The polymerization of fibrin occurs primarily via intermolecular non-covalent binding between knobs ‘A’ exposed by cleavage of fibrinopeptides A in the Aα chains of fibrinogen and holes ‘a’ constitutively present in the γ chains [1]. We have previously investigated the interactions between knobs ‘A’ and holes ‘a’ at the single-molecule level and have found that they are strong and stable [2] with complex forced unbinding mechanisms [3]. The structural complexity and thermodynamics of these interactions must be reflected in the non-trivial dynamics of the mechanical response of the knob-hole bond to stress. By applying an original model system to quantify the mechanical dissociation of individual knob-hole complexes, we revealed an unusual strengthening of A:a knob-hole bonds in response to increasing pulling force, referred to as a “catch” bond.
Our model system is based on an optical trap that generates pico-Newton mechanical force [4, 5]. Briefly, a single fibrinogen-coated latex bead is trapped and repeatedly brought into contact with a fibrin-coated pedestal. When fibrinogen (bearing holes ‘a’) on the bead binds to monomeric fibrin (bearing knobs ‘A’) on the pedestal, the trap exerts a force to dissociate the complex [5].
To form surface-attached monomeric fibrin, purified human fibrinogen was immobilized on stationary 5-μm-diameter pedestals [4] and treated with thrombin (1 U/ml, 30 min, 37°C) to convert it to monomeric fibrin. Thrombin was removed by washing with at least 20× volume of 20 mM HEPES, 150 mM NaCl, pH 7.4, containing 0.1% Triton X-100 buffer and/or blocked with D-Phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK). Fibrinogen was coupled to 2-μm beads [4] at a density of ∼2.0×104 molecules/μm2. The microscope chamber was equilibrated with a 20 mM HEPES buffer pH 7.4, containing 150 mM NaCl, 3 mM CaCl2, and 2 mg/ml BSA (to reduce non-specific interactions), then fibrinogen-coated beads were flowed into the chamber. A single fibrinogen-coated bead was trapped and brought close to a fibrin-coated pedestal. After starting the bead oscillation, the bead and the pedestal touched each other repeatedly with a compressive force of 20–30 pN and contact duration of 0.5s. The pulling force was varied from 5 pN to 60 pN, a range previously shown to reveal the “catch-slip” transition in bimolecular complexes [6–8]. Several tens of pedestal-bead pairs were analyzed for each experimental condition and the total number of bond lifetime values observed at each included 102–103 data points to get statistically representative data.
In our model system previously shown to represent the A:a knob-hole interactions [2], we investigated the dependence of the dissociation time (bond lifetime) of the fibrin-fibrinogen complex on tensile force. Specificity was tested by coating the interacting surfaces with an inert protein, such as bovine serum albumin (BSA), or a relevant protein lacking knobs ‘A’ (fibrinogen). The control interactions (fibrinogen/BSA, fibrin/BSA, fibrinogen/fibrinogen) as well as fibrinogen/fibrin interfaces resulted in prevailing short-lived attachment signals with bond lifetimes <40ms that were not included in the analysis. In fibrin/fibrinogen interactions the fraction of attachment events lasting >40ms comprised about 10% and varied up to tens of seconds. To segregate genuine A:a knob-hole binding from other protein-protein interactions, we measured fibrinogen/fibrin interactions in the absence and presence of the GPRPam peptide, a competitive inhibitor of these knob-hole interactions. Analysis revealed that GPRPam most effectively suppressed interactions lasting >0.5s, which were therefore considered reflecting the knob-hole binding. The bond lifetimes <0.5s reflected formation of multiple GPRPam-insensitive weaker fibrin/fibrinogen bonds irrelevant to knob-hole complexes.
The main finding of this study is that fibrin-fibrinogen interactions displayed a non-monotonic dependence of the average bond lifetimes versus tensile force, in which the average bond lifetime first increased with forces up to 30–40 pN and then decreased with larger forces (Fig. 1A). Normally, bond lifetimes diminish with force and they are referred to as “slip” bonds. Bonds whose dissociation lifetime increases with tensile force are referred to as “catch” bonds. What we observed was a “catch-slip” transition. A negative control for “catch” bonds was the streptavidin-biotin complex, which displayed a pure “slip” bond behavior characterized by a monotonic decrease of the average bond lifetimes when the pulling force ranged from 10–50 pN (Fig. 1B).
Fig. 1.
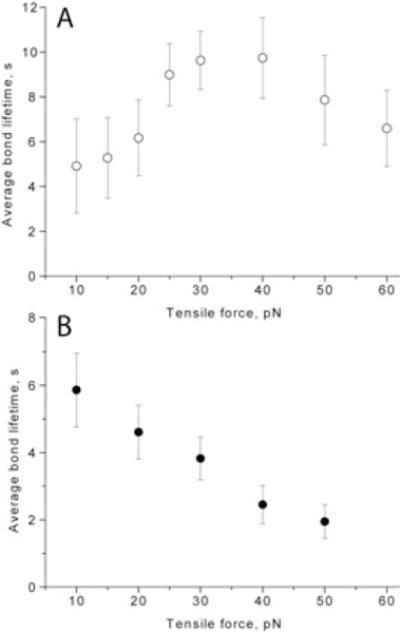
Average bond lifetimes as a function of tensile force for fibrin/fibrinogen (A) and streptavidin/biotin (B) molecular pairs.
At least two arguments have been used to support the view that single-molecule interactions were detected. First, we have adjusted the surface densities of the reacting molecules in such a way that all the bond lifetimes >0.5s (i.e., representing A:a interactions) would not exceed 1/10 of the total number of interaction cycles, which means that multiple bonds are quite infrequent due to the low surface density of the reacting molecules (∼300 nm2 per molecule) and small contact area in one touching cycle (∼450 nm2). Second, when the fibrin/fibrinogen interactions on similarly prepared surfaces were measured in the ramp-force mode, we only observed a sharp single peak in the force histograms [2], suggesting that this peak represents individual knob-hole bonds. Multiple interactions might occur infrequently, resulting in strong irreversible bead attachments.
The existence of “catch” bonds was debated for many years until evidence of their existence was found [6–9]. The physiological importance of “catch” bonds is, for instance, that they mediate firm adhesion and rolling of leukocytes on vessel walls under high shear stress so that they avoid getting stuck in capillaries where the shear stress is low [10]and shear-enhanced platelet adhesion on vWF-coated surfaces [7, 10]. Although theoretical models for the “catch-slip” transition have been proposed [11–14], “catch” bonds in biological systems remain novel, striking, and largely enigmatic, mainly because the mechanistic origin of this counterintuitive behavior is unknown.
This remarkable finding of the “catch-slip” transition for a fibrin-fibrinogen bimolecular pair has at least three important consequences. First, shear-enhanced strengthening of the A:a knob-hole bonds can prevent breakup and damage to clots that are under stress. Second, this “catch” bond behavior may favor fibrin polymerization in fast blood flow as in atherosclerotically narrowed arteries and decelerate fibrin polymerization in slow blood flow as in veins and capillaries. Third, the “catch-slip” transition is indicative of existence of at least two bound states of the knob-hole complex with different affinities, energy landscapes and association/dissociation pathways [11]. Further investigation of these new and unexplored mechano-chemical aspects of fibrin polymerization will advance our understanding of blood clotting and will provide a firm molecular-level foundation for the development of new approaches to modulate this process.
Acknowledgments
The authors thank Andrey Mekler for his help with data acquisition. The work was supported by NIH grant HL090774.
Footnotes
Addendum
R. I. L. performed experiments, R. I. L. and J. W. W. analyzed data, wrote the manuscript.
Disclosure of Conflicts of Interest
The authors state that they have no conflict of interest.
References
- 1.Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013;121:1712–9. doi: 10.1182/blood-2012-09-306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litvinov RI, Gorkun OV, Owen SF, Shuman H, Weisel JW. Polymerization of fibrin: specificity, strength, and stability of knob-hole interactions studied at the single-molecule level. Blood. 2005;106:2944–51. doi: 10.1182/blood-2005-05-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kononova O, Litvinov RI, Zhmurov A, Alekseenko A, Cheng CH, Agarwal S, Marx KA, Weisel JW, Barsegov V. Molecular mechanisms, thermodynamics, and dissociation kinetics of knob-hole interactions in fibrin. J Biol Chem. doi: 10.1074/jbc.M113.472365. Published on May 28, 2013. jbc M113.472365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litvinov RI, Bennett JS, Weisel JW, Shuman H. Multi-step fibrinogen binding to the integrin (alpha)IIb(beta)3 detected using force spectroscopy. Biophys J. 2005;89:2824–34. doi: 10.1529/biophysj.105.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litvinov RI, Barsegov V, Schissler AJ, Fisher AR, Bennett JS, Weisel JW, Shuman H. Dissociation of bimolecular alphaIIbbeta3-fibrinogen complex under a constant tensile force. Biophys J. 2011;100:165–73. doi: 10.1016/j.bpj.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–3. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 7.Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, Lopez JA, Cruz MA, Dong JF, McIntire LV, McEver RP, Zhu C. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118:3195–207. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–84. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Lou J, Zhu C. Forcing switch from short- to intermediate- and long-lived states of the alphaA domain generates LFA-1/ICAM-1 catch bonds. J Biol Chem. 2010;285:35967–78. doi: 10.1074/jbc.M110.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–96. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barsegov V, Thirumalai D. Dynamic competition between catch and slip bonds in selectins bound to ligands. J Phys Chem B. 2006;110:26403–12. doi: 10.1021/jp0653306. [DOI] [PubMed] [Google Scholar]
- 12.Thomas WE. Mechanochemistry of receptor-ligand bonds. Curr Opin Struct Biol. 2009;19:50–5. doi: 10.1016/j.sbi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, McEver RP. Catch bonds: physical models and biological functions. Mol Cell Biomech. 2005;2:91–104. [PubMed] [Google Scholar]
- 14.Sarangapani KK, Qian J, Chen W, Zarnitsyna VI, Mehta P, Yago T, McEver RP, Zhu C. Regulation of catch bonds by rate of force application. J Biol Chem. 2011;286:32749–61. doi: 10.1074/jbc.M111.240044. [DOI] [PMC free article] [PubMed] [Google Scholar]


