Abstract
Purpose
To determine the efficacy of imatinib in children with newly diagnosed chronic phase (CP) chronic myelogenous leukemia (CML).
Methods
This was an open label, multi-center phase II clinical trial. Courses were defined as consecutive 28-day intervals. Oral imatinib was administered daily at 340 mg/m2 without interruption in the absence of toxicity.
Results
Fifty-one children received 978 28-day courses of imatinib. The most common toxicities encountered were hematologic. Forty-one patients (80%) achieved a complete hematologic response by the end of course 2. Nineteen children (38%) obtained a complete cytogenetic response (CCyR) at the end of course 3. Overall, 72% achieved CCyR at a median time of 5.6 months. The rate of complete molecular response (>3 log reduction) was 27%. Progression-free and overall survival at 3 years were 72% ± 6.4% and 92% ± 3.9%, respectively.
Conclusions
Daily oral imatinib at a dose of 340 mg/m2 is well tolerated in children. In addition, imatinib therapy is effective in inducing a high percent of hematologic, cytogenetic and molecular responses, comparable to adults with CML.
Keywords: imatinib therapy, pediatric chronic myelogenous leukemia
INTRODUCTION
Childhood chronic myelogenous leukemia (CML) is rare, representing only 2% of leukemias [1]. The molecular features, clinical presentation, and response of childhood CML to standard therapies are comparable to adult CML. Similar to adult CML, the only proven curative therapy for childhood Philadelphia chromosome (Ph+) CML remains hematopoietic stem-cell transplantation (SCT) [2–5]. However, donors are not available for all patients. Additionally, high rates of transplant-related mortality (TRM) and post-transplant recurrence lead to 3-year event-free survival rates of about 40–70% [2,5].
CML is characterized by translocation of the ABL1 gene on chromosome 9 to the BCR gene on chromosome 22, resulting in a constitutively active ABL1 tyrosine kinase (TK). Imatinib is a potent, selective inhibitor designed to inhibit the TK activity of the BCR-ABL1 fusion protein associated with the Philadelphia chromosome translocation [6]. Imatinib has shown excellent activity and a good safety profile in numerous adult studies, and is established as a standard therapy for newly diagnosed adults with CML [7].
In adults with CP CML, the initial recommended dose of imatinib is 400 mg daily (~240 mg/m2). A 600 mg daily dose (roughly equivalent to 340 mg/m2) has demonstrated higher response rates in adults with AP or BC CML. Higher dose imatinib also produced better responses in some patients refractory to the standard 400 mg dose [8,9]. While studies using imatinib 400 mg twice a day reported a more rapid reduction in tumor burden than imatinib 400 mg/day at 18 months, longer follow-up is required to evaluate the benefit and possible long-term toxicities of such an approach [10].
The Children’s Oncology Group (COG) phase I study demonstrated a safety profile in children similar to adults. Imatinib showed anti-leukemic activity at all doses studied, that is, 260, 340, 440, and 570 mg/m2 [11]. One patient at the 440 mg/m2 dose level had dose-limiting weight gain. There were no other first course dose-limiting toxicities. A maximum tolerated dosage was not defined, while there was evidence of efficacy seen at all doses studied, that is, 260, 340, 440 and 570 mg/m2. Complete hematologic responses (CHR) occurred in all 14 evaluable patients with CP CML, while ten of 12 (83%) evaluable children achieved complete cytogenetic responses (CCyR). To determine the response rate to imatinib in newly diagnosed pediatric CML patients and to better delineate its toxicities, we conducted this phase II evaluation. The dose chosen, 340 mg/m2, achieves steady state plasma concentrations comparable to the 600 mg daily adult dose [11], which has produced favorable results in adults with accelerated phase (AP) or blast crisis (BC) CML [12–14].
METHODS
Trial Design
This open label, multi-center phase II study (AAML0123) incorporated a multiple-stage design, with the primary goal of estimating the response rate to imatinib administered orally once daily continuously to children with CP CML. The study was registered at ClinicalTrials.gov under identifier NCT00030394 and additional information of the study can be obtained from this link: http://clinicaltrials.gov/ct2/show/record/NCT00030394?term=aaml0123&rank=1. Initial design included patients with newly diagnosed CML; intolerant or relapsing after treatment with interferon; and those experiencing relapse following initial SCT. Patients continued therapy with imatinib in the absence of progressive disease. The latter two strata were closed due to poor enrollment and initial results of the phase III International Randomized Study of Interferon and STI571 (IRIS) trial favoring the use of imatinib [15]. The three patients enrolled in these two strata are not included in this report.
The null hypothesis was that the major cytogenetic response (MCyR = CCyR or partial cytogenetic responses (PCyR)) rate at the end of course 3 was ≤35% versus the alternative hypothesis that the MCyR rate was ≥55%. The following two-stage procedure was employed for monitoring treatment efficacy. Step 1: Seventeen patients were enrolled. If ≤3 MCyR at the “end-of-course-3” were observed, then the study would have been closed. Otherwise, step 2 followed. Step 2: An additional 17 patients were enrolled. If ≥16 MCyR at the “end-of-course-3” were observed among 34 total patients, further study of this treatment in this patient population was justified.
Inclusion and Exclusion Criteria
Patients younger than 22 years at enrollment with newly diagnosed CP CML were eligible. Prior hydroxyurea use was allowed. Other eligibility criteria included: (a) Lansky or Karnofsky score ≥50%, (b) adequate hepatic function (bilirubin ≤1.5 times normal, ALT <3 times normal, and albumin >2 g/dl), and (c) adequate renal function (creatinine ≤1.5 times normal for age or GFR >70 ml/min/1.73 m2). Patients were ineligible if they were pregnant or breastfeeding, had an uncontrolled infection, or were receiving another investigational agent. Neither concomitant anticonvulsant medication nor coumadin was allowed. Avoidance of medications known to interfere with the cytochrome P450 isoenzymes CYP 3A4 and 2D6 was recommended.
Institutional Review Boards for each participating institution approved the protocol before enrollment. Patients or their legal guardians gave written informed consent in accordance with the Declaration of Helsinki.
Drug Administration
Imatinib mesylate was supplied as 100 mg capsules (Novartis Pharmaceuticals Inc., East Hanover, NJ). Individual patient dosages were rounded to the nearest 100-mg increment. A course of imatinib therapy was defined as a consecutive 28-day interval. Imatinib was administered once daily with food.
Imatinib was withheld if a grade 2 nonhematologic adverse event (AE) failed to resolve with supportive therapy or if there was a grade 3 or 4 nonhematologic AE. After resolution of AE to a grade ≤1, imatinib was resumed with a 30% dose reduction. If Grade 2 toxicity recurred, further dose reductions could be similarly performed.
If initial toxicity did not recur after two courses with 30% dose reduction, dosage was increased to the initial dosage in two increments over two courses.
No dose modifications were allowed for hematological toxicity during the first course of therapy. In subsequent courses, imatinib was held for grade 4 hematologic toxicity (ANC < 500/μl or platelets <25,000/μl) until ANC was >1,000/μl or platelets >50,000/μl. If Grade 4 hematological toxicity did not resolve within 3 days (7 days for platelets) of withholding imatinib, a bone marrow biopsy was performed. Imatinib was resumed at the same dose if cellularity was ≥10%. Dosage was reduced 30% if grade 4 toxicity recurred or if it was associated with marrow cellularity of <10%.
If initial toxicity did not recur after two courses with 30% dose reduction, dosage was increased to the initial dosage in two increments over two courses. Antibiotics, blood products, and general supportive care were used as indicated clinically.
Dose intensity during the first year of therapy was calculated for each patient as the average daily mg/m2 dose as follows: the average daily dose was calculated as the sum of all doses divided by the total number of treatment days. This was then converted to an average mg/m2 dose by dividing by the patient’s meter-squared at the time of study enrolment.
Safety Analysis
All eligible patients who received at least one dose of study medication underwent safety analysis. AE were graded according to Common Toxicity Criteria Version 2.0 (http://ctep.info.nih.gov). Safety assessments consisted of monitoring and recording all AE and serious AEs (severity, duration, outcome, and presumed relationship to study drug), and regular assessments of laboratory parameters, vital signs, physical condition, and body weight.
Efficacy Evaluation
Patients who received at least one dose of imatinib and had at least one efficacy evaluation after baseline were assessed for response. Complete blood counts (CBC) with differential and platelets were measured at study entry, at weekly intervals during courses 1 and 2, and then at 2-week intervals for the duration of treatment. Marrow cytogenetic response was measured at end-of-course 3, 6, 9, and during the second year of therapy. If CCyR was achieved, reverse transcriptase polymerase chain reaction (RT-PCR) analysis for BCR-ABL1 was performed to assess molecular response.
Criteria for response
Hematologic response
Hematologic response (HR) was evaluated for courses 1 and 2. HR was defined as a >50% reduction in baseline WBC maintained for 2 weeks. CHR was defined as a reduction in WBC to <10,000/μl and platelets <450,000/μl maintained for 4 weeks, without progressive disease features (see below) [16].
Cytogenetic and molecular responses
Karyotypes were centrally reviewed. Cytogenetic response (CyR) was expressed as the number of metaphase cells containing the Ph+ chromosome divided by number of metaphase cells and categorized as follows: (1) CCyR, 0% Ph+ cells, with resolution of marrow and blood morphologic abnormalities; (2) PCyR, >0% to ≤35% Ph+ cells and above 35% at baseline; (3) minor CyR, >35% to ≤65% Ph+ cells and above 65% at baseline; (4) minimal CyR, >65% to ≤95% Ph+ cells and above 65% at baseline; and (5) no CyR, >95% Ph+ cells. Ideally, at least 20 metaphase cells were examined. Samples yielding <5 cells in metaphase were considered as quantity not sufficient (QNS).
In patients achieving CCyR, molecular response was measured in a centralized laboratory by quantitative RT-PCR for the major breakpoint (p210) using the Ipsogen FusionQuant kit (Ipsogen, Marseille, France) as previously described [17,18]. Molecular response was expressed as the ratio of BCR-ABL1 over ABL1. All measurements were performed in duplicate and reported as the mean. A major molecular response (MMR) has been defined as a BCR-ABL1/ABL1 ≤0.05% to 0.1% [19]. We chose a cut-off value of ≤0.05% for these analyses. All negative PCR values were repeated and verified using the Ipsogen FusionQuant assay.
Progressive disease
Progressive disease was defined as an increase of ≥30% in Ph+ marrow cells (by standard cytogenetics) or progression to AP or BC CML, defined as leukocyte doubling time <5 days; chloroma; medullary fibrosis; peripheral blood or bone marrow blasts >10%; peripheral blood or bone marrow blasts plus promyelocytes >20%; peripheral blood basophils and eosinophils >20%; progressing splenomegaly despite treatment; increasing leukocyte count despite adequate drug treatment; or blast transformation.
Statistical Considerations
Overall survival (OS) was defined as time from study enrollment to death. Patients who were alive at last contact were censored at that time. Progression-free survival (PFS) evaluated the time from study enrollment to the first occurrence of progression, relapse after response, second malignancy, or death as a first event from any cause. Patients who had no event at last contact were censored at that time. Distribution functions of OS and PFS were estimated by Kaplan and Meier method [20].
RESULTS
Patient Population
Fifty-five patients were enrolled at 37 participating COG centers in the US, Canada and Australia between December 2002 and July 2004 (participating institutions listed in Supplemental Appendix). Three patients, previously treated with interferon or who had recurrent disease following SCT at the time of enrolment, were excluded from data analysis since the study focused on newly diagnosed CP CML patients. One patient was ineligible for study entry (accelerated, not chronic phase). Another withdrew from study during the first week of treatment and had no response or safety data submitted. Therefore, this patient was not evaluated for analyses of response or toxicity, but is included in the analyses of PFS and OS. On January 23, 2006, the study was amended to limit protocol therapy to 13 courses of imatinib. All data were analyzed with a cut-off date of June 2, 2008. The median follow-up for patients alive was 3.8 years. The median age at study entry was 11.8 years (range 2.3–19.1 years). There were 29 females (57%). Table I summarizes patient characteristics at study entry.
TABLE I.
Study Entry Characteristics for 51 Eligible Patients
| N | % | |
|---|---|---|
| Gender | ||
| Male | 22 | 43% |
| Female | 29 | 57% |
| Age (yrs) | ||
| Median (range) | 11.8 | (2.3–19.1) |
| 0–2 | 3 | 6% |
| 3–10 | 19 | 37% |
| 11–21 | 29 | 57% |
| Race | ||
| American Indian or Alaska Native White | 1 | 2% |
| Asian | 4 | 9% |
| Native Hawaiian or other Pacific Islander | 1 | 2% |
| Black or African American | 6 | 13% |
| White | 34 | 74% |
| Unknown | 5 | |
| Ethnicity | ||
| Hispanic or Latino | 6 | 13% |
| Not Hispanic or Latino | 41 | 87% |
| Unknown | 4 | |
| WBC (×103/μl)—median (range) | 95.4 | (3.3–618) |
| BM Blasts %—median (range) | 1 | (0–8) |
| Platelets (×103/μl)—median (range) | 453 | (65–3,625) |
WBC, white blood cells; BM, bone marrow; yrs, years; N, number; %, percent.
Response to Imatinib
Hematologic response
Hematologic response was evaluated at the end of course 1 and 2 (48 and 49 evaluable patients, respectively). At the end of course 1, HR and CHR rates were 81% (39/48) and 19% (9/48), respectively. HR and CHR rates at the end of course 2 were 100% (49/49) and 80% (39/49), respectively.
Cytogenetic response
The primary endpoint for this study was a major cytogenetic response (CCyR or PCyR) at end-of-course-3. Among 17 initial patients evaluated in this 2-stage study design, >4 patients had a MCyR. Thus, accrual continued to 51 total eligible patients. The end-of-course-3 CyR could not be determined for 16 of 51 patients due to too few metaphase cells (QNS). Three patients lacked cytogenetic evaluation at end-of-course-3. Of 32 evaluable patients for CyR at end-of-course-3, 12 achieved CCyR, 10 achieved PCyR, 8 achieved minor/minimal responses, and 2 had no response (Table II). Twelve of 51 patients achieved a CCyR at end-of-course-3. The development of CyR of the remaining 38 patients is described in Figure 1.
TABLE II.
Cytogenetic Response (N = 51)
| At the end of course 3 (%) (N evaluable patients = 32) | Best response over all courses (N evaluable patients = 46)
|
|||
|---|---|---|---|---|
| N responses (%) | Coursea | Responses observed at the end of the course | ||
| CCyR | 12 (38%) | 33 (72%) | 3 | 12 |
| 5 | 2 | |||
| 6 | 11 | |||
| 8 | 1 | |||
| 9 | 4 | |||
| 10 | 2 | |||
| 19 | 1 | |||
| PCyR | 10 (31%) | 7 (15%) | 3 | 3 |
| 4 | 1 | |||
| 6 | 1 | |||
| 9 | 1 | |||
| 10 | 1 | |||
| Minor/minimal response | 8 (25%) | 4 (9%) | 3 | 4 |
| No response | 2 (6%) | 2 (4%) | ||
| Not evaluatedb | 3 | 3 | ||
| QNS | 16 | 2 | ||
CCyR, complete cytogenetic response; PCyR, partial cytogenetic response; N, number; %, percent; QNS, Cell quantity was not sufficient, with less than five metaphases available for analysis.
According to the protocol, cytogenetic response was measured at the end of courses 3, 6, 9/first week of courses 4 and 7, prior to course 10. However, some variation occurred;
Specimens were not submitted for 2 patients; 1 patient withdrew during first course of therapy.
Fig. 1.
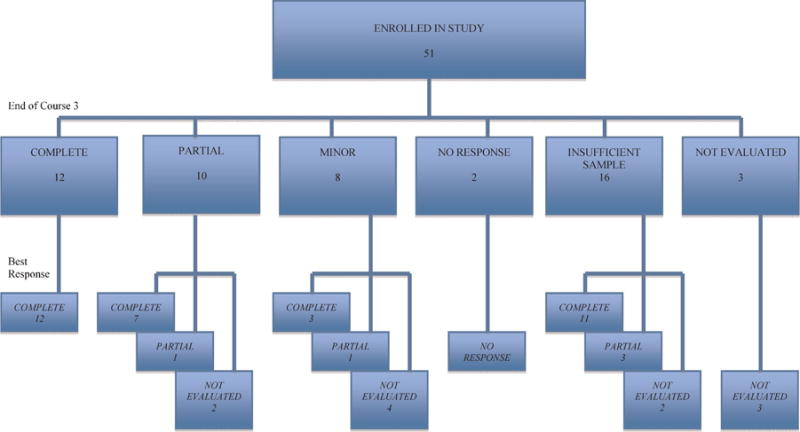
Primary endpoint for this study was a major cytogenetic response at end-of-course-3. The end-of-course-3 cytogenetic response (CyR) could not be determined for 19 patients. Of 32 evaluable patients for CyR at end-of-course-3, 12 achieved complete CyR, 10 achieved partial CyR, 8 achieved minor/minimal responses, and 2 had no response. The development of CyR following end-of-course 3 is schematized in this figure.
Best cytogenetic response
The best CyR is presented in Table II. For two patients, the CyR could not be determined because of QNS. Three other patients lacked cytogenetic evaluation. Of 46 evaluable patients, 33 (72%) achieved CCyR, at a median time of 5.6 months (all but three documented by 9 months). The majority of these patients achieved CCyR between course 3 and 7. Of note, 40 patients (87%) developed MCyR. Only 2 patients were cytogenetic non-responders to imatinib.
Molecular response
Quantitative PCR for BCR-ABL1 was available for 22 patients. Of these 22 patients, 15 achieved CCyR, 4 had minor/minimal cytogenetic response, 2 were QNS, and 1 was not evaluated for cytogenetic response. In 6 patients who achieved CCyR, 5 had undetectable BCR-ABL1 and 1 had ≤0.05% BCR-ABL1 (MMR). The patient not evaluated for cytogenetic response also had undetectable BCR-ABL1. In all, 6 patients (27%) had complete molecular response.
Safety and Toxicity
A total of 978 courses of imatinib were administered (median 14, range 1–46). For all patients, there were no significant deviations in protocol-specified treatment or dose. Most dose modifications were made due to AEs. In general, imatinib was well tolerated. Grade 3 and 4 AEs (Table III) occurring with a frequency >10% were all hematologic: neutropenia, anemia, thrombocytopenia, and lymphopenia. Of 50 patients evaluable for toxicity, 8 experienced Grade 3 or 4 lymphopenia and/or anemia during course 1. Lymphopenia and anemia were rare during later courses. Grade 3 or 4 neutropenia was seen in 8/50 (16%), 8/50, 5/48 (10%) and 9/47 (19%) patients, during courses 2–5, respectively. Other grade 3 or 4 toxicities were rarely seen.
TABLE III.
Most Common Severe Adverse Events
| Number (%) of most common Grade 3 or 4 adverse events
|
||||||
|---|---|---|---|---|---|---|
| Overall (at least 1 course) | Course 1 | Course 2 | Course 3 | Course 4 | Course 5 | |
| Total evaluable patients | 50 | 50 | 50 | 50 | 48 | 47 |
| Neutropenia | 16 (32%) | 1 (2%) | 8 (16%) | 8 (16%) | 5 (10%) | 9 (19%) |
| Anemia | 7 (14%) | 4 (8%) | 0 (0%) | 1 (2%) | 2 (4%) | 0 (0%) |
| Thrombocytopenia | 8 (16%) | 3 (6%) | 3 (6%) | 5 (10%) | 2 (4%) | 1 (2%) |
| Lymphopenia | 5 (10%) | 4 (8%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Arthralgia/myalgia | 6 (12%) | 0 (0%) | 4 (8%) | 3 (6%) | 1 (2%) | 0 (0%) |
| Vomiting | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Increased AST | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Increased ALT | 2 (4%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diarrhea | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Myalgias | 3 (6%) | 0 (0%) | 1 (2%) | 2 (4%) | 1 (2%) | 0 (0%) |
| Headache | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abdominal pain/cramping | 3 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 1 (2%) |
| Hypokalemia | 3 (6%) | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperglycemia | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypophosphatemia | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Edema/weight gain | 2 (4%) | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) |
%, percent.
The most common grade 3 and 4 non-hematologic toxicities, were arthralgia/myalgia (12%), vomiting (2%), increased AST (2%) or ALT (4%) diarrhea (2%), myalgias (6%), headache (2%), and abdominal pain/cramping (6%). Hypokalemia, hyperglycemia, and hypophosphatemia were reported in 6%, 2% and 2% of patients, respectively. One patient, diagnosed with autoimmune hepatitis while on study, discontinued therapy after 9 courses. Of note, the incidence of edema/weight gain (4%) was low. There were no cardiac toxicities reported. Eighteen (35%) patients did not require any dose modifications of imatinib 340 mg/m2 throughout the time they were in the study. The mean and median daily dose intensities (average daily dose per m2, during the first year of therapy) for patients were 320 and 328 mg/m2, respectively. Twelve out of 50 patients (24%) received less than 80% of the protocol-specified mg/m2 dose (i.e., less than 272 mg/m2) while on treatment.
Transplant
Twenty-five children withdrew from protocol therapy to undergo SCT (of whom 5 relapsed after SCT). A total of 252 courses were administered to these 25 patients (median 9, range 4–24). Thirteen patients (52%) achieved CCyR before withdrawal from protocol therapy by investigator/family choice. One patient who withdrew during the first week of imatinib had SCT 4 months later. Five additional patients received SCT after relapsing. Among these 31 children who received SCT, 7 died of transplant related complications, and one additional of acute respiratory distress syndrome (ARDS).
Progression-Free Survival and Overall Survival
Of the 51 patients, 13 developed recurrent disease, six (18.1%) after achieving CCyR. For these patients, median number of courses received on protocol was 11 (range of 1–35 courses). Documentation of recurrence of Ph+ cells occurred at 2–24 courses following initial documentation of CCyR. One additional patient developed a second malignancy. Of these 14 patients with recurrent disease, 10 were treated by SCT. Five died, 3 of TRM. Of the other 37 patients, 21 were taken off protocol therapy for SCT. Four of those died of TRM. There were a total of 9 deaths, 7 due to TRM. Therefore, PFS at 3 years was estimated as 72 ± 6.4% and OS rate at 3 years was estimated as 92 ± 3.9%. Overall survival at 3 years for the 31 SCT patients is 87 ± 12% and 100 ± 0% for the 20 who were not transplanted (Fig. 2).
Fig. 2.
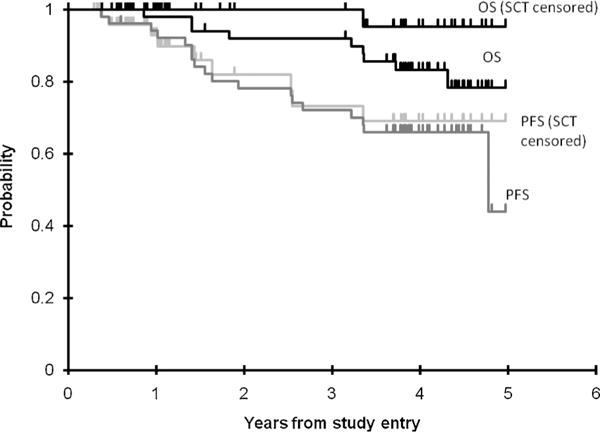
Overall survival and progression-free survival (N = 51).
DISCUSSION
In the COG phase 1 study, imatinib was safely administered daily at doses ranging from 260 to 570 mg/m2 and a maximum tolerated dose was not determined [11]. The dose of 340 mg/m2, providing systemic exposure similar to that of adults treated with 600 mg daily, was therefore selected for this phase 2 study. Randomized studies are in progress in adults comparing an initial dose of 400 mg daily versus a higher-dose; it remains uncertain at this point if a higher dose strategy is of clinical benefit.
In this study, we confirmed that a daily dose of imatinib of 340 mg/m2 was tolerable, with a mean daily dose intensity of 320 mg/m2. Only 24% (12 of 50) received <80% of the protocol-specified 340 mg/m2 dose while on treatment. The most common grade 3–4 AEs were hematologic. The non-hematologic grade 3–4 toxicities included allergic reaction, avascular necrosis and desquamating rash. In contrast with adult series, we observed a low incidence of fluid retention syndrome [21]. Overall cumulative incidence of arthralgia/myalgia was 14%, but did not generally require a significant decrease in dosing. In contrast to some reports [22], we observed a low incidence of hypophosphatemia. In the adult phase 2 study, the drug was well tolerated with 2.1% discontinuing therapy due to drug AE [21].
In the IRIS trial, OS and EFS were 89 and 83%, respectively, for patients treated initially with imatinib at a median follow up of 60 months [15]. In comparison, we estimated the 3-year OS as 92 ± 3.9% and PFS as 72 ± 6.4%. In the adult study, in contrast to this report, those patients who did not achieve a CHR within 3 months, or less than a minor response at 12 months, were allowed to increase imatinib dosage, which translates into clinical benefit [23].
We observed a significant proportion of children in whom the marrow culture yielded insufficient metaphase cells for standard cytogenetic analysis at the end-of-course 3. Hemodilution following imatinib therapy [24], and decreased bone marrow cellularity have previously been reported, without significant correlation to cytogenetic response [24–26].
BC CML, occasionally of sudden onset, is difficult to treat, and compromises other treatment options. The annual rate of progression to AP or BC was 1.5%, 2.8%, 1.6%, 0.9%, and 0.6% yearly, respectively, during the first 5 years of the IRIS study. In our cohort of 50 evaluable and eligible children, one child (2%) with initial PCyR progressed to BC after 5 imatinib courses. We did not observe progression to advanced disease in other children under study.
In children with advanced CP CML (interferon resistant or intolerant, or relapsing following SCT), imatinib induced CCyR in 12 of 20 patients, with a reduction of the BCR-ABL1/ABL1 ratio to <10−4 in 11 (50%) [27]. We previously reported that 10/12 children with advanced CML achieved CCyR [11]. Current milestones for monitoring adult patients with CP CML are rate of CHR at 3 months, MCyR at 6–12 months, CCyR at 12–18 months, and MMR at 18–24 months [19]. We similarly observed 80% CHR by 2 months, 65% MCyR by 6 months and 72% CCyR by 12 months, in children with newly diagnosed CP CML. In 27% of patients (6/22), BCR-ABL1 was undetectable by quantitative PCR (Q-PCR).
Currently, there is no evidence that imatinib therapy is curative. Discontinuation of imatinib is associated with disease relapse in most patients, including those in molecular remission, but sustained PCR negativity has been obtained in 6 of 12 patients after a median follow up of 18 months following discontinuation of imatinib [28]. Therefore, imatinib therapy in children likely implies long-term treatment. Compliance is often an issue in teenagers. In preclinical models, imatinib was shown to be teratogenic in rats (but not in rabbits), and impaired spermatogenesis has been reported [29]. Concerns about growth have also been raised [30]. However, given the significant TRM rate associated with SCT, there is no consensus on the optimal management of newly diagnosed children with CP CML[31,32].
In the IRIS trial, 57% of the imatinib CCy responders (39% of all patients) had more than a 3 log reduction by Q-PCR. At 24 months, all patients achieving CCyR with more than a 3 log reduction were alive and free of progression, while only 85% of patients not achieving CCyR were progression free. Therefore, long-term therapy in patients with documented significant response is a valuable therapeutic option [33,34].
In conclusion, our study demonstrates that a significant proportion of children with newly diagnosed CP CML treated with imatinib at a dosage of 340 mg/m2 will show favorable response with a low incidence of transformation into BC phase. Imatinib therapy, therefore, provides a safe bridging option to transplant, at no increased risk [35]. For those who are not candidates for SCT, imatinib represents a safe and effective therapy providing sustained response. Since a significant portion of patients may lose their response, continued monitoring should be performed, as alternative donor transplant and new tyrosine kinase inhibitors may be offered to those patients with clinical resistance.
Supplementary Material
Acknowledgments
This research is supported by the Chair’s Grant U10 CA98543-08 (PI Greg Reaman) and the Statistics and Data Center Grant U10 CA98413-08 (PI James Anderson) of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncoogygroup.org/admin/grantinfo.htm.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to declare.
Presented in part at American Society of Hematology, Orlando, 2006.
References
- 1.Smith MA, Gloeckler Ries LA, Gurney JG, et al. In: Leukemia in Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995, National Cancer Institute, SEER program. Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. 99–4649. 1999. pp. 17–34. [Google Scholar]
- 2.Cwynarski K, Roberts IA, Iacobelli S, et al. Stem cell transplantation for chronic myeloid leukemia in children. Blood. 2003;102:1224–1231. doi: 10.1182/blood-2002-12-3637. [DOI] [PubMed] [Google Scholar]
- 3.Davies SM, DeFor TE, McGlave PB, et al. Equivalent outcomes in patients with chronic myelogenous leukemia after early transplantation of phenotypically matched bone marrow from related or unrelated donors. Am J Med. 2001;110:339–346. doi: 10.1016/s0002-9343(01)00629-5. [DOI] [PubMed] [Google Scholar]
- 4.Dini G, Rondelli R, Miano M, et al. Unrelated-donor bone marrow transplantation for Philadelphia chromosome-positive chronic myelogenous leukemia in children: Experience of eight European Countries. The EBMT Paediatric Diseases Working Party. Bone Marrow Transplant. 1996;18:80–85. [PubMed] [Google Scholar]
- 5.Gamis AS, Haake R, McGlave P, et al. Unrelated-donor bone marrow transplantation for Philadelphia chromosome-positive chronic myelogenous leukemia in children. J Clin Oncol. 1993;11:834–838. doi: 10.1200/JCO.1993.11.5.834. [DOI] [PubMed] [Google Scholar]
- 6.Savage DG, Antman KH. Imatinib mesylate. A new oral targeted therapy. N Engl J Med. 2002;346:683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 7.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 8.Cortes J, Giles F, O’Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003;102:83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy of newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 10.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: High rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27:4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champagne MA, Capdeville R, Krailo M, et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: Results from a Children’s Oncology Group phase 1 study. Blood. 2004;104:2655–2660. doi: 10.1182/blood-2003-09-3032. [DOI] [PubMed] [Google Scholar]
- 12.Peng B, Hayes M, Resta D, et al. Clinical investigation of the pharmacokinetics and pharmacodynamics of imatinib in a phase 1 trial in chronic myeloid leukaemia patients. J Clin Oncol. 2004;22:935–942. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: Results of a phase 2 study. Blood. 2002;99:1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian HM, Cortes J, O’Brien S, et al. Imatinib mesylate (STI571) therapy for Philadelphia chromosome-positive chronic myelogenous leukemia in blast phase. Blood. 2002;99:3547–3553. doi: 10.1182/blood.v99.10.3547. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 16.Baccarini M, Dreyling M, on behalf of the ESMO guidelines working group Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010:v165–v167. doi: 10.1093/annonc/mdq201. [DOI] [PubMed] [Google Scholar]
- 17.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—A Europe Against Cancer program. Leukemia. 2003;17:2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 18.Saldanha J, Silvy M, Beaufils N, et al. Characterization of a reference material for BCR-ABL (M-BCR) mRNA quantitation by real-time amplification assays: Towards new standards for gene expression measurements. Leukemia. 2007;21:1481–1487. doi: 10.1038/sj.leu.2404716. [DOI] [PubMed] [Google Scholar]
- 19.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:475–481. [Google Scholar]
- 21.Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637–1647. doi: 10.1200/JCO.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 22.Berman E, Nicolaides M, Maki RG, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354:2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian HM, Larson RA, Guilhot F, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115:551–560. doi: 10.1002/cncr.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi S, Sunita P, Deshmukh C, et al. Bone marrow morphological changes in patients of chronic myeloid leukemia treated with imatinib mesylate. Indian J Cancer. 2008;45:45–49. doi: 10.4103/0019-509x.41769. [DOI] [PubMed] [Google Scholar]
- 25.Hasserjian RP, Boecklin F, Parker S, et al. STI571 reduces bone marrow cellularity and normalizes morphologic features irrespective of cytogenetic response. Am J Clin Pathol. 2002;117:360–367. doi: 10.1309/NR81-VCU0-CKW1-4HT9. [DOI] [PubMed] [Google Scholar]
- 26.Frater JL, Tallman MS, Variakojis D, et al. Chronic myeloid leukemia following therapy with imatinib mesylate. Am J Clin Pathol. 2003;113:833–841. doi: 10.1309/A4RG-P4LF-12GG-H8MW. [DOI] [PubMed] [Google Scholar]
- 27.Millot F, Guilhot J, Nelken B, et al. Imatinib mesylate is effective in children with chronic myelogenous leukemia in late chronic and advanced phase and in relapse after stem cell transplantation. Leukemia. 2006;20:187–192. doi: 10.1038/sj.leu.2404051. [DOI] [PubMed] [Google Scholar]
- 28.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 29.Hensley ML, Ford JM. Imatinib treatments: Specific issues related to safety, fertility and pregnancy. Semin Hematol. 2003;40:21–25. doi: 10.1053/shem.2003.50038. [DOI] [PubMed] [Google Scholar]
- 30.Li B, Boast S, de los Santos K, et al. Mice deficient in ABL are osteoporotic and have defects in osteoblast maturation. Nat Genet. 2000;24:304–308. doi: 10.1038/73542. [DOI] [PubMed] [Google Scholar]
- 31.Thornley I, Perentesis JP, Davies SM, et al. Treating children with chronic myeloid leukemia in the imatinib era: A therapeutic dilemma (editorial)? Med Pediatr Oncol. 2003;41:115–117. doi: 10.1002/mpo.10306. [DOI] [PubMed] [Google Scholar]
- 32.Pulsipher M. Treatment of CML in pediatric patients: Should imatinib mesylate (STI-571, Gleevec) or allogeneic hematopoietic cell transplant be front-line therapy? Pediatr Blood Cancer. 2004;43:523–533. doi: 10.1002/pbc.20062. [DOI] [PubMed] [Google Scholar]
- 33.Druker BJ. Circumventing resistance to kinase-inhibitor therapy. N Engl J Med. 2006;354:2594–2596. doi: 10.1056/NEJMe068073. [DOI] [PubMed] [Google Scholar]
- 34.Simonsson B. Beneficial effects of cytogenetic and molecular response on long-term outcome in patients with newly diagnosed chronic myeloid leukemia in chronic phase treated with imatinib: Update from the IRIS study. Blood. 2006;106:52a. abstract. [Google Scholar]
- 35.Oehler VG, Gooley T, Snyder DS, et al. The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood. 2007;109:1782–1789. doi: 10.1182/blood-2006-06-031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


