Abstract
Emotion attribution (EA) from faces is key to social cognition, and deficits in perception of emotions from faces underlie neuropsychiatric disorders in which cerebellar pathology is reported. Here we test the hypothesis that the cerebellum contributes to social cognition through EA from faces.
We examined fifty-seven patients with cerebellar disorders and 57 healthy controls. Thirty-one patients had complex cerebrocerebellar disease (CD); 26 had disease isolated to cerebellum (ID). EA was measured with the Reading the Mind in the Eyes task (RMET), and informants were administered a novel questionnaire, the Cerebellar Neuropsychiatric Rating Scale (CNRS).
EA was impaired in all patients (CD p<.001, ID p<.001). When analyzed for valence categories, both CD and ID missed more positive and negative stimuli. Positive targets produced the highest deficit (CD p<0.001, ID p=0.004). EA impairments correlated with CNRS measures of deficient social skills (p<.05) and autism spectrum behaviors (p<.005). Patients had difficulties with emotion regulation (CD p<.001, ID p<.001), autism spectrum behaviors (CD p<.049, ID p<.001), and psychosis spectrum symptoms (CD p<.021, ID p<.002). ID informants endorsed deficient social skills (CD p<.746, ID p<.003) and impaired attention regulation (CD p<.144, ID p<.001). Within the psychosis spectrum domain, CD patients were worse than controls for lack of empathy (CD p=0.05; ID p=0.49).
Thus, patients with cerebellar damage were impaired on an EA task associated with deficient social skills and autism spectrum behaviors, and experienced psychosocial difficulties on the CNRS. This has relevance for ataxias, the cerebellar cognitive affective / Schmahmann syndrome, and neuropsychiatric disorders with cerebellar pathology.
Keywords: cerebellum, cerebellar cognitive affective syndrome, emotion attribution, dysmetria of thought, social cognition, social perception
Introduction
Social cognition is defined as the set of mental processes required to understand, generate and regulate social behavior [1, 2]. It includes emotion attribution (EA), the detection or decoding of the mental states of others based on immediately available observable information [3]. Emotion attribution can be understood in terms of three related processes [4]: a) the identification of emotionally salient information in the environment, b) the generation of subjective emotional experience and response to this information, and c) the regulation of subjective emotional experiences and responses. Deficits in emotion attribution are believed to be at the core of neuropsychiatric disorders such as schizophrenia and autism spectrum disorder (ASD) [5, 6].
The neural circuitry underlying social cognition involves frontolimbic connections [1, 7, 8], mirror neurons in ventral premotor and rostral posterior parietal cortices [9], the amygdala [10], insula [11], medial temporal gyrus [12], and middle temporal gyrus [11, 13]. Processing of emotions from faces is subserved by higher order cortices in the temporal lobe, the ventromedial prefrontal cortex (VMPFC) and in the amygdala [14]. The cerebellum is incorporated into all these associative and paralimbic circuits by way of feed forward connections from these cerebral cortical areas to cerebellum via the pons (corticopontocerebellar projections), and by feedback connections from cerebellum through thalamus back to the cerebral cortex (cerebellothalamocerebral projections) [15–19]. The cerebellar vermis, adjacent regions of the posterior cerebellar hemispheres, and the associated fastigial nuclei, collectively referred to as the cerebellar limbic system [20, 21], are linked anatomically with the amygdala, septum and hippocampus [22, 23]; stimulation of the cerebellar vermis modulates firing patterns in these areas [24, 25]; patients with midline lesions demonstrate social-emotional aberrant behaviors [26–31] and earlier studies using cerebellar vermal stimulation ameliorated aggression in patients [32].
Functional neuroimaging evidence for a cerebellar role in social cognition was presented by van Overwalle and colleagues [33–37]. These investigators performed meta-analyses of task-based functional magnetic resonance imaging (fMRI) and resting state functional connectivity MRI (rsfcMRI). They included in their analysis studies of the mirror system recruited when moving body parts are observed – subserved by the mirror network and related sensory-motor system, and the mentalizing system recruited when subjects try to infer the intentionality of others but no motion input is available – subserved by the mentalizing/default network (as in Bucker et al. 2011 [38]). The finding of an overlap between the areas of the cerebellum involved in social cognition and the default/mentalizing network led these investigators [35] to conclude that the cerebellum performs a domain-specific mentalizing process involving abstract and complex mentalizing inferences.
Clinical investigations support these neuroimaging studies suggesting a role for the cerebellum in social cognition. Baribeau and colleagues [39] used a facial emotion attribution task, the revised version of the Reading the Mind in the Eyes task (RMET) developed by Baron-Cohen [40] to study 265 children with autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and obsessive-compulsive disorder (OCD). Cerebellar pathology has been reported in ASD and ADHD [41–44], but is less well defined in OCD [45–49]. The ASD and ADHD children scored lower than patients with OCD and controls (p < .001) on the RMET total score, and on items with positive valence (p < .01). Greater social communication impairment and hyperactivity/impulsivity, but not OCD traits/symptoms, were associated with lower scores on the RMET, irrespective of diagnosis [39].
The cerebellar cognitive affective syndrome (CCAS) [27], now also referred to by the eponym ‘Schmahmann syndrome’ [28], is characterized by deficits in executive function, linguistic processing, and visual spatial cognition as well as impairments in affect regulation, The affective component of the CCAS, conceptualized as the neuropsychiatry of the cerebellum, comprises impairments in five major domains of behavior – attentional control, emotional control, psychosis spectrum, autism spectrum, and social skill set [21]. Many of these behaviors are impacted directly by the individual’s ability to understand, generate, and control social behavior, the essence of social cognition.
Studies of social cognition using story-based assessments of EA in patients with primary cerebellar disease or injury [50] have produced conflicting results. Patients with spinocerebellar ataxia (SCA) 2 and SCA7 were shown to be impaired [51], whereas patients with superficial siderosis [52] and SCA3 and SCA6 [53] were not. In contrast, EA performance was shown to be impaired in small studies of cerebellar patients using picture-based face emotion attribution tasks. Adamaszek and colleagues [54] administered a picture-based battery of a series of photographs of faces adapted from the Florida Affect Battery [55] to 15 patients with cerebellar stroke and demonstrated impaired emotional facial expression and emotional prosody in response to stimuli with negative valence. Agata and colleagues [56] administered the Ekman 60 Faces battery and the Tamietto 50 Faces test [57] to 13 patients with SCA 2 (n=9), SCA6 (n=5), SCA 7 (n = 2), and SCA 8 (n=4). This study reported a significant difference between patients and controls in the recognition of all emotional stimuli presented, and within the cerebellar cohort, impairments were most pronounced for recognition of negative and positive emotions.
Little is known about emotion attribution from faces and social cognition in patients with primary cerebellar disease or injury. In this study we set out to explore the cerebellar contribution to social cognition adopting the a priori hypothesis that deficits in emotion attribution are associated with deficient social skills, lack of empathy and behavioral alterations [58–63]. We note, however, that alternative theories have been proposed for the inability to accurately interpret the mental state others. It has been argued, for example, that a lack of cognitive empathy, or the inability to adopt another person's perspective (i.e., Theory of Mind (ToM) deficit), is the core hallmark of a lack of social skills, and that this impairment underlies other problems including the inability to understand another person's mind and emotions [58].
In this study we hypothesized that 1) patients with cerebellar pathology are impaired on tasks of EA, 2) cerebellar pathology is sufficient to produce deficits in EA, and 3) EA impairments may be associated with impaired social skills, emotion dysregulation, and impairments in social cognition that have been associated with autism spectrum behaviors and with blunted empathy.
Participants and Methods
Participants
Patients were recruited from the Ataxia Unit in the Department of Neurology of the Massachusetts General Hospital if they had hereditary or other neurodegenerative ataxias, or acquired injury to the cerebellum. Medically ill ataxia patients and those with developmental neurological disorders were not considered. In all cases, detailed history was elicited and neurological examination performed (JDS). Group assignments of patients into isolated cerebellar disease and complex cerebrocerebellar disease were based on analysis of genotypes in patients with genetic neurodegenerative cerebellar disease, published pathologic features of the SCAs and related disorders [64], and expert consensus criteria [65]. Analysis of clinically available magnetic resonance brain imaging in patients with injury to the cerebellum complemented the group assignment.
Of the 57 patients, 31 were categorized as having complex cerebrocerebellar degeneration as the pathology of their disorders (genetic or acquired) is known to involve cerebellum as well as cerebral and brainstem structures [65–67]. This group included patients with spinocerebellar ataxia (SCA)1, SCA2, SCA3, SCA7, SCA17, ataxia oculomotor apraxia type 2 (AOA2), multiple system atrophy of the cerebellar type (MSAc), Friedreich's ataxia, complex cerebrocerebellar degeneration of unknown etiology, and left cerebellar injury with brainstem involvement. Twenty six patients had disease confined to cerebellum. Of these, 18 had isolated cerebellar degeneration in which the pathology of the genetic or other neurodegenerative disorder has been shown to be confined to cerebellum (isolated cerebellar hypoplasia, SCA6, SCA8, idiopathic late onset cerebellar ataxia (ILOCA), autosomal recessive cerebellar ataxia type 1 (ARCA-1), episodic ataxia type 2 (EA-2)). A further 8 had isolated injury confined to the cerebellum (right hemisphere, left hemisphere, midline or bilateral) as determined by neurological examination and magnetic resonance brain imaging.
Fifty-seven healthy controls were recruited from the panel of volunteers at the Massachusetts General Hospital, matched for age, gender, education and handedness.
See Table 1 for a detailed description of patient demographics, diagnoses, and age, educational level and gender of the study subjects.
Table 1.
Patient demographics and clinical data
| Disease entity | Total number of patients n (number female /number male) | Age (mean/SD) | Education years (mean/SD) | |
|---|---|---|---|---|
| Complex cerebellar group (CD) | SCA1 | 5 (2/3) | 59.2+/−13.2 | 14 +/− 2 |
| SCA2 | 5 (2/3) | 54.4+/−9.5 | 14.8 +/−2.4 | |
| SCA3 | 9 (5/4) | 53.2+/−9 | 15 +/−2.6 | |
| SCA7 | 1 (0/1) | 63+/−0 | 16 +/−0 | |
| SCA17 | 1 (1/0) | 48+/−0 | 16 +/−0 | |
| Friedreich's ataxia | 2 (0/2) | 41.5+/− 16.2 | 16 +/−0 | |
| MSAc | 4 (2/2) | 55+/−9.5 | 14.33 +/−2.5 | |
| AOA-2 | 1 (1/0) | 20 +/− 0 | 14 +/−0 | |
| Complex cerebrocerebellar degeneration with gene variants | 2 (1/1) | 54.6 +/− 6.6 | 15.66 +/−2 | |
| Cerebellar and brainstem hemorrhage | 1 (0/1) | 65+/−0 | 20 +/−0 | |
| Isolated cerebellar group (ID) | SCA6 | 4 (3/1) | 64+/−4.8 | 17.25 +/−2 |
| SCA8 | 4 (3/1) | 55.75+/−6.4 | 15 +/−1.4 | |
| ARCA-1 | 2 (0/2) | 50.5 +/−10.6 | 15 +/−1.4 | |
| EA-2 | 2 (1/1) | 23.5+/−0.7 | 13 +/−1.4 | |
| ILOCA | 5 (2/3) | 47.2+/−9.8 | 14.8 +/−2 | |
| Nonprogressive isolated cerebellar ataxia | 1 (1/0) | 18+/−0 | 13 +/−0 | |
| Left cerebellar injury (L-PICA stroke, L-SCA stroke) | 2 (1/1) | 48.5 +/−0.7 | 18.5 +/−2 | |
| Bihemispheric cerebellar injury (hemorrhage (n=2), medulloblastoma) | 3 (1/2) | 28. 3+/−9.8 | 15 +/−3 | |
| Right cerebellar injury (R-PICA stroke, R-SCA stroke (n=2)) | 3 (2/1) | 43 +/−15.6 | 16 +/−0 |
Reported as means (standard deviations in parentheses). Abbreviations: AOA-2, ataxia oculomotor apraxia type 2; ARCA-1, autosomal recessive cerebellar ataxia type 1; EA-2, episodic ataxia type 2; ILOCA, idiopathic late onset cerebellar ataxia; L, left; SCA, superior cerebellar artery; MSA-c, multiple system atrophy of the cerebellar type; PICA, posterior inferior cerebellar artery; R, right; SCA, superior cerebellar artery; SCA-1, spinocerebellar ataxia type 1.
This study was approved by the Institutional Review Board of the Massachusetts General Hospital. Informed consent was obtained from all participants.
Social cognitive assessment
In order to evaluate social cognition, specifically EA, we administered the revised Reading the Mind in the Eyes task (RMET) [40]. The RMET consists of 36 black-and-white photographs of the eye region of faces taken from magazine photos. Each pair of eyes is standardized to the same size (15 cm x 6 cm) and edited such that the eye region is visible from just above the eyebrow down to midway along the bridge of the nose. Participants are asked to determine which of four words (the standardized correct response and three distracters) describe what the person in the photograph might be thinking or feeling. Response items can be divided into three categories of varying emotional valence (positive, negative, and neutral) [68]. Total scores range from 0 (no correct answers) to 36 (maximum correct). Subscores range from 0 to 12 (negative valence), 0 to 8 (positive valence), and 0 to 16 (neutral valence), depending on the number of items in each category.
Neuropsychiatric assessment
Neuropsychiatric behaviors were evaluated by means of a novel questionnaire derived from an earlier study which introduced the concept of the neuropsychiatry of the cerebellum [21]. In that analysis, patients demonstrated, or family members reported, neuropsychiatric phenomena that were categorized according to five domains of behavior – attentional control, emotional control, autism spectrum, psychosis spectrum, and social skill set. Within each of these five domains, symptoms were further grouped according to negative spectrum symptoms (diminished, hypometric, or undershoot) and positive spectrum symptoms (exaggerated, hypermetric or overshoot) (Table 2). Schmahmann et al. (in preparation) have used these symptoms, scored on a 4-point Likert scale to develop a novel instrument, the Cerebellar Neuropsychiatric Rating Scale (CNRS). Here we used a preliminary version of this new CNRS, completed by first degree relatives of the patients and healthy controls, to assess deficits in the five identified domains of behavior in our cerebellar cohorts and correlated responses on this scale with subjects’ performance on the RMET. The questions probing depression and dysphoria on the CNRS were used to test for confounding influences of depression on the interpretation of valence categories of the RMET task. Within the psychosis spectrum domain, symptoms of lack of empathy were evaluated separately.
Table 2.
Domains assessed in the Cerebellar Neuropsychiatric Rating Scale
| Positive (exaggerated) symptoms | Negative (diminished) symptoms | |
|---|---|---|
| Attentional Control | Inattentiveness | Ruminativeness |
| Distractibility | Perseveration | |
| Hyperactivity | Difficulty shifting focus of attention | |
| Compulsive and ritualistic behaviors | Obsessional thoughts | |
| Emotional control | Impulsiveness, disinhibition | Anergy, anhedonia |
| Lability, unpredictability | Sadness, hopelessness | |
| Incongruous feelings, pathological laughing/crying | Dysphoria | |
| Anxiety, agitation, panic | Depression | |
| Autism spectrum | Stereotypical behaviors | Avoidant behaviors, tactile defensiveness |
| Self stimulation behaviors | Easy sensory overload | |
| Psychosis spectrum | Illogical thought | Lack of empathy |
| Paranoia | Muted affect, emotional blunting | |
| Hallucinations | Apathy | |
| Social skill set | Anger, aggression | Passivity, immaturity, childishness |
| Irritability | Difficulty with social cues and interactions | |
| Overly territorial | Unawareness of social boundaries | |
| Oppositional behavior | Overly gullible and trusting |
Neuropsychatric symptoms and signs arranged according to five major domains, each with positive (hypermetric) and negative (hypometric). Adapted from Table 1 in Schmahmann et al., 2007.
Statistical analysis
Behavioral data were analyzed using SSPS v21 (SSPS Inc.). Parametric data for patient groups and matched controls were compared via one way analysis of variance (ANOVA) of the means of each group based on a priori hypotheses followed by paired student’s t-tests if the F ratio was significant. Following Cohen’s guidelines for effect size, the effect size was calculated (d=(x̄ 1-x̄ 2)/SDaverage); r=√(d2/(4+d2): small d>0.2/r>0.1, medium d>0.5/r>0.243, large d>0.8/r>0.371) [69]. Effect size was calculated using means and standard deviations instead of t-values and degrees of freedom to prevent an overestimation of effect sizes [70]. A p<.05 was considered statistically significant.
We further modeled the RMET error probability using random effects logistics regression, using the “R” statistical software (Version 3.1.0) [71], with the ‘lme4’ and ‘lmerTest’ libraries [72, 73]. We adjusted for multiple comparisons using the approach of Hothorn et al. [74], as implemented in the ‘multcomp’ R library.
The Pearson product-moment correlation coefficient (Pearson’s correlation) was used to measure the strength of associations between RMET and four selected aspects of the CNRS adopting the a priori hypothesis [58–63] that deficits in emotion attribution are associated with deficient social skills, lack of empathy, emotional control, and autism spectrum behaviors. This was done in order to avoid effects of multiple comparison [69].
Results
Performance on the RMET
EA was impaired in all patients when compared to controls when analyzed for total scores (CD patients p<.001, ID patients p<.001) (Table 3). Patients missed more negative and positive targets than neutral targets when analyzed separately from controls (Figure 4).
Table 3.
Reading the mind in the eyes test (RMET)
| RMET | Patients | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Raw Score (/36) | ANOVA F | p | Mean | SD | Mean | SD | t | df (adjusted) | p |
| CD patients | 18.96 | <.001 | 21.94 | 5.66 | 27.68 | 3.27 | − 5.41 | 30 | <.001 |
| ID patients | 21.04 | 5.45 | 28.11 | 3.011 | − 5.18 | 25 | <.001 | ||
Comparison of the total scores on the RMET (maximum score = 36) in patients with complex (CD) and isolated (ID) cerebellar pathology.
When modeling the RMET error probability using random effects logistic regression, both CD and ID patients had a significantly smaller average probability of correct response for positive valence eyes than their corresponding control groups (p=0.004 and p<0.001, respectively, adjusting for multiple comparisons), but not for neutral or negative valence (p>0.27). The estimated differences from controls in these positive valence probabilities, 0.79–0.63 = 0.16 for the CD group, and 0.81–0.50 = 0.31 for the ID group, were not significantly different (p=0.32) (Table 5, Figure 1).
Table 5.
Estimated mean probability of correct RMET response
| Patient group | RMET Valence | Mean probability patients | Mean probability controls | p |
|---|---|---|---|---|
| CD | Neutral | 0.75 | 0.77 | 0.98 |
| CD | Positive | 0.63 | 0.79 | 0.004 |
| CD | Negative | 0.56 | 0.63 | 0.47 |
| ID | Neutral | 0.74 | 0.81 | 0.27 |
| ID | Positive | 0.50 | 0.81 | <.001 |
| ID | Negative | 0.57 | 0.62 | 0.88 |
Comparison of RMET error probability in patients with complex (CD) and isolated (ID) cerebellar pathology and controls was modeled using random effects logistic regression followed by adjustment for multiple comparisons [72–74]. A p <.05 was considered statistically significant. Note: Cerebellar patients were significantly less likely to score correct on positive stimuli than healthy controls with large effect sizes. ID patients were even less likely to score correct on positive valence stimuli than CD patients with the difference between them not being significant (ID=CD).
Figure 1. Plot of mean probability of correct RMET response per valence category.
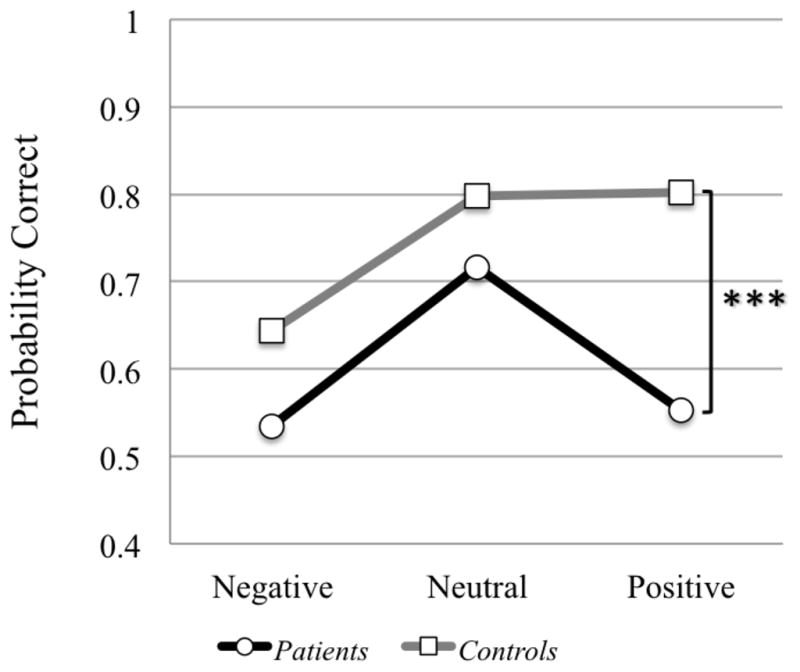
Comparison of probability of correct answers on RMET valence categories (negative, neutral and positive stimuli) for all patients in comparison to controls. Cerebellar patients were significantly less likely to score correct on positive stimuli than healthy controls (*** = p <.001). This pattern was consistent within all patient groups (CD=ID). A p <.05 was considered statistically significant.
Within the ID group eight patients had focal lesions of the left or right cerebellar hemispheres, and midline / bilateral cerebellum. These patients were as impaired on the RMET as patients with degeneration confined to the cerebellum. No significant difference in performance was found between patients with lesions of the right or left hemispheres, but the number of subjects with focal lesions was small.
Cerebellar Neuropsychiatric Rating Scale (CNRS)
Social and emotional skills were assessed by administering a questionnaire to first degree relatives of patients and controls, targeting the previously identified domains of affective impairment in a population of cerebellar patients. Note that higher scores on the symptoms / sign questionnaire indicate a higher degree of pathology (for a summary of symptoms investigated on the novel questionnaire see Table 2).
CNRS domains impaired in both ID and CD patients
Patients in both the CD and ID groups scored higher than controls on the symptom questionnaire for hypometric emotional control (CTL mean = 0.97; CD mean = 3.47, p<0.001; ID mean = 4.60, p<0.001) and hypermetric emotional control (CTL mean = 1.86; CD mean = 3.42, p=0.013; ID mean = 4.10, p<0.001complex). Effect sizes were large for both hypometric (CD r = .55; ID r = .62) and hypermetric (CD r = .39; ID r = .55) measures.
Both patients groups scored higher than controls on the symptom questionnaire for hypometric autism spectrum symptoms (CTL mean = 0.95; CD mean = 1.68, p=0.049; ID mean = 2.50, p<0.001). Effect sizes were medium for CD (r = .30) and large for ID (r = .60).
They also scored higher when compared to controls on the symptom questionnaire for hypometric psychosis spectrum (CTL mean = 1.16; CD mean = 2.32, p=0.021; ID mean = 3.20, p=0.002). Effect sizes were medium for CD (r = .31) and large for ID (r = .46).
CNRS domains impaired only in ID patients
Patients in the isolated group, but not in the complex group, scored higher compared to controls on the symptom questionnaire for hypermetric psychosis spectrum (CTL mean = 0.30; CD mean = 0.89, p=0.064; ID mean = 1.00, p=0.002). Effect sizes were medium for CD (r = .32) and large for ID (r = .45).
ID patients scored higher when compared to controls on the symptom questionnaire for hypometric social skills (CTL mean = 1.43; CD mean = 1.63, p=0.746; ID mean = 3.80, p=0.003). Effect sizes were negligible for CD (r = .05) and large for ID (r = .48).
ID patients also scored higher than controls on the symptom questionnaire for hypometric attention regulation (CTL mean = 2.84; CD mean = 4.16, p=0.144; ID mean = 7.00, p<0.001). Effect sizes were small for CD (r = .28) and large for ID (r = .68).
CNRS impairments only in CD patients
Within the psychosis spectrum domain on the symptom questionnaire, patients in the CD group, but not in the ID group, scored higher than controls for the report of lack of empathy (CTL mean = 0.35; CD mean = 0.80, p=0.036; ID mean = 0.50, p=0.491). Effect sizes were medium for CD (r = .32) and small for ID (r = .13).
No significant differences were found between ID or CD patients and controls on the symptom questionnaire for hypermetric social skill set (CTL mean = 1.95; CD mean = 3.21, r = .26; ID mean = 3.60, r = .49; p=0.058), hypermetric autism spectrum (CTL mean = 0.68; CD mean = 0.74, r = .03; ID mean = 1.10, r = .22; p=0.240) and hypermetric attention spectrum (CTL mean = 3.08; CD mean = 3.42, r = .08; ID mean = 4.70, r = .34; p=0.240).
RMET and CNRS Correlation
Correlation analysis was performed between the RMET and selected domains identified as being impaired on the CNRS: namely, emotional control, lack of empathy, autism spectrum behaviors, and deficient social skills.
Performance on the RMET had strong negative correlation with autism spectrum behaviors (hypermetric range) (Pearson‘s r=−.428; p=.005) and deficient social skills (hypometric range) (Pearson’s r = −0.387; p=0.011).
No significant correlation was found between performance on the RMET and symptoms of emotional dysregulation or lack of empathy.
Further, symptoms of depression and dysphoria were not significantly correlated with missed RMET targets when analyzed according to their valence categories (negative, neutral, positive).
Discussion
EA deficits in patients with cerebellar disease
Our results show that patients with cerebellar damage were impaired on a test of emotion attribution, assessed using the revised RMET [40]. This was true for patients with complex cerebrocerebellar degeneration as well as for those with isolated cerebellar disease or injury. This finding supports our hypothesis that patients with cerebellar pathology are impaired on tasks of emotion attribution (EA), and that cerebellar pathology alone is sufficient to produce these deficits in EA.
Our findings are in line with earlier reports in smaller cohorts of cerebellar patients that demonstrated EA impairments using picture-based emotion attribution tasks [54, 56]. When analyzing the profile of performance for the patient group only, despite the differences in picture-based tasks used in the present and earlier studies, we replicate the observation that cerebellar patients have more difficulty recognizing emotions with negative and positive valence than they do for emotionally neutral stimuli (Figure 1) [54, 56].
The impaired decoding of negative emotions on the RMET in the patient group is in line with our earlier observation that the cerebellum is embedded in an aversion-related circuitry that processes unpleasant emotions [75]. Studies measuring fMRI activity pattern in response to emotionally salient stimuli show deficits in accuracy of decoding negative stimuli [75–77]. Further, with regard to processing negative emotions from faces, patients with pathology in cerebellar-limbic networks are impaired on extracting information from the eyes, the primary decoding region for negative emotions [78].
However, when analyzing patient performance in comparison to controls, the difference for valence category accuracy on the RMET shifts towards an even larger impairment for positive emotions. We show that both patient groups (CD and ID) demonstrate a significantly smaller average probability of correct response for positive valence than their corresponding control groups (Figure 1). This is consistent with the recent finding of Baribeau et al. [39] that patients with ASD and ADHD which include cerebellar pathology [41–44] are impaired on overall scores of the RMET in addition to significant impairment on items with positive valence. These two studies are the first to show that patients with cerebellar pathology are impaired in their ability to recognize happy faces on the RMET. This observation may be explained by the nature of the RMET itself. Patients performing the RMET rely solely on visual cues from the region around the eyes to decode emotions without other regions of the face. The developing brain relies heavily on visual cues from the mouth area to recognize happiness [79] and during development, cues from the mouth area become less important and are largely replaced by visual decoding of the eye region [78]. Previous studies of whole face perception in patients with cerebellar stroke and degeneration [54, 56] did not demonstrate this deficit in decoding positive stimuli that we describe in our study using the RMET alone. This discrepency suggests that cerebellar patients utilize additional perioral facial cues to decode positive facial stimuli. The results provide novel insights into the cerebellar contribution to social perception from faces. They emphasize the importance of the eye region for emotion recognition, and suggest further that patients with cerebellar pathology may be evaluating the evaluating the mouth region as a compensatory strategy for recognizing positive emotions. This opens the way for future interventions and cognitive behavioral training targeting decoding of the eyes as well as the mouth in patients with cerebellar pathology, perhaps including those with schizophrenia and autism.
Altogether, the findings using the RMET stand in contrast to those using story-based tasks [50] that have produced conflicting results: Patients with spinocerebellar ataxia (SCA) 2 and SCA7 were shown to be impaired [51], whereas patients with superficial siderosis [52] and SCA3 and SCA6 [53] were not. This difference may be explained by our larger sample size, but it may also be related to the structure of tasks. Story-based tasks allow subjects to utilize other reasoning abilities to compensate for a weakness in EA [80] whereas the RMET is a more pure measure of emotion attribution which has been widely used and validated in experimental studies of EA [40].
The cerebellum’s role in social cognition is facilitated by its anatomical connections to the limbic system [20, 22, 26] which is necessary for recognition of emotionally salient stimuli on the opposite ends of the emotional spectrum – i.e., sadness and happiness [10, 81]. The amygdala and anterior insula are particularly important for the identification of emotional stimuli [10]. The amygdala is also engaged with identifying faces and direction of gaze [82, 83], emotional expressions displayed by others and the generation of appropriate emotional and behavioral responses to emotionally salient stimuli [10]. Our patients had more difficulty recognizing emotions with negative and positive valence than they did neutral stimuli, indicating that cerebellar pathology was sufficient to induce a profile of EA impairments similar to that resulting from pathology in cerebral hemispheric limbic structures [84]. This is consistent with the notion of a limbic cerebellum, and the view that cerebellar-limbic connectivity facilitates the cerebellar influence on processes subserving emotion recognition [20–22, 26].
Report of symptoms using the CNRS
On the novel cerebellar neuropsychiatric rating scale (CNRS), cerebellar patients exhibited a range of deficits when compared to healthy controls. Hypometric symptoms predominated in all five domains: both CD and ID patients were affected in the domains of attentional control, emotional control, autism spectrum and psychosis spectrum, while for the ID group deficits were reported in social skill set. Hypermetric symptoms were reported in the domain of emotional control (CD and ID) and psychosis spectrum (ID only). There was no domain in which ID was spared but CD affected, with the single exception of the feature of lack of empathy (within the hypometric psychosis spectrum), which was higher in CD patients but not in ID patients.
These findings in our cerebellar patients make it unlikely that the neuropsychiatic features identified by the CNRS resulted from cerebral (i.e., other than cerebellar) pathology. Indeed, the ID group alone reported impairments in the domains of attentional control and social skill set (hypometric features), and psychosis spectrum disorders (hypermetric). The pure, or at least relatively pure, cerebellar basis for neuropsychiatric phenomeona provide support for the formulation of the constellation of symptoms and signs that are the basis of the notion of the neuropsychiatry of the cerebellum (Schmahmann et al., 2007 [21], building on Schmahmann and Sherman 1998 [27, 28] and Levisohn et al., 2000 [29]). We note that the present adult patient cohorts did not differ significantly from healthy controls with respect to hypermetric symptoms in the domains of attentional control, autism spectrum and social skill set. This stands in contrast to our previous study [21] that included children with cerebellar tumors, cerebellar agenesis, and childhood onset cerebellar degenerative diseases, which found evidence of hypermetric impairments in these domains. It is possible that the neuropsychiatric spectrum of the CCAS may differ in children compared to adults. This is suggested by reports of developmental CCAS in children with cerebellar disorders that describe neurobehavioral deficits in attentional control, autism spectrum and social skill set [29–31, 85, 86].
RMET performance correlates with deficient social skills and autism spectrum behaviors
Our finding that impairments in EA were significantly associated with deficient social skills and autism spectrum behaviors is in line with the current understanding of the cognitive processes involved in emotion recognition. The recognition of emotionally salient stimuli (EA) prompts the generation of an emotional subjective experience and response that need to be regulated so that the affective state and response are contextually appropriate [4]. EA deficits leading to, or at least related to, autism spectrum behaviors and deficient social skills are relevant when considering the presence of cerebellar pathology in a range of neuropsychiatric disorders with impaired emotion recognition [87–89]. The impairment in EA in patients with pure cerebellar disease highlights the potential pathophysiological role of the cerebellum in autism which consistently shows impairments on the RMET and other tasks of emotion recognition [87–89]. Similarly, patients with Huntington’s disease score lower than controls on the RMET [90], as do patients with schizophrenia who also show impairments in responses to face stimuli on the NimStim Face Stimulus Set [90]. Cerebellar pathology is a component of the underlying neurobiology in each of these disorders (autism [91], schizoprenia [92], and Huntington’s disease [93]) and therefore the impairment in social cognition in these disorders that is dependent upon EA, may result, at least in part, from cerebellar pathology.
We note that cerebellar patients who endorsed symptoms of depression and dysphoria on the CNRS did not perform more poorly on the RMET, and were no different from the overall patient cohort in their distribution of errors in each of the valence categories (positive, neutral and negative). Whereas studies in patients with depression report a bias towards identification of emotional stimuli as negative [94], there was no evidence in our cohort of an influence of depressed mood on the selection of emotional valence for the RMET stimuli.
Cerebellar role in EA and social cognition
At a mechanistic level, the RMET requires subjects to match the eyes in each picture at an unconscious, rapid and automatic level in order to make judgments regarding facial emotional expression [40]. The cerebellum is necessary for implicit learning, automatic processing and higher-order control [95], and automaticity and categorization are important determinants of the meaning of gesture, speech and expression [96]. These features relate directly to the dysmetria of thought theory and the notion of the universal cerebellar transform (UCT) [20, 26, 97], in which the essential function of the cerebellum is to maintain behavior around a homeostatic baseline, appropriate to context, automatically and without conscious awareness. Suboptimal performance by cerebellar patients on the EA task may thus reflect loss or impairment of the UCT required for the processing of the emotionally salient information. Given the importance of the EA for social cognition, the disruption of the cerebellar influence on EA represents a potential direct link between cerebellar pathology and deficits in social cognition.
Strengths and limitations
This is the largest cohort to date of adult patients with cerebellar disease in which social cognition has been investigated, including emotion attribution and neuropsychiatric and psychosocial behaviors. We employed a widely used experimental test of emotion attribution (RMET) [40] along with a preliminary version of an experimental neuropsychiatric questionnaire, the CNRS, developed in our lab and based on prior findings from our research regarding the nature of emotional and social deficits in patients with cerebellar disorders (Schmahmann et al., in preparation).
Limitations of this study also result from the experimental approach inherent in currently available methods of social cognitive testing. It should be acknowledged that EA is not synonymous with social cognition, although it is an essential component in the process of reaching conclusions about social situations. Traditional tests of EA are limited to static protocols in an experimental setting that parse social cognitive functions which are usually dynamic and fleeting in daily life. Social cognitive demands in real life do not occur in isolation, nor are they decontextualized as a single photographic image, but rather are contextually dependent and continuously changing [98]. Assessing social cognitive functions in isolation in an experimental setting may therefore over- or under-estimate an individual’s capability. Nevertheless, the RMET is one of the most frequently used tests of EA and provides a reliable measure of EA that enables comparison of subjects across disease entities. The EA task may require cognitive processing, such as visual-spatial reasoning as well as executive functions, attention, working memory or language skills [98]. Further, it has been suggested that social cognitive performance per se is ameliorated by operationalization of cognitive networks in addition to recruitment of a social cognitive core circuitry [99, 100]. This question will need to be addressed by evaluating whether cognitive networks support EA in patients with cerebellar disease.
Whereas the RMET assesses decontextualized emotion attribution, we developed the CNRS in our lab to evaluate social and emotional cognition in patients with cerebellar pathology in their everyday life. The results of this study, specifically that the CNRS correlates with performance on the RMET, adds validity to this newly developed measure. This assessment instrument remains to be further validated in prospective studies in our lab and elsewhere.
Recent investigations [33–37] indictate that there are network specific locations within cerebellum for different aspects of social cognition. It would be informative to perform a detailed analysis of structure-function correlation in the domains of social cognition in patients with focal cerebellar lesions to determine if there is unique or preferential overlap with lesions in the somatomotor, limbic and default networks. Our cohort was mostly degenerative, however, and there were insufficient numbers of patients with focal lesions to perform this analysis with anatomic specificity with respect to cerebellar lobules or hemispheric asymmetry. Given what is now known about cerebellar functional topography for sensorimotor, cognitive and affective behaviors [102], and specifically for social cognition [33–37], further investigations are planned in patients with focal cerebellar lesions to follow up our present observations in patients with more diffuse cerebellar damage.
Conclusions
Patients with primary cerebellar disease are impaired on the Reading the Mind in the Eyes Task, a test of Emotion Attribution which is important for social cognition. The profile of suboptimal performance on EA in the RMET is similar to that in patients with pathology confined to limbic circuitry, underscoring the functional importance of reciprocal cerebellar interconnections with the limbic, paralimbic and cerebral association areas [102].
Judgment of facial emotional expression in the RMET requires rapid, automatic and implicit processing [40] which is also the essence of the proposed universal cerebellar transform [20, 26, 97]. EA deficits in cerebellar patients thus provide support for the notion of the universal cerebellar transform, and indicate that the cerebellum is essential for social cognition. The difficulties with social cognition reported by family members on the CNRS suggest that performance on this measure relates to challenges that patients encounter in their everyday life.
These findings have relevance for social emotional functioning in patients with cerebellar disorders, and for the cerebellar contribution to neuropsychiatric disorders including autism and schizophrenia in which social cognition is affected.
Table 4.
Reading the mind in the eyes test (RMET) accuracy on valence categories (raw scores)
| RMET Valence Category | Patients | Controls | ||
|---|---|---|---|---|
| Correct negative (%) | Mean | SD | Mean | SD |
| CD patients | 52.15 | 20.41 | 67.20 | 16.23 |
| ID patients | 50.32 | 21.79 | 65.71 | 14.59 |
| Correct neutral (%) | Mean | SD | Mean | SD |
| CD patients | 70.16 | 15.20 | 78.83 | 9.50 |
| ID patients | 71.39 | 12.14 | 82.45 | 10.61 |
| Correct positive (%) | Mean | SD | Mean | SD |
| CD patients | 59.27 | 21.16 | 81.05 | 9.05 |
| ID patients | 46.15 | 25.19 | 82.69 | 10.05 |
Comparison of the percent accuracy of correct answers per valence category of presented stimuli (negative, neutral, positive) in patients with complex (CD) and isolated (ID) cerebellar pathology and controls.
Acknowledgments
The contributions to this project of Jessica A. Harding, Jason MacMore and Bruna Olson Bressane are gratefully acknowledged.
Funding
This work was supported in part by the National Ataxia Foundation, the Cerebellar Research Consortium for the Spinocerebellar Ataxias (RC1 NS068897-02), and the Birmingham and MINDlink Foundations.
Abbreviations
- CCAS
cerebellar cognitive affective syndrome
- CD
complex cerebrocerebellar disease group
- CNRS
Cerebellar Neuropsychiatric Rating Scale
- EA
emotion attribution
- ID
isolated cerebellar disease group
- RMET
reading the mind in the eyes test
- SD
standard deviation
- UCT
Universal Cerebellar Transform
Footnotes
Conflict of interest
The authors declare no conflict of interests.
References
- 1.Beer JS, Ochsner KN. Social cognition: a multi level analysis. Brain Res. 2006;1079:98–105. doi: 10.1016/j.brainres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Fiske ST, Taylor SE. Social Cognition. New York: McGraw-Hill; 1991. [Google Scholar]
- 3.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 4.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiat. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 5.Brüne M. Social cognition and psychopathology in an evolutionary perspective. Current status and proposals for research. Psychopathology. 2001;34:85–94. doi: 10.1159/000049286. [DOI] [PubMed] [Google Scholar]
- 6.Frith CD, Friston KJ, Liddle PF, Frackowiak RS. PET imaging and cognition in schizophrenia. J R Soc Med. 1992;85:222–24. doi: 10.1177/014107689208500414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 8.Beer JS, Mitchell JP, Ochsner KN. Special issue: Multiple perspectives on the psychological and neural bases of social cognition. Brain Res. 2006;1079:1–3. doi: 10.1016/j.brainres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Rizzolatti G, Fogassi L, Gallese V. Mirrors of the mind. Sci Am. 2006;295:54–61. doi: 10.1038/scientificamerican1106-54. [DOI] [PubMed] [Google Scholar]
- 10.Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–63. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 11.Kipps CM, Duggins AJ, McCusker EA, Calder AJ. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington's disease. J Cogn Neurosci. 2007;19:1206–17. doi: 10.1162/jocn.2007.19.7.1206. [DOI] [PubMed] [Google Scholar]
- 12.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci. 1998;265:1927–31. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone T, van Reekum CM, Oakes TR, Davidson RJ. The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc Cogn Affect Neurosci. 2006;1:242–9. doi: 10.1093/scan/nsl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adolphs R. Processings of emotional and social information by the human amygala. In: Gazzaniga MS, editor. The new cognitive neurosciences III. 3. Massachusetts: MIT Press; 2004. pp. 1005–16. [Google Scholar]
- 15.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–98. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Schmahmann JD, Pandya DN. Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. J Comp Neurol. 1989;289:53–73. doi: 10.1002/cne.902890105. [DOI] [PubMed] [Google Scholar]
- 17.Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997;17:438–58. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmahmann JD, Pandya DN. The cerebrocerebellar system. The cerebellum and cognition. In: Schmahmann JD, editor. Int Rev Neurobiol. Vol. 41. 1997. pp. 31–60. [DOI] [PubMed] [Google Scholar]
- 19.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 20.Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguist. 2000;13:189–214. [Google Scholar]
- 21.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6:254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 22.Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974;45:268–87. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- 23.Snider RS, Maiti A, Snider SR. Cerebellar pathways to ventral midbrain and nigra. Exp Neurol. 1976;53:714–28. doi: 10.1016/0014-4886(76)90150-3. [DOI] [PubMed] [Google Scholar]
- 24.Zanchetti A, Zoccolini A. Autonomic hypothalamic outbursts elicited by cerebellar stimulation. J Neurophysiol. 1954;17:475–83. doi: 10.1152/jn.1954.17.5.475. [DOI] [PubMed] [Google Scholar]
- 25.Bobée S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–17. doi: 10.1016/s0166-4328(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 26.Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–87. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- 27.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 28.Manto M, Mariën P. Schmahmann's syndrome - identification of the third cornerstone of clinical ataxiology. Cerebellum. 2015;2:2. doi: 10.1186/s40673-015-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levisohn L, Cronin-Golomb A, Schmahmann J. Neuropsychological consequences of cerebellar tumour resection in children: Cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–50. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 30.Pollack IF. Posterior fossa syndrome. Int Rev Neurobiol. 1997;41:411–32. doi: 10.1016/s0074-7742(08)60362-1. [DOI] [PubMed] [Google Scholar]
- 31.Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–60. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- 32.Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol Psychiatry. 1978;13:501–29. [PubMed] [Google Scholar]
- 33.Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–72. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Cerebellar areas dedicated to social cognition? A comparison of meta-analytic and connectivity results. Soc Neurosci. 2015;10(4):337–44. doi: 10.1080/17470919.2015.1005666. [DOI] [PubMed] [Google Scholar]
- 36.Van Overwalle F, D'aes T, Mariën P. Social Cognition and the Cerebellum: A Meta-analytic Connectivity analysis. Human Brain Mapping. Hum Brain Mapp. 2015 doi: 10.1002/hbm.23002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Overwalle F, Mariën P. Functional connectivity between the cerebrum and cerebellum in social cognition: A multi-study analysis. Neuroimage. 2015;124:248–255. doi: 10.1016/j.neuroimage.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baribeau DA, Doyle-Thomas KA, Dupuis A, Iaboni A, Crosbie J, McGinn H, et al. Examining and comparing social perception abilities across childhood-onset neurodevelopmental disorders. J Am Acad Child Adolesc Psychiatry. 2015;54:479–86. doi: 10.1016/j.jaac.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–51. [PubMed] [Google Scholar]
- 41.Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatr. 2003;160:1768–74. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- 43.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1263. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Tobe RH, Bansal R, Xu D, Hao X, Liu J, Sanchez J, et al. Cerebellar morphology in Tourette syndrome and obsessive-compulsive disorder. Ann Neurol. 2010;67:479–87. doi: 10.1002/ana.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;4:853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 46.Nabeyama M, Nakagawa A, Yoshiura T, Nakao T, Nakatani E, Togao O, et al. Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res: Neuroimaging. 2008;163:236–47. doi: 10.1016/j.pscychresns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Tolin DF, Kiehl KA, Worhunsky P, Book GA, Maltby N. An exploratory study of the neural mechanisms of decision making in compulsive hoarding. Psychol Med. 2009;39:325–36. doi: 10.1017/S0033291708003371. [DOI] [PubMed] [Google Scholar]
- 48.Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, et al. Grey matter abnormalities in obsessive-compulsive disorder - statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–4. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- 49.Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–30. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 50.Blair J, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 51.Sokolovsky N, Cook A, Hunt H, Giunti P, Cipolotti L. A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav Neurol. 2010;23:17–29. doi: 10.3233/BEN-2010-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Harskamp NJ, Rudge P, Cipolotti L. Cognitive and social impairments in patients with superficial siderosis. Brain. 2005;128:1082–92. doi: 10.1093/brain/awh487. [DOI] [PubMed] [Google Scholar]
- 53.Garrard P, Martin NH, Giunti P, Cipolotti L. Cognitive and social cognitive functioning in spinocerebellar ataxia : a preliminary characterization. J Neurol. 2008;255:398–405. doi: 10.1007/s00415-008-0680-6. [DOI] [PubMed] [Google Scholar]
- 54.Adamaszek M, D'Agata F, Kirkby KC, Trenner MU, Sehm B, Steele CJ, et al. Impairment of emotional facial expression and prosody discrimination due to ischemic cerebellar lesions. Cerebellum. 2014;13:338–45. doi: 10.1007/s12311-013-0537-0. [DOI] [PubMed] [Google Scholar]
- 55.Bowers D, Blonder LX, Heiman KM. The role of the right hemisphere in emotional communication. Brain. 1991;114:1115–27. doi: 10.1093/brain/114.3.1115. [DOI] [PubMed] [Google Scholar]
- 56.D’Agata F, Caroppo P, Baudino B, Caglio M, Croce M, Bergui M, et al. The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum. 2011;10:600–10. doi: 10.1007/s12311-011-0276-z. [DOI] [PubMed] [Google Scholar]
- 57.Tamietto M, Adenzato M, Geminiani G, de Gelder B. Fast recognition of social emotions takes the whole brain: interhemispheric cooperation in the absence of cerebral asymmetry. Neuropsychologia. 2007;45(4):836–43. doi: 10.1016/j.neuropsychologia.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Golan O, Sinai-Gavrilov Y, Baron-Cohen S. The Cambridge Mindreading Face-Voice Battery for Children (CAM-C): complex emotion recognition in children with and without autism spectrum conditions. Mol Autism. 2015;6:22. doi: 10.1186/s13229-015-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6:248–54. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 60.Boraston Z, Blakemore SJ, Skuse D. Impaired sadness recognition is linked to social interaction deficit in autism. Neuropsychologia. 2007;45:1501. doi: 10.1016/j.neuropsychologia.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 63.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–16. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 64.Koeppen AH. In: The cerebellum and its disorders. Manto MU, Pandolfo M, editors. Cambridge: Cambridge University Press; 2002. pp. 387–406. [Google Scholar]
- 65.Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders. New York: Springer; 2013. [Google Scholar]
- 66.Lin DJ, Hermann KL, Schmahmann JD. Multiple system atrophy of the cerebellar type: Clinical state of the art. Mov Disord. 2014;29:294–304. doi: 10.1002/mds.25847. [DOI] [PubMed] [Google Scholar]
- 67.Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rüb U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- 68.Harkness K, Sabbagh M, Jacobson Jrey N, Chen T. Enhanced accuracy of mental state decoding in dysphoric college students. Cognition emotion. 2010;19:999–1025. [Google Scholar]
- 69.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 70.Dunlop WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–77. [Google Scholar]
- 71.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. http://www.R-project.org/ [Google Scholar]
- 72.Bates D, Maechler M, Bolker B, Walker S. _lme4: Linear mixed-effects models using Eigen and S4_. R package version 2014. 2014;1:1–7. http://CRAN.R-project.org/package=lme4. [Google Scholar]
- 73.Kuznetsova A, Brockhoff P, Christensen HB. lmerTest: Tests for random and fixed effects for linear mixed effect models. R package version. 2014:2.0–11. http://CRAN.R-project.org/package=lmerTest.
- 74.Hothorn T, Bretz F, Westfall P. Simultaneous Inference in General Parametric Models. Biometrical Journal. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 75.Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. 2011;31:3795–804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baumann O, Mattingley JB. Functional topography of primary emotion processing in the human cerebellum. Neuroimage. 2012;61:805–11. doi: 10.1016/j.neuroimage.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 77.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 78.Smith ML, Cottrell GW, Gosselin F, Schyns PG. Transmitting and Decoding Facial Expressions. Psychol Science. 2005;16:184–189. doi: 10.1111/j.0956-7976.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 79.Evers K, Kerkhof I, Steyaert J, Noens I, Wagemans J. No differences in emotion recognition strategies in children with autism spectrum disorder: evidence from hybrid faces. Autism Res Treat. 2014;2014:345878. doi: 10.1155/2014/345878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith NV, Hermlein B, Tsimpli IM. Dissociation of social affect and theory of mind in a case of Asperger’s syndrome. UCL Working Papers in Linguistics. 2003;15:357–77. [Google Scholar]
- 81.Calder AJ, Ewbank M, Passamonti L. Personality influences the neural responses to viewing facial expressions of emotion. Philos Trans R Soc Lond B Biol Sci. 2011;366:1684–701. doi: 10.1098/rstb.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 83.Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Ito K, et al. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;4:779–83. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- 84.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;15:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 85.Brossard-Racine M, du Plessis AJ, Limperopoulos C. Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum. 2015;14:151–64. doi: 10.1007/s12311-014-0597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoche F, Frankenberg E, Rambow J, Theis M, Harding JA, Qirshi M, et al. Cognitive phenotype in ataxia-telangiectasia. Pediatr Neurol. 2014;51:297–310. doi: 10.1016/j.pediatrneurol.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 87.Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS One. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holt RJ, Chura LR, Lai MC, Suckling J, von dem Hagen E, Calder AJ, et al. Reading the Mind in the Eyes': an fMRI study of adolescents with autism and their siblings. Psychol Med. 2014;44:3215–27. doi: 10.1017/S0033291714000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loukusa S, Mäkinen L, Kuusikko-Gauffin S, Ebeling H, Moilanen I. Theory of mind and emotion recognition skills in children with specific language impairment, autism spectrum disorder and typical development: group differences and connection to knowledge of grammatical morphology, word-finding abilities and verbal working memory. Int J Lang Commun Disord. 2014;49:498–507. doi: 10.1111/1460-6984.12091. [DOI] [PubMed] [Google Scholar]
- 90.Eddy CM, Sira Mahalingappa S, Rickards HE. Is Huntington's disease associated with deficits in theory of mind? Acta Neurol Scand. 2012;126:376–83. doi: 10.1111/j.1600-0404.2012.01659.x. [DOI] [PubMed] [Google Scholar]
- 91.Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, Mostofsky SH. Behavioural and neural basis of anomalous motor learning in children with autism. Brain. 2015;138:784–97. doi: 10.1093/brain/awu394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirjak D, Wolf RC, Kubera KM, Stieltjes B, Maier-Hein KH, Thomann PA. Neurological soft signs in recent-onset schizophrenia: Focus on the cerebellum. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:18–25. doi: 10.1016/j.pnpbp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 93.Rüb U, Hoche F, Brunt ER, Heinsen H, Seidel K, Del Turco D, et al. Degeneration of the cerebellum in Huntington's disease (HD): possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol. 2013;23:165–77. doi: 10.1111/j.1750-3639.2012.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–51. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- 95.Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–62. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 96.Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Hum Brain Mapp. 2007;28:915–30. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 98.Byom LJ, Mutlu B. Theory of mind: mechanisms, methods, and new directions. Front Hum Neurosci. 2013;7:413. doi: 10.3389/fnhum.2013.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 100.Siegal M, Varley R. Neural systems involved in “theory of mind”. Nat Rev Neurosci. 2002;3:436–71. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- 101.Stoodley CJ, Desmond JE, Schmahmann JD. Functional topography of the human cerebellum revealed by functional neuroimaging studies. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders. New York: Springer; 2013. pp. 735–765. [Google Scholar]
- 102.Schmahmann JD. The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. Int Rev Psychiatr. 2001;13:247–260. [Google Scholar]


