microimplants. Lateral ventricle infusion of Kp-271 or vehicle (120 μl/h) was achieved through the use of portable electronic syringe pumps (Graseby MS16A; Smiths Medical, Dublin, OH, USA) as described previously (57). Syringe pumps were secured to the backs of animals by means of a harness. Syringes were connected to lateral ventricle cannulae with sterile SILASTIC tubing (Dow Corning, Midland, MI, USA). Infusions were initiated 1 h prior to the insertion of senktide microimplants and terminated at the end of the blood sampling procedure. Microimplants were removed and animals were treated prophylactically with gentamicin sulfate (4 ml, i.m.; Patterson Veterinary, Devens, MA, USA). Two days later, two CIDRs were reinserted, and the protocol was repeated after 10 days of elevated progesterone, with the ewes that were given saline during the first treatment period receiving Kp-271 and vice versa.
Tissue collection
Hypothalamic tissue was collected for immunocytochemistry and histological determination of treatment sites as described previously (60). Briefly, ewes were heparinised and euthanised with an i.v. overdose (8–12 ml) of sodium pentobarbital (Euthasol; Patterson Veterinary). When the breathing stopped, the head was removed and perfused via the internal carotids with 6 l of 4% paraformaldehyde in 0.1 M phosphate buffer containing 0.1% NaNO3. Tissue blocks were removed and stored in fixative (as above) at 4 °C overnight and then in 30% sucrose. After sucrose infiltration, 45-μm thick frozen coronal sections were cut using a freezing microtome. For Experiments 1 and 3, every fifth section through the area of interest was stained with cresyl violet and examined to determine the position of the cannula. For immunocytochemistry, five parallel series of sections (225 μm apart) were stored at −20 °C in cryoprotectant (10% polyvinyl pyrrolidone [PVP-40; Sigma-Aldrich], 30% ethylene glycol and 30% sucrose in 0.1 M PB) (61).
LH radioimmunoassay
LH concentrations were measured in duplicate, using 100–200 μl of plasma per sample, by means of a double-antibody radioimmunoassay using reagents supplied by the National Hormone and Peptide Program (NHPP; Harbor-UCLA Medical Center, Torrance, CA, USA), which has been validated for use in sheep (62,63). Values are expressed in terms of the reference standard preparation NIH-LH-S12. The sensitivity limit of the LH radioimmunoassay averaged 0.1 ng/tube and the inter- and intra-assay coefficients of variation were 9.5% and 3.5%, respectively.
Immunocytochemistry
For all protocols, washes and incubations were performed on free-floating hypothalamic sections at room temperature under gentle agitation and sections were washed extensively between incubations in 0.1 M phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked with 1% H2O2 (in 1 M PBS, 10 min; Sigma-Aldrich). Nonspecific binding was blocked with an antibody incubation solution consisting of 4% normal goat serum (NGS; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and 0.4% Triton X-100 (Sigma-Aldrich) in 0.1 M PBS (1 h). After processing, sections were mounted onto Superfrost plus glass slides (Fisher Scientific, Pittsburgh, PA, USA) and coverslipped with gelvatol containing the anti-fading agent, 1,4-diazabicyclo(2,2)octane (DABCO; Sigma-Aldrich; 50 mg/ml), for all immunofluorescence detection or dehydrated and coverslipped with dibutyl phthalate xylene (Electron Microscopy Sciences, Hatfield, PA, USA) for chromogen detection. For each of the staining procedures, sections from all animals were processed simultaneously.
c-Fos and kisspeptin
This protocol was similar to that used in our previous studies (53). Series of sections containing the ARC or POA (three sections/area/ewe) were incubated successively with rabbit polyclonal antibody against c-Fos (dilution 1 : 2500 in 0.1 M PBS, 17 h; sc-253; Santa Cruz Biotechnology, Santa Cruz, CA, USA), biotinylated goat anti-rabbit IgG (dilution 1 : 500 in 0.1 M PBS, 1 h; BA-1000; Vector Laboratories, Burlingame, CA, USA), avidin-biotin-horseradish peroxidase complex (dilution 1 : 500 in 0.1 M PBS, 1 h; ABC-Elite; Vector Laboratories) and 0.2 mg/ml 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich) in 0.1 M PB containing 0.015% H2O2 and 62.5 μg/ml NiSO4 (10 min). Next, sections were incubated with rabbit anti-sheep kisspeptin (dilution 1: 50 000 in 0.1 M PBS, 17 h; no. 564, gift from Dr A. Caraty, ex-INRA, Nouzilly, France), biotinylated goat anti-rabbit IgG, ABC-Elite and DAB. Both primary antibodies have been previously validated for use in sheep (7,53). For each tissue section, single- (kisspeptin only) and dual- (kisspeptin/c-Fos) labelled cells were counted under bright-field illumination as described previously (53). A cell was considered dual-labelled when the black nickel-enhanced reaction product within the nucleus was observed to be surrounded by a brown reaction product in the cytoplasm in the same focal plane. The results were expressed as a percentage of kisspeptin-immunoreactive (-IR) cells coexpressing c-Fos.
BDA injection sites
Sections containing the RCh were immunostained for BDA and tyrosine hydroxylase (TH), as the marker of A15 dopamine neurones, a prominent cell group within the RCh (60,64–66). Sections were incubated successively with antibody incubation solution (17 h), ABC-Elite (1 h) and DAB containing NiSO4 (10 min). Next, sections were incubated successively with mouse anti-TH (dilution 1 : 10 000 in 0.1 M PBS, 17 h; MAB 5280; Millipore, Billerica, MA, USA), biotinylated goat anti-mouse (dilution 1 : 500 in 0.1 M PBS, 1 h; BA-9200; Vector Laboratories), ABC-Elite (1 h) and DAB (10 min).
BDA and kisspeptin (peroxidase)
Sections containing the ARC were incubated successively with ABC-Elite (1 h) and DAB containing NiSO4 (10 min) to visualise BDA. Next, sections were incubated successively with rabbit anti-sheep kisspeptin (dilution 1 : 50 000 in 0.1 M PBS, 17 h), biotinylated goat anti-rabbit IgG, ABC-Elite and DAB to visualise kisspeptin.
BDA and kisspeptin (fluorescence)
Sections containing the ARC were incubated successively with rabbit anti-sheep kisspeptin (dilution 1 : 1000 in 0.1 M PBS, 17 h) and Alexa Fluor 488-conjugated goat anti-rabbit (dilution 1 : 100 in 0.1 M PBS, 30 min; A11008; Life Technologies, Carlsbad, CA, USA) to visualise kisspeptin, and then successively with an ABC, tyramide sample amplification system (dilution 1 : 250 in 0.1 M PBS containing 1 μl of 3% H2O2/ml, 10 min; NEN Life Sciences, Boston, MA, USA) and Alexa Fluor 555-conjugated streptavidin (dilution 1 : 200 in 0.1 M PBS, 30 min; S32355; Life Technologies) to visualise BDA.
Tract-tracing and image collection
The location and spread of BDA was investigated in a series of sections throughout the RCh. Axon terminals concentrating BDA were examined in the ARC in four to six sections per ewe at × 20 magnification using a brightfield microscope (DM500B; Leica Microsystems, Wetzlar, Germany). Images of injection sites and BDA labelling in the ARC were captured using NEUROLUCIDA (MicroBrightfield Bioscience, Williston, VT, USA) and a digital camera (Microfire A/R; Optronics, Goleta, CA, USA) attached to a brightfield microscope (DM500B; Leica Microsystems).
Confocal imaging
Sections processed for dual immunofluorescence were imaged using a laser-scanning confocal system (D-Eclipse C1; Nikon Instruments, Melville, NY, USA) attached to a biological research microscope (Eclipse E800; Nikon Instruments). Fluorophores were excited by two lasers at wavelengths of 488 and 543 nm. Confocal Z-stacks of optical sections (1 μm at × 60 magnification) were captured. Images of both RCh and ARC were not altered in any way, except for slight adjustments to the brightness for optimal illustration.
Statistical analysis
In Experiment 1, all 12 ewes in the RCh group and 10 of 11 ewes in the POA group had correct placement of guide cannulae, as described in detail elsewhere (47). In Experiment 2, all three ewes had BDA injections restricted to the RCh. In Experiment 3, seven of eight ewes had correct placement of guide cannulae. Only ewes with proper guide cannula placement were included in the statistical analyses. The statistical significance of the effect of treatment on the mean ± SEM LH concentrations during the 3 h after microimplant insertion and on relative kisspeptin/c-Fos coexpression in Experiment 1 was assessed using individual Mann–Whitney rank sum tests. Cumulative changes in LH concentrations after the insertion of microimplants in Experiment 3 were expressed as the mean ± SEM area under the curve (AUC) of all replicates within the two treatment groups, and were compared using a Wilcoxon signed rank test. Mean ± SEM LH levels at each timepoint in Experiment 3 were compared using two-way ANOVA with repeated measures (influence of time and treatment) and with Holm–Sidak pairwise multiple comparison. P < 0.05 was considered statistically significant.
Results
Experiment 1: Which population of kisspeptin neurones is activated by RCh or POA administration of senktide?
Mean LH concentrations in ewes that received empty implants in the RCh were 0.43 ± 0.19 ng/ml, whereas, in ewes with RCh senktide implants, mean LH levels were 11.2 ± 3.7 ng/ml (Fig. 1A), which is both a significant increase and consistent with previous reports from our group (47,50). Senktide did not significantly alter the number of cells expressing kisspeptin in either the ARC or the POA (see Supporting information, Table S1). However, fewer kisspeptin neurones were observed within the POA compared to the ARC, in line with previous reports (46,53). Senktide microimplants in the RCh significantly increased the coexpression of c-Fos in kisspeptin-immunoreactive-IR neurones in the ARC compared to empty implants (62.6 ± 5.3% versus 9.7 ± 2.9%) (Fig. 1B). Senktide-containing microimplants in the RCh also significantly increased POA kisspeptin/c-Fos coexpression relative to empty implants (7.1 ± 1.5% versus 2.1 ± 1.8%, respectively) (Fig. 1B), albeit to a much lesser extent than in the RCh.
Fig. 1.
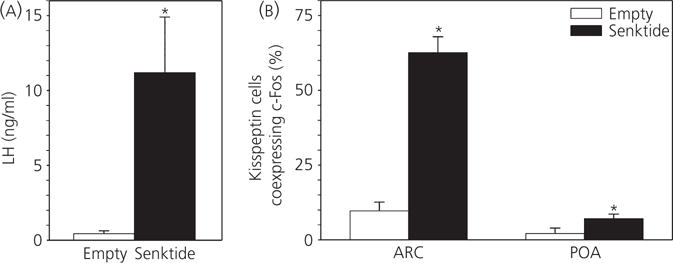
Effect of retrochiasmatic area (RCh) senktide administration on kisspeptin cell activation in anoestrous ewes. (A) Mean ± SEM luteinising hormone (LH) secretion in anoestrous ewes during the first 3 h after receiving bilateral senktide-containing (n = 7) or empty (n = 5) implants in the RCh. (B) Percentage (mean ± SEM) of arcuate nucleus (ARC) or preoptic area (POA) kisspeptin neurones coexpressing c-Fos in those two treatment groups. *P < 0.05 versus corresponding value in control group (Mann–Whitney rank sum test).
After senktide microimplantation in the POA, the average LH concentration was 8.8 ± 1.5 ng/ml, which is significantly higher than after placement of empty implants (1.1 ± 0.2 ng/ml) (Fig. 2A), as reported previously (47). No differences in the number of cells expressing kisspeptin were observed between treatment groups (see Supporting information, Table S1). The percentage of ARC kisspeptin cells coexpressing c-Fos was significantly greater in senktide-treated ewes compared to those receiving empty implants (40.6 ± 6.9% versus 19.1 ± 5.9%) (Fig. 2B). Senktide microimplantation in the POA did not alter the percentage of local kisspeptin neurones expressing c-Fos (7.6 ± 2.9% versus 6.4 ± 3.7%) (Fig. 2B).
Fig. 2.
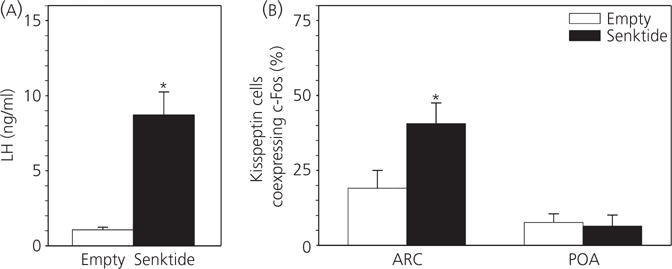
Effect of preoptic area (POA) senktide administration on kisspeptin cell activation in follicular phase ewes. (A) Mean ± SEM luteinising hormone (LH) secretion in follicular phase ewes during the first 3 h after receiving bilateral intra-POA senktide-containing (n = 6) or empty (n = 5) implants. (B) Percentage (mean ± SEM) of arcuate nucleus (ARC) or POA kisspeptin neurones coexpressing c-Fos in those two treatment groups. *P < 0.05 versus corresponding value in control group (Mann–Whitney rank sum test). Note that the scale of the y-axis is the same as in Fig. 1 to facilitate comparison of the magnitudes of the response to senktide in both areas.
Experiment 2: Do RCh neurones project to ARC kisspeptin neurones?
All three ewes had BDA injections restricted to the RCh, although the location and spread of the BDA infusion within the RCh varied (Fig. 3). In all animals, BDA-labelled axon terminals could be traced to the ARC, ipsilateral to the injection site. BDA-labelled boutons were observed in close proximity to kisspeptin-immunoreactive soma and dendrites (Fig. 4). Confocal analysis of fluorescence stained sections confirmed that BDA-labelled boutons were found in close apposition to kisspeptin soma and dendrites in the ARC (Fig. 5). Moreover, in the two animals in which greater spread of BDA from the injection site was evident (#48 and #602), 70–80% of ARC kisspeptin cells received RCh inputs and often more than one input was noted. Although predominantly an anterograde tracer, BDA is also known to produce retrograde labelling (56,67). In ewes #48 and #602, BDA not only labelled anterograde projections of RCh neurones, but also cell bodies and dendrites of neurones that project to the RCh; such retrograde projections were found in the ARC (Figs 4 and 5); however, none contained kisspeptin immunoreactivity.
Fig. 3.
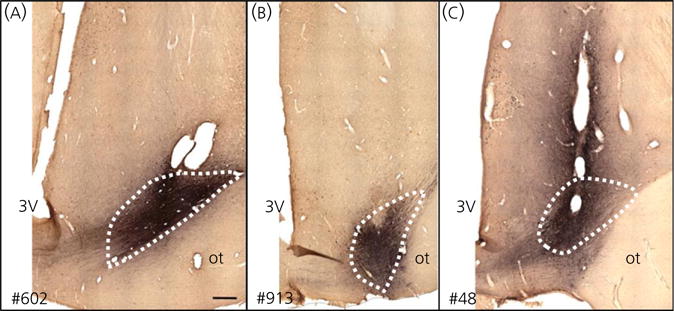
Retrochiasmatic area (RCh) biotinylated dextran amine injection sites in three ewes. (A) Ewe #602: rostral–caudal spread of approximately 1.2 mm (B). Ewe #913: rostral–caudal spread of approximately 0.5 mm. (C) Ewe #48: rostral–caudal spread of approximately 1.5 mm. Dotted lines indicate the approximate boundaries of the RCh based on matched Nissl-stained sections at the same level. Scale bar = 500 μm. 3V, third ventricle; ot, optic tract.
Fig. 4.
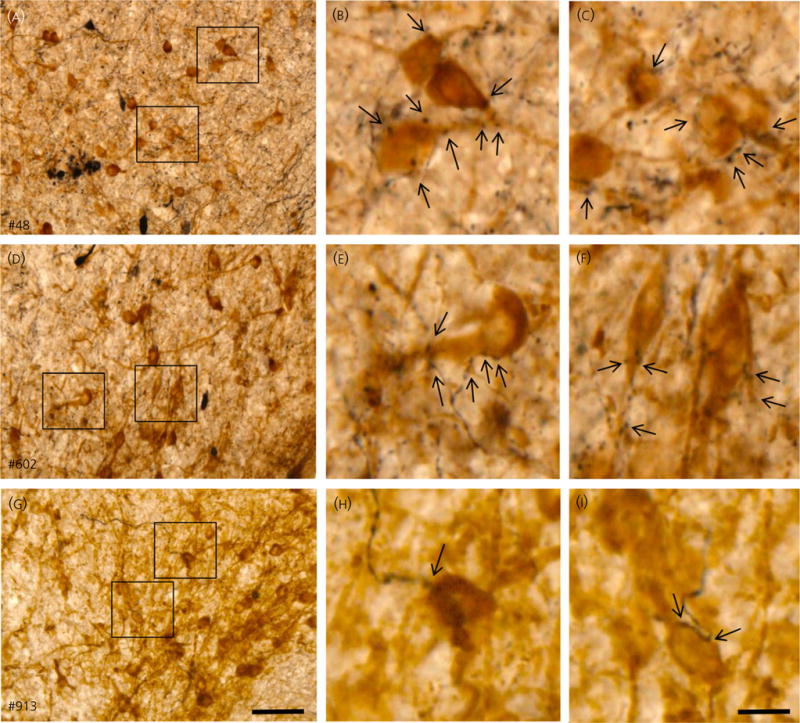
Arcuate nucleus (ARC) projections of retrochiasmatic area neurones in three ewes. Demonstration of biotinylated dextran amine (BDA)-labelled axons in ARC in close proximity to kisspeptin immunoreactive cell bodies in ewes #48 (A–C), #602 (D–F), and #914 (G–I). (A, D, G) show low magnification overview of labelling in ARC; scale bar = 50 μm. (B, C, E, F, H, I) show higher magnifications of areas indicated by boxes in (A, D, G) and illustrate BDA-labelled boutons in close proximity to kisspeptin neurones (indicated by arrows); scale bar = 15 μm.
Fig. 5.
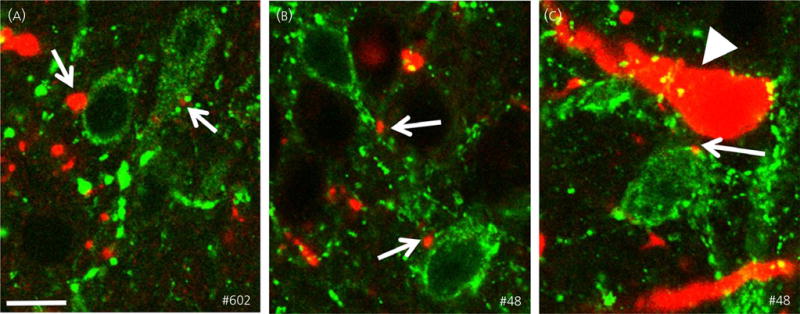
Close contacts between retrochiasmatic area neurones and arcuate nucleus kisspeptin neurones (indicated by arrows). Confocal images (optical sections of 1 μm) illustrating biotinylated dextran amine (BDA)-labelled axon terminals (red) in close apposition to kisspeptin-immunoreactive soma and dendrites (green) in ewes #602 (A) and #48 (B, C). Retrogradely-labelled cells and dendrites containing BDA (arrow head) are shown in (C). Note that this neurone is negative for kisspeptin immunoreactivity. Scale bar = 15 μm.
Experiment 3: Does endogenous kisspeptin mediate surge-like LH secretion induced by RCh microimplantation of senktide?
Correct placement of guide cannulae was observed in seven of eight ewes (Fig. 6A), with one animal in which the pair of cannulae was confirmed to terminate within the optic chiasm (not shown) excluded from the analysis. Overall, LH secretion in senktide-treated ewes was significantly suppressed by Kp-271 infusion compared to saline-infused controls (AUC: 12.9 ± 1.4 versus 25.6 ± 4.6, respectively) (Fig. 6B). Mean LH concentrations prior to senktide treatment were similar between the saline- and Kp-271-infused groups (3.7 ± 0.2 versus 3.3 ± 0.3 ng/ml, respectively). LH concentrations tended to increase 90 min after the insertion of senktide microimplants in the saline-infused group (Fig. 6C,E), reaching statistical significance within 2 h of senktide administration (Fig. 6E). No such increase was observed in the Kp-271-infused group (Fig. 6D,E). LH levels after senktide administration peaked at 18.8 ± 4.0 ng/ml in the saline-infused controls and at 7.7 ± 1.3 ng/ml in Kp-271-infused ewes.
Fig. 6.
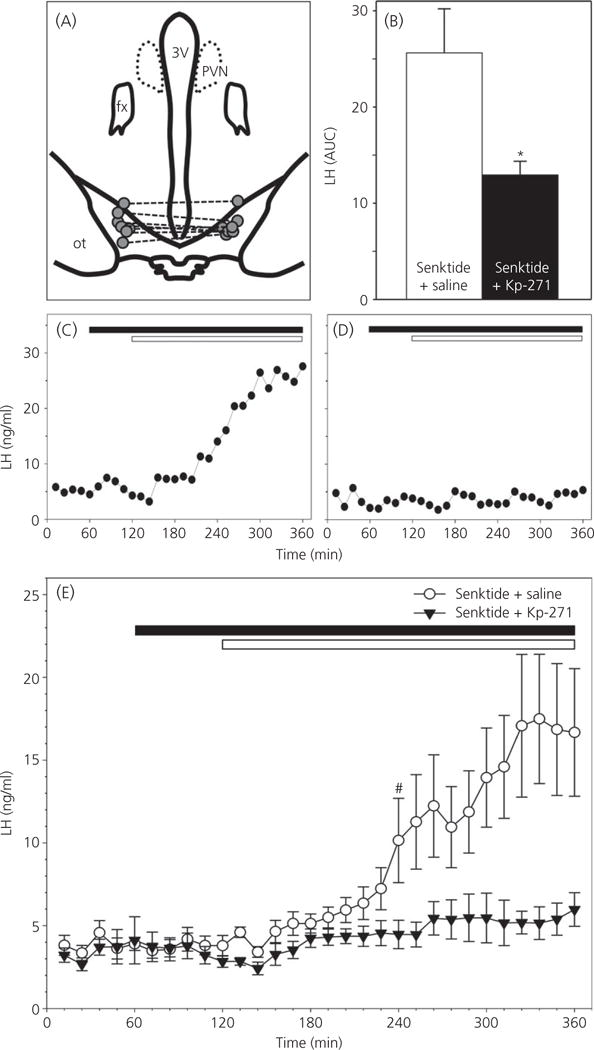
Effect of Kiss1R antagonist on retrochiasmatic area (RCh) senktide-induced luteinising hormone (LH) secretion in follicular phase ewes. (A) Camera lucida representation of ovine hypothalamus showing correct placement of bilateral guide cannulae within the RCh in seven animals. Dashed lines connect bilateral microimplant loci in the same animal. 3V, third ventricle; PVN, paraventricular nucleus; fx, fornix; ot, optic tract. (B) LH secretion, expressed as the area under the curve [mean ± SEM area under the curve (AUC)] in ovary-intact ewes (n = 7 per treatment group) receiving senktide implants in the RCh during i.c.v. infusion of the Kiss1R antagonist, Kp-271 (black bar) or vehicle (white bar) during the follicular phase of the oestrous cycle. (C, D) Representative LH profiles demonstrating the effect of intra-RCh implantation (white bar) of senktide, during i.c.v. infusion (black bar) of the Kiss1R antagonist, Kp-271, or vehicle (saline), on LH secretion in ovary-intact ewes during the follicular phase of the oestrous cycle. (E) Concentrations of LH (mean ± SEM) in ovary-intact ewes (n = 7 per treatment group) bilaterally implanted with senktide in the RCh (white bar) during i.c.v. infusion (black bar) of the Kiss1R antagonist, Kp-271 (black triangles), or vehicle (saline; white circles) during the follicular phase of the oestrous cycle. *P < 0.05 versus corresponding value in saline-treated control group (Wilcoxon signed rank test); #first value in the time-series that is significantly different (P < 0.05 versus corresponding value in saline-treated control group [two-way ANOVA with repeated measures (influence of time and treatment) with Holm–Sidak pairwise multiple comparison], all subsequent values are also significantly different from controls).
Discussion
Using c-Fos as an index of neuronal activation, we showed that senktide treatment in either the RCh or POA increases activation of ARC kisspeptin (KNDy) neurones concomitant with an increase in LH secretion. We then described a novel neuroanatomical connection consistent with the activation of KNDy neurones by RCh senktide treatment, showing that RCh neurones project directly to and form close contacts with KNDy neurones. Finally, we have demonstrated that surge-like LH secretion induced by intra-RCh administration of senktide is mediated by kisspeptin/Kiss1r signalling. Taken together, these results provide the first evidence indicating that NK3R activation in the RCh stimulates GnRH/LH secretion via KNDy neurones.
Kisspeptin has been suggested to mediate the effects of NKB in several species. Indeed, NK3R is expressed in KNDy neurones in rats (23), mice (68) and sheep (44). By contrast, although NK3R was detected in 16% of GnRH neurones in rats (23), there is no evidence for NK3R expression in GnRH neurones in ewes (44). Moreover, senktide-induced stimulation of LH secretion has been shown to be diminished by Kiss1r desensitisation in monkeys (52), Kiss1r antagonist administration in rats (51) and GPR54 knockout in mice (69). It is important to note that these studies proposed that senktide acts directly on KNDy neurones and, as such, relate to pulsatile LH secretion. Our data, however, indicate that, in sheep, senktide can act in the RCh or POA to stimulate KNDy neurones indirectly to induce surge-like LH secretion. We have demonstrated that direct activation of ARC NK3R most likely stimulates episodic LH secretion with considerably lower peak LH levels, as achieved by senktide microimplants placed in the ARC than in the RCh or POA (47). To this end, it is of little surprise that i.c.v. infusion of the NK3R antagonist MRK-08, which is more likely to reach NK3R in the ARC than in the RCh or POA, suppressed LH pulses but did not alter the amplitude of E2-induced LH surges in ewes (70).
Although senktide treatment in the POA increased activation of KNDy neurones in conjunction with increased LH secretion, the role of POA NK3R in the preovulatory LH surge is unclear because SB222200 microimplants in the POA did not affect the E2-induced LH surge (47). The increase in the percentage of KNDy neurones expressing c-Fos after POA administration of senktide (5.5-fold) is relatively small compared to the 14-fold increase after RCh administration, which is consistent with a more important role for NK3R in the RCh. Moreover, during an endogenous preovulatory surge in ewes, there is an increase in the activation of both ARC and POA kisspeptin neurones (53); this effect is also observed after RCh but not POA placement of senktide microimplants. Alternatively, the more pronounced response to senktide in the RCh might be explained by the fact that NK3R-positive cells are more concentrated there compared to the POA, where they are more widely scattered (44). This anatomical difference could account for the observation that LH levels elevated by the POA administration of senktide took approximately 1 h longer to peak and reach significance above controls than did RCh administration of senktide (47). This difference raises the possibility that c-Fos induction in KNDy neurones is equally retarded and does not fully manifest within the 3-h post-treatment in this experiment. Finally, the fact that the RCh group comprised anoestrous ewes and the POA group comprised follicular phase ewes is unlikely to explain the discrepancy in the magnitude of KNDy c-Fos induction, as a result of the comparable magnitude of the LH response to senktide administration in both cases (47). However, we cannot rule out an effect of photoperiod on c-Fos induction in KNDy neurones. In light of these regional differences, subsequent experiments focused on the RCh because the NK3R in this region has been clearly implicated in the endogenous LH surge (47).
The results of the final two experiments provide functional and anatomical evidence showing that NKB-responsive neurones in the RCh act via KNDy neurones (Fig. 7). The results of Experiment 3 demonstrate that kisspeptin is entirely responsible for the potent effect of RCh NK3R activation on LH secretion in follicular phase ewes. This conclusion is consistent with previous work giving rise to the notion that kisspeptin plays an important role in the generation of the GnRH surge in sheep (46,53). Moreover, the results of Experiments 1 and 3, taken together, indicate that the stimulatory actions of NKB in the RCh occur via kisspeptin release from KNDy neurones and support a role for the latter in the preovulatory LH surge in ewes (53). This conclusion is also supported by the results of the tract-tracing experiment. Although this experiment demonstrates a direct projection of RCh neurones to KNDy neurones, determining their neurochemical phenotype requires further study. However, it is known that the NK3R-containing neurones in the ovine RCh are distinct from A15 dopamine neurones that also project to the ARC and are implicated in the seasonal control of reproduction (64,71) because the A15 neurones do not express this receptor (50). Similarly, although NKB-IR fibres have been reported to form close contacts with NK3R neurones in the RCh (44), the identity of neurones supplying these projections remains to be identified. In the ewe, the ARC appears to contain the majority of NKB-immunoreactive neurones in the diencephalon (35,72). However, our tract-tracing study revealed that projections from the ARC to the RCh did not contain kisspeptin, and so KNDy neurones do not appear to supply the RCh with NKB. Notwithstanding, the ovine ARC contains a small percentage of NKB-immunoreactive neurones that do not coexpress kisspeptin and/or dynorphin (7,72). The identification of further hypothalamic and extrahypothalamic populations of NKB-expressing neurones by immunohistochemistry and in situ hybridisation, as well as the characterisation of their efferent projections, remains a priority.
Fig. 7.
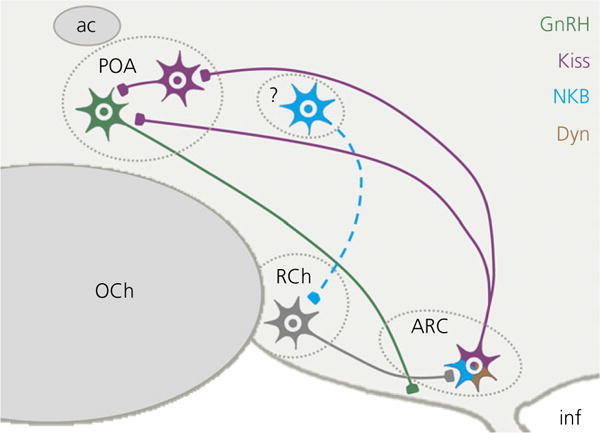
Schematic representation of connectivity between neurones implicated in the luteinising hormone surge in sheep. Retrochiasmatic area (RCh) neurones (grey) project to KNDy neurones in the arcuate nucleus (ARC), which coexpress kisspeptin (purple), NKB (blue) and dynorphin (Dyn) (brown), and innervate kisspeptin and gonadotrophin-releasing hormone neurones (GnRH) (green) in the preoptic area (POA). The source of NKB that activates NK3R on RCh neurones is currently unknown. ac, anterior commissure; inf, infundibular recess; OCh, optic chiasm.
Senktide administered in the RCh induced c-Fos expression in over 60% of KNDy neurones, which is commensurate with the extent of activation observed during endogenous LH surges in ovary-intact ewes (53). However, neither RCh, nor POA senktide administration elevated c-Fos expression in POA kisspeptin neurones to levels seen during the endogenous LH surge (53,73). This might account for the discrepancy between peak LH concentrations achieved after RCh administration of senktide (22–32 ng/ml) and those expected during endogenous or E2-induced LH surges (often in excess of 100 ng/ml) (47,74). Although the mechanism by which ovine POA kisspeptin neurones are activated during the endogenous LH surge is unknown, it is generally considered to be dependent on the positive-feedback effects of E2 (38,53,73), and might involve KNDy neurones that project to them (29). Other neural systems are also likely involved because activation of 60% of KNDy neurones with RCh senktide microimplants induced c-Fos expression in only a small percentage of POA kisspeptin neurones. We suggest that, during seasonal anoestrus, when this particular experiment was performed, circulating E2 levels are inadequate for the activation of POA kisspeptin neurones (38), which is necessary for the full LH surge. Indeed, in the presence of surge-inducing levels of exogenous E2 that trigger LH surges in OVX ewes, RCh administration of SB222200 inhibited the amplitude of E2-induced LH surges by only 42% (47). Other neuronal populations (75), including A1 noradrenergic (76–79) and ARC β-endorphin neurones (16,80,81), contribute to the positive-feedback effects of E2 on LH secretion in sheep. These systems may act independently of the NKB/kisspeptin pathway because antagonism of central Kiss1r reduced the amplitude of the oestrogen-induced LH surge by only 50% (46). If so, they could account for the partial effect of RCh NK3R blockade on the amplitude of the LH surge (47) and the limited activation of POA kisspeptin neurones after RCh administration of senktide. Thus, the RCh NK3R-KNDy axis described in the present study, in addition to previously characterised surge-regulating neural systems (82), is an important component of the preovulatory LH surge generation mechanism.
In summary, we report evidence that further implicates RCh NKB/NK3R signalling in regulating the preovulatory LH surge, and we also elaborate on the mechanism of such actions by demonstrating their dependency on the stimulatory effect of kisspeptin of KNDy origin. Activation of NK3R-containing neurones in the POA also stimulates KNDy neurones, although the role of these neurones in the endogenous LH surge remains to be determined. Based on these data and previous work (47), we propose that one pathway for induction of the preovulatory LH surge involves NKB release from axonal boutons and terminals in the vicinity of RCh NK3R neurones. The NK3R neurones of the RCh in turn stimulate kisspeptin secretion from KNDy neurones, thereby increasing the amplitude of the LH surge.
Supplementary Material
Table S1. Effect of retrochiasmatic area (RCh) or preoptic area (POA) senktide microimplantation on the total number of kisspeptin cells.
Acknowledgments
We thank Heather Bungard (West Virginia University Food Animal Research Facility), Gail Nesselrod (Department of Physiology & Pharmacology, West Virginia University) and Dr Margaret Minch (Division of Animal and Nutritional Sciences, West Virginia University) for the care of the animals, as well as Tina Smith (Department of Neurobiology & Anatomical Sciences, The University of Mississippi Medical Center) and Dr Miroslav Valent (Department of Physiology & Pharmacology, West Virginia University) for assistance with the LH radioimmunoassay. We also thank Dr Al Parlow and the National Hormone and Peptide Program (NIDDK, Bethesda, MD, USA) for the reagents used to measure LH and Dr Alain Caraty (ex-INRA, Nouzilly, France) for the generous gift of anti-sheep kisspeptin.
This work was supported by National Institutes of Health grants R01-HD039916, R01-HD017864 and R01-HD082135 (RLG and MNL). The funders were not involved in the study design, the acquisition and interpretation of data, the decision to publish the work or the preparation of the manuscript.
Footnotes
Disclosure
The authors have nothing to disclose.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 3.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O’Rahilly S, Reimann F, Semple RK, Topaloglu AK. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94:3633–3639. doi: 10.1210/jc.2009-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 6.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 8.Ronnekleiv OK, Kelly MJ. Kisspeptin excitation of GnRH neurons. Adv Exp Med Biol. 2013;784:113–131. doi: 10.1007/978-1-4614-6199-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol. 2013;784:297–323. doi: 10.1007/978-1-4614-6199-9_14. [DOI] [PubMed] [Google Scholar]
- 10.Piet R, de Croft S, Liu X, Herbison AE. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front Neuroendocrinol. 2015;36:15–27. doi: 10.1016/j.yfrne.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153:5105–5118. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99:18–32. doi: 10.1159/000355285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 14.Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE. 2009;4:e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- 17.Simorangkir DR, Ramaswamy S, Marshall GR, Pohl CR, Plant TM. A selective monotropic elevation of FSH, but not that of LH, amplifies the proliferation and differentiation of spermatogonia in the adult rhesus monkey (Macaca mulatta) Hum Reprod. 2009;24:1584–1595. doi: 10.1093/humrep/dep052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramaswamy S, Plant TM, Marshall GR. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta) Biol Reprod. 2000;63:82–88. doi: 10.1095/biolreprod63.1.82. [DOI] [PubMed] [Google Scholar]
- 19.Oussaid B, Lonergan P, Khatir H, Guler A, Monniaux D, Touze JL, Beckers JF, Cognie Y, Mermillod P. Effect of GnRH antagonist-induced prolonged follicular phase on follicular atresia and oocyte developmental competence in vitro in superovulated heifers. J Reprod Fertil. 2000;118:137–144. [PubMed] [Google Scholar]
- 20.Dobson H, Campbell BK, Scaramuzzi RJ. Use of a GnRH antagonist in conjunction with low amplitude, high frequency LH pulses to induce follicular growth without an LH surge and ovulation in ewes. Anim Reprod Sci. 1997;46:213–222. doi: 10.1016/s0378-4320(96)01624-7. [DOI] [PubMed] [Google Scholar]
- 21.Oussaid B, Mariana JC, Poulin N, Fontaine J, Lonergan P, Beckers JF, Cognie Y. Reduction of the developmental competence of sheep oocytes by inhibition of LH pulses during the follicular phase with a GnRH antagonist. J Reprod Fertil. 1999;117:71–77. doi: 10.1530/jrf.0.1170071. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56:729–737. doi: 10.1507/endocrj.k09e-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 24.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borsay BA, Skrapits K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Hrabovszky E. Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance p immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology. 2014;100:141–152. doi: 10.1159/000368362. [DOI] [PubMed] [Google Scholar]
- 27.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154:4259–4269. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. doi: 10.1007/978-1-4614-6199-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27:624–635. doi: 10.1111/jne.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- 31.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 32.Herbison AE, Robinson JE, Skinner DC. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology. 1993;57:751–759. doi: 10.1159/000126433. [DOI] [PubMed] [Google Scholar]
- 33.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- 34.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141:4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- 36.Smith JT. Sex steroid regulation of kisspeptin circuits. Adv Exp Med Biol. 2013;784:275–295. doi: 10.1007/978-1-4614-6199-9_13. [DOI] [PubMed] [Google Scholar]
- 37.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150:5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- 39.Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- 40.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 41.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2011;23:293–301. doi: 10.1111/j.1365-2826.2011.02107.x. [DOI] [PubMed] [Google Scholar]
- 43.Kallo I, Vida B, Deli L, Molnar CS, Hrabovszky E, Caraty A, Ciofi P, Coen CW, Liposits Z. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol. 2012;24:464–476. doi: 10.1111/j.1365-2826.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 44.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22:1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn T, Fergani C, Coolen LM, Padmanabhan V, Lehman MN. Prenatal testosterone excess decreases neurokinin 3 receptor immunoreactivity within the arcuate nucleus KNDy cell population. J Neuroendocrinol. 2015;27:100–110. doi: 10.1111/jne.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 47.Porter KL, Hileman SM, Hardy SL, Nestor CC, Lehman MN, Goodman RL. Neurokinin-3 receptor activation in the retrochiasmatic area is essential for the full pre-ovulatory luteinising hormone surge in ewes. J Neuroendocrinol. 2014;26:776–784. doi: 10.1111/jne.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grachev P, Millar RP, O’Byrne KT. The role of neurokinin B signalling in reproductive neuroendocrinology. Neuroendocrinology. 2014;99:7–17. doi: 10.1159/000357734. [DOI] [PubMed] [Google Scholar]
- 49.Navarro VM. Interactions between kisspeptins and neurokinin B. Adv Exp Med Biol. 2013;784:325–347. doi: 10.1007/978-1-4614-6199-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151:3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, Millar RP, Lightman SL, O’Byrne KT. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS ONE. 2012;7:e44344. doi: 10.1371/journal.pone.0044344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153:5406–5414. doi: 10.1210/en.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 55.Karsch FJ, Foster DL, Legan SJ, Ryan KD, Peter GK. Control of the preovulatory endocrine events in the ewe: interrelationship of estradiol, progesterone, and luteinizing hormone. Endocrinology. 1979;105:421–426. doi: 10.1210/endo-105-2-421. [DOI] [PubMed] [Google Scholar]
- 56.Coolen LM, Jansen HT, Goodman RL, Wood RI, Lehman MN. A new method for simultaneous demonstration of anterograde and retrograde connections in the brain: co-injections of biotinylated dextran amine and the beta subunit of cholera toxin. J Neurosci Methods. 1999;91:1–8. doi: 10.1016/s0165-0270(99)00055-2. [DOI] [PubMed] [Google Scholar]
- 57.Nestor CC, Coolen LM, Nesselrod GL, Valent M, Connors JM, Hileman SM, Cheng G, Lehman MN, Goodman RL. Evidence that orphanin FQ mediates progesterone negative feedback in the ewe. Endocrinology. 2013;154:4249–4258. doi: 10.1210/en.2013-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodman RL, Holaskova I, Nestor CC, Connors JM, Billings HJ, Valent M, Lehman MN, Hileman SM. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology. 2011;152:3451–3460. doi: 10.1210/en.2011-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roseweir AK, Millar RP. Kisspeptin antagonists. Adv Exp Med Biol. 2013;784:159–186. doi: 10.1007/978-1-4614-6199-9_8. [DOI] [PubMed] [Google Scholar]
- 60.Goodman RL, Maltby MJ, Millar RP, Hileman SM, Nestor CC, Whited B, Tseng AS, Coolen LM, Lehman MN. Evidence that dopamine acts via kisspeptin to hold GnRH pulse frequency in check in anestrous ewes. Endocrinology. 2012;153:5918–5927. doi: 10.1210/en.2012-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 62.Whisnant SC, Havern RL, Goodman RL. Endogenous opioid suppression of luteinizing hormone pulse frequency and amplitude in the ewe: hypothalamic sites of action. Neuroendocrinology. 1991;54:587–593. doi: 10.1159/000125964. [DOI] [PubMed] [Google Scholar]
- 63.Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- 64.Lehman MN, Durham DM, Jansen HT, Adrian B, Goodman RL. Dopaminergic A14/A15 neurons are activated during estradiol negative feedback in anestrous, but not breeding season, ewes. Endocrinology. 1996;137:4443–4450. doi: 10.1210/endo.137.10.8828506. [DOI] [PubMed] [Google Scholar]
- 65.Lehman MN, Ladha Z, Coolen LM, Hileman SM, Connors JM, Goodman RL. Neuronal plasticity and seasonal reproduction in sheep. Eur J Neurosci. 2010;32:2152–2164. doi: 10.1111/j.1460-9568.2010.07530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weems PW, Goodman RL, Lehman MN. Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Front Neuroendocrinol. 2015;37:43–51. doi: 10.1016/j.yfrne.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dolleman-Van der Weel MJ, Wouterlood FG, Witter MP. Multiple anterograde tracing, combining Phaseolus vulgaris leucoagglutinin with rhodamine- and biotin-conjugated dextran amine. J Neurosci Methods. 1994;51:9–21. doi: 10.1016/0165-0270(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 68.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenrohr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153:316–328. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 70.Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that neurokinin B controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinology. 2015;101:161–174. doi: 10.1159/000377702. [DOI] [PubMed] [Google Scholar]
- 71.Thiery JC, Gayrard V, Le Corre S, Viguie C, Martin GB, Chemineau P, Malpaux B. Dopaminergic control of LH secretion by the A15 nucleus in anoestrous ewes. J Reprod Fertil Suppl. 1995;49:285–296. [PubMed] [Google Scholar]
- 72.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology. 2011;152:214–222. doi: 10.1210/en.2010-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodman RL, Pickover SM, Karsch FJ. Ovarian feedback control of follicle-stimulating hormone in the ewe: evidence for selective suppression. Endocrinology. 1981;108:772–777. doi: 10.1210/endo-108-3-772. [DOI] [PubMed] [Google Scholar]
- 75.Goodman RL, Inskeep EK. Control of the ovarian cycle of the sheep. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4th. San Diego, CA: Academic Press; 2015. pp. 1259–1305. [Google Scholar]
- 76.Jackson GL. Blockage of estrogen-induced release of luteinizing hormone by reserpine and potentiation of synthetic gonadotropin-releasing hormone-induced release of luteinizing hormone by estrogen in the ovariectomized ewe. Endocrinology. 1975;97:1300–1307. doi: 10.1210/endo-97-5-1300. [DOI] [PubMed] [Google Scholar]
- 77.Jackson GL. Effect of adrenergic blocking drugs on secretion of luteinizing hormone in the ovariectomized ewe. Biol Reprod. 1977;16:543–548. [PubMed] [Google Scholar]
- 78.Robinson JE, Kendrick KM, Lambart CE. Changes in the release of gamma-aminobutyric acid and catecholamines in the preoptic/septal area prior to and during the preovulatory surge of luteinizing hormone in the ewe. J Neuroendocrinol. 1991;3:393–399. doi: 10.1111/j.1365-2826.1991.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 79.Clarke IJ, Scott CJ, Pereira A, Pompolo S. The role of noradrenaline in the generation of the preovulatory LH surge in the ewe. Domest Anim Endocrinol. 2006;30:260–275. doi: 10.1016/j.domaniend.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144:3663–3676. doi: 10.1210/en.2002-0188. [DOI] [PubMed] [Google Scholar]
- 81.Weesner GD, Malven PV. Intracerebral immunoneutralization of beta-endorphin and met-enkephalin disinhibits release of pituitary luteinizing hormone in sheep. Neuroendocrinology. 1990;52:382–388. doi: 10.1159/000125609. [DOI] [PubMed] [Google Scholar]
- 82.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effect of retrochiasmatic area (RCh) or preoptic area (POA) senktide microimplantation on the total number of kisspeptin cells.


