Abstract
Objectives
To assess the criterion validity and responsiveness of Patient Reported Outcomes Measurement Information System (PROMIS) in a web-based cohort of children with Crohn’s disease.
Study design
We recruited children, ages 9 to 17, with Crohn’s disease and their parents from the web-based Crohn’s and Colitis Foundation of America Kids & Teens Study cohort. Upon entry into the cohort and 6 months later, children self-reported Crohn’s disease activity, health-related quality of life and PROMIS domains of pain interference, anxiety, depression, fatigue and peer relationships.
Results
Mean PROMIS scores for the 276 participating patients were worse among those with worse self-reported Crohn’s disease activity (per Short Crohn’s disease Activity Index, p<0.005 for all), Crohn’s disease activity in the prior 6 months (per Manitoba Index, p<0.01 for all) and health related quality of life (per IMPACT-35, p<0.001 for all). One hundred forty-three patients and their parents completed follow-up questionnaires, 75% of whom reported stable disease activity. Those with improved Crohn’s disease activity reported improved PROMIS scores, and those with worsened Crohn’s disease activity reported worse PROMIS scores for all domains except anxiety. All participants reported improved anxiety from baseline, but those with stable or worsened Crohn’s disease activity reported less improvement (p=0.07).
Conclusions
PROMIS scores were significantly associated with known groups of Crohn’s disease activity in a linear and clinically meaningful manner, and responded to change in Crohn’s disease activity over a 6-month period. This supports the criterion validity and responsiveness of pediatric PROMIS.
Keywords: patient reported outcomes, PROMIS, Crohn’s disease, anxiety, depression, fatigue, peer relationships, quality of life
Patient-reported outcomes (PROs) are measures of how patients feel and function, obtained directly from patients without interpretation, capturing outcomes of importance to patients. PROs both complement standard primary outcomes, such as survival or physiologic measures, and provide primary outcomes that are necessarily patient-focused, such as patient function or quality of life (QoL).(1)
The National Institutes of Health’s Patient Reported Outcomes Measurement Information System (PROMIS) is a set of non-disease specific instruments – one for adults and one for pediatric patients - assessing domains of physical, psychological, and social health, and QoL.(2) PROMIS instruments are unique in that they have been developed using modern measurement theory, including rigorous qualitative and quantitative methods.(3) They are not disease-specific, and are standardized to a reference population, allowing for comparison between different domains of health and across a wide range of chronic diseases.(2), (4) Pending additional clinical validation in children with chronic disease, PROMIS pediatric measures may serve as endpoints in clinical, observational, comparative effectiveness, and health services research.(5, 6) Researchers and clinicians will then be able to use these endpoints to identify previously unrecognized psychological, social, or functional health disorders, reveal correlations between these disorders and underlying chronic disease activity, and prompt appropriate interventions.(7), (8), (9, 10)
When the onset of chronic disease is in childhood or adolescence, it can affect physical, psychological, and social development, as well as school performance, resulting in impaired or delayed achievement as an adult in interpersonal relationships, education, and employment.(11), (12) PROMIS pediatric instruments, which have been developed to assess these domains of health in children, have been studied in several disease states, including cancer, obesity, asthma, and nephrotic syndrome, and discriminate well among known groups of disease activity and severity.(13), (10), (14) However, establishing the validity of PROMIS in children requires research in additional chronic diseases.
Children with inflammatory bowel disease (IBD) have been found to have lower quality of life and social function than their healthy peers, and experience higher rates of depression than children with other chronic diseases.(15), (16) (17), (18) (19) Crohn’s disease has no definitive cure and commonly relapses, leading to intermittent disruptions to life and wellbeing, affecting a child’s function, development, and QoL; thus, it is an important model of a relapsing pediatric chronic disease in which to further evaluate PROMIS.
In this study, we first aimed to evaluate the concurrent criterion validity of PROMIS -how PROMIS instrument scores relate to established measures of Crohn’s disease activity and health-related QoL- by studying a national, prospective cohort of children with Crohn’s disease. Second, we sought to explore the responsiveness of PROMIS instruments to change over time amongst cohort members who participated in 6-month follow-up.
Methods
We performed cross-sectional analyses to evaluate associations between PROMIS measures, disease activity indices, disease-specific health-related QoL measures, therapy types, and remission status. We also performed exploratory longitudinal analyses to evaluate the associations between change in disease activity indices and the same QoL measures. The study protocol was reviewed and approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
The Crohn’s and Colitis Foundation of America (CCFA) is a non-profit, volunteer organization that funds, publishes, and advocates for IBD research, and provides support for patients with IBD of all ages. In 2011, the CCFA sponsored the development and maintenance of CCFA Partners, a web-based cohort of over 13,000 adults with self-identified IBD (ie Crohn’s disease and ulcerative or indeterminate colitis [UC/IC])(20, 21). Through a web-based portal, adults in the cohort complete research surveys every six months, receive research updates, and access CCFA educational resources.
In 2013, we launched a parallel, web-based cohort of pediatric patients with IBD and their parents, the CCFA Partners Kids & Teens Study. The purpose of this cohort is to create a long-term community of children with IBD engaged in research focused on the relationships between patient-reported exposures, health behaviors, and outcomes.(22) We recruited patients with self-reported IBD and their parents via the CCFA by use of their website, email rosters, various social media outlets, promotional efforts and word-of-mouth at CCFA educational and fundraising events. Patients with at least one participating parent were eligible to enter the study. Patients and parents could access the cohort entry portal through the study website (www.ccfapartners.org) or through a promotional email link. Enrollment began in August of 2013 and remains ongoing. Parents completed the section of survey questions regarding the child’s diagnosis of Crohn’s disease, demographic information, and family history. Children 9 to 17 years of age completed all symptom-related questions themselves, including PROMIS instruments specifically designed for child respondents. Six months after entering the cohort, participating patients and parents received email reminders to complete follow-up questionnaires of the same disease-related questions and PROMIS instruments. For the present analyses, we included all patients age 9 years or older with self-reported Crohn’s disease who completed all initial surveys, including child-reported PROMIS instruments and parent-reported demographic information, by November 2013.
PROMIS
Participating patients completed 4 items (questions), from each of 5 selected PROMIS pediatric instruments: anxiety, depression, fatigue, pain interference (a measure of the consequences of pain on various aspects of life, including social, cognitive, emotional, physical, recreational activities, sleep, and enjoyment of life), and peer relationships (a measure of the quality of relationships with friends and other acquaintances).(2) We selected these instruments in collaboration with pediatric IBD specialists and experts in PROMIS methodology(3), because they measure domains of health and health-related QoL affected by Crohn’s disease in children. In an effort to minimize respondent burden and enhance long-term cohort retention, we chose to use four-item short forms rather than longer forms or computer adaptive testing. Although four-item short forms are less precise at the individual level, they can be effective utilized in studies of moderate to large populations such as ours. PROMIS instruments are calibrated using a T-score metric with the mean of the original calibration population equal to 50, and the standard deviation (SD) in the calibration population equal to 10.(2) Minimally Important Differences (MIDs) are the smallest differences in PRO scores able to detect a clinically meaningful change in the outcome that the PRO is designed to measure. Any differences in PRO scores smaller than the MID are likely due to measurement error, rather than a true change in the outcome. Studies in adults suggest MIDs for many PROMIS items to be in the range of 2–6.(23) Though MIDs are not yet well established in PROMIS pediatric measures, new research utilizing adolescent patients, parents, and physicians as judges of clinically important differences in scores, suggests MIDs of 2–3 for multiple pediatric PROMIS instruments.(24) Higher scores in any PROMIS domain indicate more of the domain being measured – therefore, higher scores for anxiety, depression, fatigue, and pain interference indicate poorer wellbeing, whereas higher scores for peer relationships indicate better relationships with peers and therefore better wellbeing.
Other Variables
We administered the IMPACT-35 questionnaire to assess health-related QOL and we measured disease activity the Short Crohn’s Disease Activity Index (SCDAI). Both of these indices have been validated and are in wide use in both research and clinical care. A SCDAI < 150 indicates clinical remission, and values above this threshold indicate active disease.(25) We did not utilize the Pediatric Crohn’s Disease Activity Index(26), (25), which requires physical examination and laboratory assessments, and is therefore not feasible for survey research. We also assessed IBD activity within the past 6-months via the validated Manitoba IBD Index, which is a single-item, patient-reported indicator of IBD activity within the last 6-months, with scores ranging from one (constantly active) to six (inactive).(27) Additionally, we collected information regarding patient demographics, past IBD-related surgery, and both past and current IBD medication use -oral 5-aminosalicylates, oral corticosteroids, immunomodulators (Methotrexate, Azathioprine and 6-Mercaptopurine), and biologic therapies (Infliximab, Adalimumab, Certolizumab Pegol, and Natalizumab). In an effort to minimize survey length and respondent burden, we did not question patients about conditions or treatments other than IBD, such as depression, anxiety, or other mental health diagnoses.
Statistical Analyses
We first performed cross-sectional analyses using descriptive statistics and bivariate comparisons to assess the relationships between PROMIS T-scores and patient demographics, indices of disease activity (SCDAI), symptom activity within the last six months (Manitoba IBD Index) and QoL (IMPACT-35), current medication use, and other disease characteristics, including remission status, sex, age, age at diagnosis, time since diagnosis, and current steroid use. As disease activity indices and QoL scores were not normally distributed, we categorized these values into quartiles. We compared mean PROMIS scores between patients in remission and active disease by Pearson’s chi-square test. We then compared mean PROMIS scores across quartiles of disease activity and QoL scores using one-way analysis of variance (ANOVA) and non-parametric tests of trend (NPTOT) for the ranks across ordered groups. We investigated possible interactions between potential confounders (sex, age, age at diagnosis, time since diagnosis, and current steroid use) and PROMIS scores using a likelihood-ratio method. We then used multinomial logistic regression to evaluate associations between PROMIS measures and disease activity. After fitting models that included all potential confounders, we used a change-in-effect method to remove any variable that did not confound the relationship between PROMIS scores and disease activity or health-related QoL.(28)
We next performed exploratory longitudinal analyses by grouping patients into categories of stable disease, worsening disease, or improving disease, based on a threshold change of 70 points between baseline and follow-up SCDAI scores.(29) We then compared change in mean PROMIS scores across these groups using ANOVA and a NPTOT for the ranks across ordered groups. All data were prepared using SAS version 9.3 and analyzed using Stata version 13.1.
Results
A total of 276 patients with self-reported Crohn’s disease in children 9 years of age or older joined the CCFA Partners Kids & Teens cohort through November 2013. The mean age of participating patients was 13 and the mean age at diagnosis was 10 years (Table I); 44% were female, and 83% were in remission as determined by SCDAI score (102 ± 71). The mean Manitoba IBD Index score was 4, corresponding to occasional disease activity in the 6 months prior to the survey. Accordingly, only 10% were being treated with oral corticosteroids at the time of the survey, 54% were being treated with a biologic, and 47% with immunomodulators (Methotrexate, Azathioprine or 6-Mercaptopurine). When asked about prior medication use, the majority of patients (87%) reported being treated with oral corticosteroids at some point since their diagnosis.
Table 1.
Characteristics of the Study Population
| n = 276 | ||
|---|---|---|
| Demographics | Age, mean (SD) | 13.2 (2.4) |
| Sex, % female | 43.8 | |
| Race White African-American Hispanic Asian Multiracials |
90.6 4.1 6.9 0.3 5.0 |
|
| Highest parental education 12th grade Some college College Graduate school |
2.8 13.2 37.6 46.4 |
|
| Disease Characteristics | % In remission | 82.9 |
| Age at diagnosis, mean (SD) | 9.8 (3.0) | |
| Therapy | Historical therapy, % 5-Aminosalicylates Immunomodulators Biologics Corticosteroids Surgery |
63.4 74.6 61.2 86.6 16.7 |
| Current therapy, % 5-Aminosalicylates Immunomodulators Biologics Corticosteroids |
27.9 47.1 54.0 9.8 |
|
| Disease-Specific Measures | Disease-Specific Activity Scores, mean (SD)SCDAIa | 102 (71) |
| Health Related Quality of Live Scores, mean (SD) IMPACT-35b | 131 (22) | |
| Disease Activity within the last 6 months, mean (SD) Manitobac | 4 (2) | |
| PROMIS measures | PROMIS T-scores, mean (SD) Anxiety Depression Fatigue Pain interference Peer relationships |
47 (11) 43 (8) 48 (12) 48 (11) 49 (9) |
3-item questionnaire of Crohn’s disease activity, score < 150 indicates remission
35-item, self-administered questionnaire of health-related QoL in children with IBD, scores range 35 (poor) to 175 (best)
1-item questionnaire of IBD activity within the prior 6 months. A score of 4 indicates “occasional” activity
PROMIS Results
Mean baseline PROMIS scores for this cohort are displayed in Table I. Compared with boys, girls reported clinically or statistically worse mean PROMIS scores in all domains but peer relationships. Older children (age 14–17), compared with younger children (age 9–13 years), reported clinically or statistically worse PROMIS scores in all domains except depression and pain interference (Table II). Those on current corticosteroid treatment reported clinically or statistically worse fatigue, pain interference, and peer relationships, but not anxiety or depression (Table II).
Table 2.
Relationship Between Patient Characteristics and PROMIS T-scores
| Patient Characteristics | PROMIS scores | |||||
|---|---|---|---|---|---|---|
| Anxiety (n= 276), mean (SD) |
Depression (n= 276), mean (SD) |
Fatigue (n= 276), mean (SD) |
Pain interference (n= 276), mean (SD) |
Peer relationships (n= 276), mean (SD) |
||
| Demographics | Age 9–13 years 14–17 years |
46 (11) 48 (11) |
43 (9) 44 (9) |
47 (12)* 50 (12)* |
48 (12) 48 (11) |
50 (9)* 48 (8)* |
| sex Male Female |
45 (10)¶ 49 (12)¶ |
42 (8) 45 (9) |
47 (11) 49 (13) |
47 (11)* 50 (11)* |
49 (9) 49 (9) |
|
| Highest parental education 12th grade Some college College Graduate school |
45 (10) 48 (11) 47 (11) 48 (12) |
44 (10) 46 (10) 43 (8) 44 (9) |
53 (11) 53 (13) 48 (11) 48 (12) |
54 (9) 52 (12) 50 (10) 48 (11) |
51 (9) 48 (9) 49 (9) 50 (9) |
|
| Disease Characteristics | Crohn’s disease status Remission Active |
45 (11)¶ 55 (11)¶ |
42 (8)¶ 50 (10)¶ |
45 (11)¶ 60 (11)¶ |
45 (10)¶ 59 (8)¶ |
50 (9)* 46 (9)* |
| Current therapy 5-Aminosalicylates Immunomodulators Biologics Corticosteroids |
46 (12) 48 (12) 46 (11) 48 (9) |
42 (8) 43 (9) 43 (8) 43 (7) |
45 (11)* 49 (12) 48 (12) 51 (14) |
47 (12) 48 (11) 48 (11) 53 (12)* |
50 (9) 49 (8) 49 (9) 47 (10) |
|
Statistically significant difference between group means as determined by one-way Analysis of Variance (ANOVA), p-value ≤ 0.05
Statistically significant difference between group means as determined by one-way ANOVA, p-value ≤ 0.001
Associations with Disease Severity
Mean PROMIS scores for all domains were worse, indicating worse health or more dysfunction, among patients with worse reported Crohn’s disease activity, as measured by the SCDAI (Table III). Notable, those who reported the best Crohn’s disease activity also reported mean PROMIS scores in all domains that were equivalent to or better than that of the reference population (Table III). For all PROMIS domains, there was both an overall statistically significant difference in mean PROMIS scores amongst the quartiles of disease activity (F <0.001 for all ANOVA), as well as a significant trend of higher PROMIS scores across rank-ordered SCDAI quartiles (z <0.001 for all non-parametric tests of trend (NPTOT)). Therefore, patients who reported worse Crohn’s disease activity correspondingly reported worse anxiety, depression, fatigue, pain interference, and peer relationships. For all domains except peer relationships, the differences in PROMIS scores between any two disease activity quartiles represented clinically meaningful differences, based on the proposed pediatric MID range of two to three. These same statistically significant and clinically meaningful differences in scores for all PROMIS domains were maintained when patients were grouped by remission status, as determined by reported SCDAI score (Table VI; available at www.jpeds.com).
Table 3.
Relationship between Crohn’s disease Activity and PROMIS T-scores
| Quartile SCDAIa | ANOVA | Trendb | ||||
|---|---|---|---|---|---|---|
| PROMIS | 1 | 2 | 3 | 4 | Prob>F | Prob>|z| |
| Anxiety | 42 | 45 | 49 | 54 | <0.001 | <0.001 |
| Depression | 39 | 41 | 44 | 49 | <0.001 | <0.001 |
| Fatigue | 42 | 45 | 49 | 58 | <0.001 | <0.001 |
| Pain interference | 39 | 46 | 50 | 58 | <0.001 | <0.001 |
| Peer relationshipsc | 51 | 49 | 49 | 46 | 0.005 | <0.001 |
Highest quartile indicates higher degree of disease activity
Non-parametric test of trend
Higher score indicates better peer relationships
Online Table 6.
Relationship between Remission status and PROMIS T-scores
| Remission Statusa | |||
|---|---|---|---|
| PROMIS | Remission (n=218) | Active (n=45) | p-value |
| Anxiety | 45 (11) | 55 (11) | <0.001 |
| Depression | 42 (8) | 50 (10) | <0.001 |
| Fatigue | 45 (10) | 60 (11) | <0.001 |
| Pain interference | 45 (8) | 59 (8) | <0.001 |
| Peer relationshipsb | 50 (8) | 46 (9) | 0.02 |
Remission status determined by SCDAI score <150
Higher score indicates better peer relationships
Analogous to the findings across SCDAI quartiles, PROMIS scores for all domains of health were worse among patients who reported more active disease in the prior 6 months, as measured by the Manitoba IBD Index (Table IV; available at www.jpeds.com). For all domains, there was both an overall statistically significant difference in mean PROMIS scores amongst the 6 categories (F ≤0.01 for all ANOVA), as well as a trend of higher PROMIS scores across rank-ordered Manitoba IBD Index categories (z ≤0.001 for all NPTOT). The differences in PROMIS scores between any two Manitoba IBD Index categories, for all domains except peer relationships, represented clinically meaningful differences (Table IV).
Online Table 4.
Relationship between Crohn’s disease disease activity within the last 6 months and PROMIS T-scores
| Manitoba IBD Index scorea | ANOVA | Trendb | ||||||
|---|---|---|---|---|---|---|---|---|
| PROMIS | 1 | 2 | 3 | 4 | 5 | 6 | Prob>F | Prob>|z| |
| Anxiety | 54 | 53 | 49 | 47 | 44 | 41 | <0.001 | <0.001 |
| Depression | 49 | 47 | 46 | 42 | 41 | 39 | 0.01 | <0.001 |
| Fatigue | 57 | 55 | 51 | 46 | 45 | 41 | <0.001 | <0.001 |
| Pain interference | 59 | 55 | 51 | 49 | 42 | 40 | <0.001 | <0.001 |
| Peer relationshipsc | 46 | 47 | 48 | 48 | 49 | 53 | 0.001 | <0.001 |
- 1 = constantly
- 2 = often
- 3 = sometimes
- 4 = occasional
- 5 = rarely
- 6 = inactive
Non-parametric test of trend
Higher score indicates better peer relationships
Despite potential differences in disease activity based on sex, age, age at diagnosis, time since diagnosis, and current steroid therapy (Table II), we did not find any independent effect of these patient characteristics on the relationship between PROMIS scores and disease activity using multinomial logistic regression and change-in-effect methods (data not shown).
Associations with Health Related QoL
Mean PROMIS scores for all domains were worse among patients reporting worse pediatric health related QoL, as measured by the IMPACT-35 (Table V; available at www.jpeds.com). For all domains, there was both an overall statistically significant difference in mean PROMIS scores between the quartiles (F <0.001 for all ANOVA), as well as a trend of higher PROMIS scores across rank-ordered quartiles of QoL (z <0.001 for NPTOT). As with the differences in PROMIS scores across SCDAI and Manitoba IBD Index groups, the differences in PROMIS scores between any two IMPACT-35 quartiles were not only statistically significant, but also represented clinically meaningful differences for all domains except peer relationships.
Online Table 5.
Relationship between Health-Related QoL and PROMIS T-scores
| Quartile IMPACT-35a | ANOVA | Trendb | ||||
|---|---|---|---|---|---|---|
| PROMIS | 1 | 2 | 3 | 4 | Prob>F | Prob>|z| |
| Anxiety | 58 | 49 | 42 | 39 | <0.001 | <0.001 |
| Depression | 52 | 44 | 39 | 37 | <0.001 | <0.001 |
| Fatigue | 60 | 51 | 45 | 38 | <0.001 | <0.001 |
| Pain interference | 59 | 51 | 43 | 40 | <0.001 | <0.001 |
| Peer relationshipsc | 46 | 46 | 50 | 54 | <0.001 | <0.001 |
Increasing quartile indicates better health-related QoL
Non-parametric test of trend
Higher score indicates better peer relationships
Longitudinal Evaluation of PROMIS Measures
Of the 143 patients who completed six-month follow-up questionnaires, 11% reported worsened, 76% stable, and 13% improved Crohn’s disease activity from baseline, based on change in SCDAI score. The patients who reported improved Crohn’s disease activity from baseline also reported meaningfully improved anxiety, depression, fatigue, pain interference, and peer relationships, with improvement in mean PROMIS scores ranging from 4–11 (Figure and Table VII; available at www.jpeds.com). Similarly, patients who reported worsened Crohn’s disease activity from baseline also reported meaningfully worse PROMIS scores in all domains (Figure and Table VII). Patients with stable Crohn’s disease activity reported little to no change in PROMIS scores, as demonstrated by a change in PROMIS scores of 0–1 compared with baseline (Figure and Table VII). For all domains, the changes in PROMIS scores across groups of patients by change in disease activity were also statistically significant and linear (Table VII).
Figure 1.
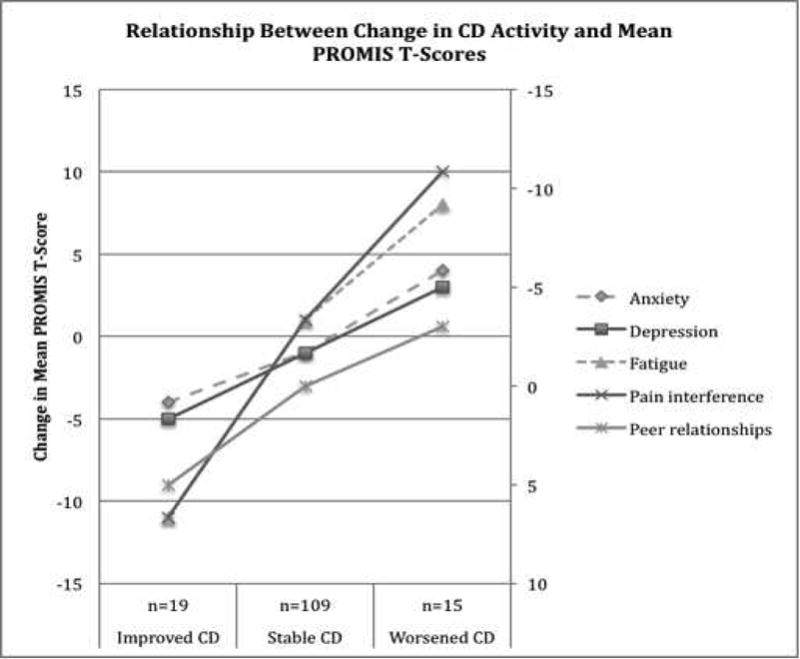
a. “Improved” patients reported a 70-point improvement in SCDAI score from baseline to time of 6-month follow-up, “Worsened” reported as 70-point worsening in SCDAI score from baseline
b. Higher T-score score indicates worse anxiety, depression, fatigue, pain interference, and better peer relationships
c. Secondary/Right-sided axis for peer relationships scores only
Online Table 7.
Relationship between change in Crohn’s disease activity and PROMIS T-scores
| Change in mean PROMIS score at 6-month follow-up (SD) | ANOVA | Trendb | |||
|---|---|---|---|---|---|
| Change in CD Activitya | Improved n=19 | Stable n=109 | Worsened n=15 | Prob>F | Prob>|z| |
| Anxiety | −4 (8) | −1 (9) | 4 (10) | 0.04 | 0.04 |
| Depression | −5 (8) | −1 (7) | 3 (10) | 0.01 | 0.01 |
| Fatigue | −11 (14) | 1 (8) | 8 (13) | <0.001 | <0.001 |
| Pain interference | −11 (13) | 1 (9) | 10 (12) | <0.001 | <0.001 |
| Peer relationshipsc | 5 (9) | 0 (8) | −3 (7) | 0.01 | 0.001 |
70-point change in SCDAI score from baseline to time of 6-month follow-up
Non-parametric test of trend
Higher score indicates better peer relationships
Discussion
In this study, we described a cross-sectional and longitudinal evaluation of PROMIS in a pediatric population with Crohn’s disease. We found that mean PROMIS scores were significantly associated with known groups of Crohn’s disease activity and health-related QoL, in a predictable, linear and clinically meaningful manner, thus supporting the concurrent criterion validity of PROMIS in our study population. We also observed that patients with well-controlled Crohn’s disease reported better PROMIS scores in the domains of psychological and physical functioning than the reference population, suggesting that with adequate disease control, children with Crohn’s disease lead normal, healthy lives. In our exploratory longitudinal analyses, we found that changes in mean PROMIS scores were also significantly associated with change reported Crohn’s disease activity over a 6-month period, in a predictable, linear and clinically meaningful manner, demonstrating the responsiveness of PROMIS in this cohort.
Although baseline scores for all PROMIS domains of health varied significantly and linearly across rank-ordered groups of Crohn’s disease severity and health-related QoL, associations with peer relationships were less dramatic than for other domains. This pattern is consistent with our clinical experience, as well as PROMIS pediatric studies in other chronic disease settings where peer relationships are less affected by disease severity than other domains.(14),(30),(7) Although incremental changes in Crohn’s disease activity and health-related QoL are associated with differences in patient-reported anxiety, depression, fatigue, and pain interference, peer relationships tend to remain intact except in those whose Crohn’s disease is very active or QoL very impaired.
Measuring QoL, physical function, psychological, and social health in a valid, reliable, and patient-centered way is centrally important to understanding chronic disease in children and improving their care. When adolescents with IBD and their physicians have been surveyed about what factors most affect their QoL, there was almost no correlation between adolescent and physician reports.(31) Given these disparate viewpoints, it is unlikely that physicians and researchers can capture what is important to patients regarding QoL and disease control without high-quality PROs. If appropriately captured, PROs of depression and other health domains have been shown to independently predict chronic disease activity, including in IBD.(32) In addition, PROs can also help identify barriers to remission and treatment adherence. In IBD, for example, the subset of patients who continue to have impaired health-related QoL despite documented mucosal healing are those with significantly more fatigue, anxiety, and depression.(33) In addition, those patients IBD with elevated anxiety and depressive symptoms have significantly lower treatment adherence.(34) Interventions for anxiety or depressive symptoms are known to be as effective in patients with chronic disease, including IBD, as in the general population, yet remain underused.(35),(36),(37)
We sought to validate PROMIS instruments in a pediatric population with Crohn’s disease by surveying patients in our CCFA Kids & Teens cohort - a large, geographically diverse study population, which allowed for longitudinal assessments of PROMIS instruments. The fact that our cohort is a volunteer, internet-based cohort, potentially limits its generalizability. In another internet-based cohort of patients with psoriasis, participants reported that their disease was less of a burden, despite being more extensive, and were significantly better-informed regarding psoriasis therapies compared with nonparticipants.(38) Therefore, we may suspect that our population is better informed and less likely to consider their disease a burden than the general population with Crohn’s disease. Another potential limitation of our study is that a patient’s Crohn’s disease status was identified by self-report, rather than review of medical records. However, in our validation study of the adult CCFA Partners cohort, self-reported IBD status was confirmed by medical records in 96% of participants, suggesting that patients who are involved in the CCFA are reliable reporters of their diagnoses. In order to minimize respondent burden and improve retention in the Kids & Teens Study cohort, we did not assess comorbid diagnoses of anxiety, depression, or other mental health disorders and did not collect data on non-IBD medication use. However, such data are not needed to assess criterion validity and explore longitudinal responsiveness of PROMIS. Another potential limitation of our study is a feature of PROMIS pediatric instruments themselves. Unlike PROMIS adult instruments, which are standardized to a general population, PROMIS pediatric T-scores are currently standardized to a population that includes a higher proportion of children with chronic disease.(39) Although this did not affect our conclusions, it did not allow us to compare our study cohort with the general population.
This study supports the criterion validity and responsiveness of child-reported PROMIS instruments in Crohn’s disease, adding to our understanding of how PROMIS functions in a pediatric population with a relapsing chronic disease. With this basis, important patient-centered research can proceed, including studies of how incorporating PROMIS in the clinical care of children with chronic diseases effects the recognition and treatment of psychological, social, and functional burdens of chronic disease, and ultimately, patient wellbeing.
Acknowledgments
Supported by the Crohn’s and Colitis Foundation of America, the National Institute for Diabetes and Digestive and Kidney Diseases (P30 DK034987 [to R.S.]), and the Health Resources and Service Administration National Research Service Award (T32 HP14001).
Abbreviations
- CCFA
Crohn’s and Colitis Foundation of America
- IC
Indeterminate Colitis
- PROs
Patient Reported Outcomes
- PROMIS
Patient Reported Information System
- SCDAI
Short Crohn’s Disease Activity Index
- UC
Ulcerative Colitis
- QoL
Quality of Life
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of the study were presented at Digestive Disease Week, <city, state, dates>; and the meeting of the Pediatric Academic Societies, <city, state, dates>.
References
- 1.Patrick DGG, Acquadro C. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 ed. Julian PT, Higgins SG, editors. The Cochrane Collaboration, John Wiley & Sons Ltd.; 2008. [Google Scholar]
- 2.Staff PN. PROMIS. Research: PROMIS Network. [Available from: http://www.nihpromis.org/researchers/InResearch.
- 3.Staff PN. PROMIS Instrument Development and Validation Scientific Standards Version 2.0: PROMIS Network. 2013 [updated May 2013. 72]. Available from: http://www.nihpromis.org/Documents/PROMISStandards_Vers2.0_Final.pdf.
- 4.Rothrock N. What is PROMIS? PROMIS: PROMIS Network. 2013 [Google Scholar]
- 5.Validation of Pediatric Patient Reported Outcomes in Chronic Disease (PEPR) Consortium (U19) National Institutes of Health; 2015. [Google Scholar]
- 6.Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1246–56 e6. doi: 10.1016/j.cgh.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatric blood & cancer. 2014;61:1282–8. doi: 10.1002/pbc.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christodoulou C, Schneider S, Junghaenel DU, Broderick JE, Stone AA. Measuring daily fatigue using a brief scale adapted from the Patient-Reported Outcomes Measurement Information System (PROMIS (R)) Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23:1245–53. doi: 10.1007/s11136-013-0553-z. [DOI] [PubMed] [Google Scholar]
- 9.Fischer HF, Klug C, Roeper K, Blozik E, Edelmann F, Eisele M, et al. Screening for mental disorders in heart failure patients using computer-adaptive tests. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23:1609–18. doi: 10.1007/s11136-013-0599-y. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Huang IC, Thompson L, Tuli S, Huang SW, DeWalt D, et al. The relationships between asthma control, daytime sleepiness, and quality of life among children with asthma: a path analysis. Sleep medicine. 2013;14:641–7. doi: 10.1016/j.sleep.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stam H, Hartman EE, Deurloo JA, Groothoff J, Grootenhuis MA. Young adult patients with a history of pediatric disease: impact on course of life and transition into adulthood. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2006;39:4–13. doi: 10.1016/j.jadohealth.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Hummel TZ, Tak E, Maurice-Stam H, Benninga MA, Kindermann A, Grootenhuis MA. Psychosocial developmental trajectory of adolescents with inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2013;57:219–24. doi: 10.1097/MPG.0b013e3182935474. [DOI] [PubMed] [Google Scholar]
- 13.Hinds PS, Nuss SL, Ruccione KS, Withycombe JS, Jacobs S, DeLuca H, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatric blood & cancer. 2013;60:402–8. doi: 10.1002/pbc.24233. [DOI] [PubMed] [Google Scholar]
- 14.Gipson DS, Selewski DT, Massengill SF, Wickman L, Messer KL, Herreshoff E, et al. Gaining the PROMIS perspective from children with nephrotic syndrome: a Midwest pediatric nephrology consortium study. Health and quality of life outcomes. 2013;11:30. doi: 10.1186/1477-7525-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackner LM, Greenley RN, Szigethy E, Herzer M, Deer K, Hommel KA. Psychosocial issues in pediatric inflammatory bowel disease: report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of pediatric gastroenterology and nutrition. 2013;56:449–58. doi: 10.1097/MPG.0b013e3182841263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackner LM, Crandall WV. Psychological factors affecting pediatric inflammatory bowel disease. Current opinion in pediatrics. 2007;19:548–52. doi: 10.1097/MOP.0b013e3282ef4426. [DOI] [PubMed] [Google Scholar]
- 17.Kugathasan S, Judd RH, Hoffmann RG, Heikenen J, Telega G, Khan F, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. The Journal of pediatrics. 2003;143:525–31. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 18.Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, et al. Inflammatory bowel disease: a Canadian burden of illness review. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2012;26:811–7. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. Journal of clinical gastroenterology. 2012;46:581–9. doi: 10.1097/MCG.0b013e318247c32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long MD, Kappelman MD, Martin CF, Lewis JD, Mayer L, Kinneer PM, et al. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflammatory bowel diseases. 2012;18:2099–106. doi: 10.1002/ibd.22895. [DOI] [PubMed] [Google Scholar]
- 21.Kappelman MD, Long MD, Martin C, DeWalt DA, Kinneer PM, Chen W, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1315–23 e2. doi: 10.1016/j.cgh.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partners C. About CCFA Partners. [Available from: https://ccfa.med.unc.edu.
- 23.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. Journal of clinical epidemiology. 2011;64:507–16. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thissen D, Liu Y, Magnus B, Quinn H, Gipson DS, Dampier C, et al. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2015 doi: 10.1007/s11136-015-1058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thia K, Faubion WA, Jr, Loftus EV, Jr, Persson T, Persson A, Sandborn WJ. Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflammatory bowel diseases. 2011;17:105–11. doi: 10.1002/ibd.21400. [DOI] [PubMed] [Google Scholar]
- 26.Otley A, Smith C, Nicholas D, Munk M, Avolio J, Sherman PM, et al. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2002;35:557–63. doi: 10.1097/00005176-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Clara I, Lix LM, Walker JR, Graff LA, Miller N, Rogala L, et al. The Manitoba IBD Index: evidence for a new and simple indicator of IBD activity. The American journal of gastroenterology. 2009;104:1754–63. doi: 10.1038/ajg.2009.197. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S. Modeling and variable selection in epidemiologic analysis. American journal of public health. 1989;79:340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thia KT, Sandborn WJ, Lewis JD, Loftus EV, Jr, Feagan BG, Steinhart AH, et al. Defining the optimal response criteria for the Crohn’s disease activity index for induction studies in patients with mildly to moderately active Crohn’s disease. The American journal of gastroenterology. 2008;103:3123–31. doi: 10.1111/j.1572-0241.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- 30.Selewski DT, Collier DN, MacHardy J, Gross HE, Pickens EM, Cooper AW, et al. Promising insights into the health related quality of life for children with severe obesity. Health and quality of life outcomes. 2013;11:29. doi: 10.1186/1477-7525-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervesi C, Battistutta S, Martelossi S, Ronfani L, Ventura A. Health priorities in adolescents with inflammatory bowel disease: physicians’ versus patients’ perspectives. Journal of pediatric gastroenterology and nutrition. 2013;57:39–42. doi: 10.1097/MPG.0b013e31828b5fd4. [DOI] [PubMed] [Google Scholar]
- 32.Morrison G, Van Langenberg DR, Gibson SJ, Gibson PR. Chronic pain in inflammatory bowel disease: characteristics and associations of a hospital-based cohort. Inflammatory bowel diseases. 2013;19:1210–7. doi: 10.1097/MIB.0b013e318280e729. [DOI] [PubMed] [Google Scholar]
- 33.Casellas F, Barreiro de Acosta M, Iglesias M, Robles V, Nos P, Aguas M, et al. Mucosal healing restores normal health and quality of life in patients with inflammatory bowel disease. European journal of gastroenterology & hepatology. 2012;24:762–9. doi: 10.1097/MEG.0b013e32835414b2. [DOI] [PubMed] [Google Scholar]
- 34.Gray WN, Denson LA, Baldassano RN, Hommel KA. Treatment adherence in adolescents with inflammatory bowel disease: the collective impact of barriers to adherence and anxiety/depressive symptoms. Journal of pediatric psychology. 2012;37:282–91. doi: 10.1093/jpepsy/jsr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reigada LC, Bruzzese JM, Benkov KJ, Levy J, Waxman AR, Petkova E, et al. Illness-specific anxiety: implications for functioning and utilization of medical services in adolescents with inflammatory bowel disease. Journal for specialists in pediatric nursing : JSPN. 2011;16:207–15. doi: 10.1111/j.1744-6155.2011.00292.x. [DOI] [PubMed] [Google Scholar]
- 36.Szigethy E, McLafferty L, Goyal A. Inflammatory bowel disease. Child and adolescent psychiatric clinics of North America. 2010;19:301–18, ix. doi: 10.1016/j.chc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Bennebroek Evertsz F, Thijssens NA, Stokkers PC, Grootenhuis MA, Bockting CL, Nieuwkerk PT, et al. Do Inflammatory Bowel Disease patients with anxiety and depressive symptoms receive the care they need? Journal of Crohn’s & colitis. 2012;6:68–76. doi: 10.1016/j.crohns.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Nijsten T, Rolstad T, Feldman SR, Stern RS. Members of the national psoriasis foundation: more extensive disease and better informed about treatment options. Archives of dermatology. 2005;141:19–26. doi: 10.1001/archderm.141.1.19. [DOI] [PubMed] [Google Scholar]
- 39.Irwin DE, Stucky BD, Thissen D, Dewitt EM, Lai JS, Yeatts K, et al. Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2010;19:585–94. doi: 10.1007/s11136-010-9618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


