Abstract
Central noradrenergic (NA) signaling contributes critically to multiple behavioral effects of cocaine administration, particularly stress- and anxiety-related effects. The present study examined the ability of acute cocaine to induce the immediate early gene product, cFos, in NA neurons and stress-related neural circuits in rats that were cocaine-naïve, or had a history of cocaine self-administration with or without extinction. Rats implanted with jugular catheters were trained to self-administer cocaine (0.5 mg/kg/infusion), with a subset subsequently trained on extinction. Cocaine-naïve controls were handled daily. After a final day of self-administration, extinction, or handling, rats received an i.p. injection of either cocaine (20 mg/kg) or saline, and 90 minutes later were anesthestized and perfused. Tissue sections were processed for immunoperoxidase labeling of nuclear cFos with either immunoperoxidase or immunofluorescent cytoplasmic labeling of dopamine beta hydroxylase or tyrosine hydroxylase. Acute cocaine increased the number of activated NA neurons within the caudal nucleus of the solitary tract (NTS; A2 cell group) in cocaine-naïve and extinguished rats, but not in rats that only self-administered. Extinction attenuated cocaine-induced cFos activation in NA neurons of the caudal ventrolateral medulla (A1/C1 cell groups), and attenuated cFos within the paraventricular nucleus of the hypothalamus, the apex of the central neuroendocrine stress axis. Cocaine consistently increased cFos in the bed nucleus of the stria terminalis, regardless of history. NA neurons of the locus coeruleus (A6 cell group) were not activated after cocaine in any experimental group. Thus, the ability of cocaine to activate central stress circuitry is altered after cocaine self-administration. Our results suggest a unique role for the NTS in cocaine-induced reinstatement, as extinction training enhanced the ability of cocaine to activate NA neurons within this region. These findings suggest central NA systems originating in the caudal brainstem as potential targets for the treatment of cocaine addiction.
Cocaine addiction is a major health and social problem that affects millions of individuals. Cocaine blocks monoaminergic reuptake transporters (1) which leads to increased monoaminergic synaptic transmission (2, 3). Although cocaine increases transmission of all monoamines, investigations into the effects of cocaine on brain activity have classically focused on mesolimbic and mesocortical dopaminergic systems. More recent studies have revealed an important role for noradrenergic (NA) systems in mediating the behavioral effects of cocaine exposure [cf. (4, 5)]. For example, NA enzymes, transporters, and receptors are critical for cocaine-induced locomotion and sensitization (6), and manipulations of the NA system alter the ability of cocaine to support conditioned place preference (7) or aversion (8). Increased NA transmission has been identified as a significant contributor to aversive and stressful effects of acute cocaine administration (9), and to anxiety produced by cocaine withdrawal (10). Further, decreased NA transmission can interfere with cocaine self-administration (11), while increases can enhance such behavior (12). A role for NA transmission in reinstatement of cocaine-seeking behavior after extinction has been particularly well documented. Central injections of norepinephrine elicit reinstatement of cocaine-seeking behavior (13), and decreased NA signaling via adrenergic receptor blockade attenuates stress-induced (14) or cocaine-primed reinstatement (15). Inhibition of dopamine-beta hydroxylase (DbH, the rate-limiting enzyme for NA synthesis) decreases multiple forms of reinstatement (11), while selective inhibition of NA reuptake decreases cocaine-seeking behavior triggered by exposure to cocaine-paired cues (16).
Despite strong evidence that NA participates in the effects of acute cocaine, cocaine self-administration, and reinstatement of cocaine-seeking, little is known regarding how cocaine affects NA neurons in either cocaine-naïve animals, or animals with a history of cocaine self-administration and extinction. Previous reports indicate that acutely administered cocaine increases cFos, a marker of neuronal activation, within the nucleus of the solitary tract (NTS) and locus coeruleus (LC) (17, 18). However, the phenotypic identity of activated neurons has not been reported. The present study sought to elucidate the effect of acute cocaine on medullary and pontine NA cell groups, and in two primary projection targets of medullary NA neurons (19-22), i.e., the paraventricular nucleus of the hypothalamus (PVN), and the anterior ventral lateral subdivision of the bed nucleus of the stria terminalis (vlBNST). We also examined how a history of cocaine self-administration and extinction might alter the ability of acute cocaine to activate neurons in these regions.
2.0 Materials and Methods
2.1 Subjects
Male Sprague-Dawley rats (Harlan, Indianapolis) weighing 225-250g upon arrival were individually housed in a humidity- and temperature-controlled (21-22° C) vivarium on a reversed light-dark cycle (lights off 07:00 hours, on 19:00 hours) with unlimited access to water. Rats received 20g of food daily between the hours of 14:00-16:00. Training and experimental sessions were conducted during the dark phase at the same time each day (09:00-15:00) and perfusions were performed between 11:00-14:00. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and under local IACUC approval.
2.2 Surgery
A control group of rats (n=14) was handled daily for 5 days before acute cocaine or vehicle injection. A second group (n=29) was anesthetized with isoflurane and implanted with jugular catheters as described previously (23). These rats were allowed at least 7 days to recover from surgery. For the first 2 weeks after surgery, catheters were flushed daily with 0.1 ml of sterile saline containing heparin (30units/ml), timentin (66.67mg/ml), and streptokinase (9333 U/ml) to maintain catheter patency and prevent infection. Thereafter, catheters were flushed with heparinized saline before and after each experimental session throughout the cocaine self-administration experiments.
2.3 Self-administration training
Self-administration and extinction training was conducted in operant conditioning chambers within sound-attenuating, ventilated cubicles (Med Associates, St. Albans, VT, USA). The chambers were equipped with two retractable response levers on one side panel, with a 28-V white light above each lever, and a red house light near the chamber ceiling. Intravenous cocaine infusions were delivered via syringe pump controlled by a drug delivery system (Med Associates). Experimental events and data collection were automatically controlled by interfaced computer software (Med Associates).
After recovery from surgery, rats were trained to intravenously self-administer cocaine on a fixed-ratio 1 (FR1) schedule in operant chambers. Daily self-administration sessions were initiated by the extension of two levers into the chamber and illumination of the red house light. During each 2 hr session, each active lever press resulted in an infusion of cocaine (0.2 mg/kg/50ul infusion) along with presentation of a 5-sec light-tone stimulus complex, followed by a 20-sec timeout period during which active lever responses were recorded but resulted in no programmed consequences. Inactive lever presses were always recorded but had no programmed consequences. Rats received 12 2-hr cocaine self-administration sessions (one session per day), during which all rats achieved a criterion of receiving least 10 infusions of cocaine.
2.4 Extinction training
After completing the self-administration phase, a subset of rats (n=13) was subjected to daily extinction sessions. Extinction sessions were initiated by extension of the two levers into the chamber and illumination of the red house light. Sessions lasted 2 hr, during which active and inactive lever presses were recorded but resulted in no programmed consequences. All rats received 8 days of extinction training, during which responding decreased to <20% of responding recorded during the final 3 days of cocaine self-administration.
2.5 Treatment-induced cFos induction
After 5 days of daily handling (controls, n=14), 12 days of cocaine self-administration without extinction (n=16), or 12 days of self-administration followed by 8 days of extinction (n=13), rats were injected with either cocaine (20 mg/kg, i.p.) or saline, then returned to their home cage for 90-120 minutes before perfusion. This post-treatment survival time is similar to that used in previous studies examining the effects of acute cocaine on cFos induction (24, 25). Perfusion and tissue collection have been previously described (26). Briefly, rats were deeply anesthetized with sodium pentobarbital (Nembutal, 10 mg/kg BW i.p.) and transcardially perfused using aqueous 0.15M NaCl for 1 min followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) for 10 min. Fixed brains were removed from the skull, blocked, frozen, and sectioned coronally (35 μm) on a freezing-stage microtome. Sections from the upper cervical spinal cord through the rostral corpus callosum were collected in six adjacent sets and stored in cryopreservant solution (27) at −20°C prior to processing for immunohistochemistry.
2.6 Immunohistochemistry
One set of tissue sections from each rat (i.e., representing brain regions sampled at 210 μM intervals) was processed for dual immunoperoxidase localization of nuclear cFos and cytoplasmic DbH, as previously described (26), the latter to identify NA neurons and fibers. DbH immunolabeling also was used as a guide to standardize selection of anatomical regions and subnuclei for quantitative analyses. A second set of sections was processed for immunoperoxidase localization of cFos followed by immunofluorescent localization of cytoplasmic tyrosine hydroxylase (TH) to identify both NA neurons within the LC. This procedure was used to obviate the problem of overly dark DbH peroxidase staining within the LC, that can impede visualization of nuclear cFos immunoperoxidase labeling (see Results). cFos protein was localized using a rabbit antiserum provided by Dr. Phillip Larsen, Denmark (1:50K). The specificity of this antibody has been reported (28). Tissue sections were processed as previously described (21) and reacted with nickel-intensified diaminobenzidine (DAB) to produce a blue-black nuclear peroxidase label for cFos protein. DbH was subsequently localized in cFos-reacted tissue sections using a mouse monoclonal antibody (1:30K; Millipore, AB1585) as previously described (21). Sections were reacted using plain DAB to produce a brown cytoplasmic peroxidase label. An alternate set of cFos-reacted tissue sections was labeled using a monoclonal antibody raised against TH (1:5K, Sigma-Aldrich, T2928), followed by donkey anti-mouse IgG conjugated to Dylight-488; Jackson ImmunoResearch, 1:300). Double-labeled tissue sections were then rinsed and mounted, dehydrated in a graded series of ethanols, defatted in xylene, and placed under coverslips using Cytoseal (Richard Allan Scientific).
2.7 Quantification
Cell counting in double-labeled tissue sections was performed as previously described to document the extent of treatment-induced cFos immunoreactivity in the NTS, caudal ventrolateral medulla (VLM), LC, PVN, and anterior vlBNST (26). Anatomical landmarks were defined with reference to the atlas of Paxinos and Watson (29), with sampling regions further standardized across animals based on the subregional distribution of DbH immunolabeling. DbH-positive neurons exhibited a brown cytoplasm with a clearly defined nucleus, and were classified as cFos-positive if the nucleus contained visible blue-black labeling, independent of intensity. Alternate sections labeled for TH immunofluorescence were analyzed to identify any NA neurons within the LC that displayed cFos-positive nuclei.
Each area of interest was sampled bilaterally in sections spaced by 210 μM. The NTS and VLM were partitioned along their rostral-caudal extent, based on previous evidence for rostrocaudal differences in NA activation within the NTS and VLM in rats after stressful or rewarding stimuli (30, 31). For this purpose, data were collected and averaged for the caudal NTS/VLM (i.e., 5-6 tissue sections per rat located caudal to the area postrema, AP), mid NTS/VLM (i.e., 3-4 sections per rat through the level of AP), and rostral NTS/VLM (i.e., approximately 3 sections per rat located rostral to the AP but caudal to the level at which the NTS pulls away from the walls of the 4th ventricle). cFos-positive cells within the PVN and anterior vlBNST were counted bilaterally in 2-3 sections per region, with counts standardized to include only the subregions that are most densely innerved by DbH-positive NA fibers (i.e., see Fig. 2A, C). For each brain region, the total number of neurons counted was divided by the number of sections in which counts were made in order to derive the average number of immunopositive cells per section for each rat.
Figure 2.
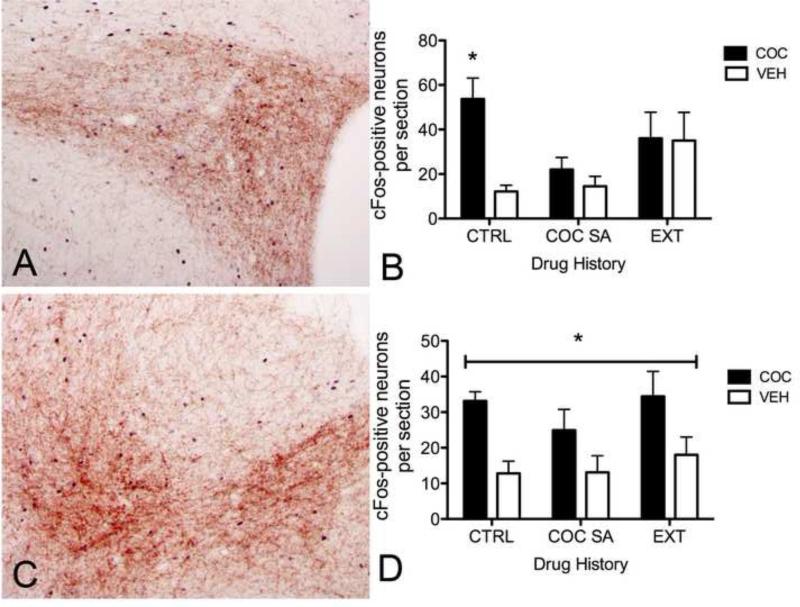
Photographic images (A, C) and summary data (B, D) demonstrating cocaine-induced cFos (black nuclear label) within the PVN and anterior vlBNST. Cocaine-induced cFos activation in the PVN in a cocaine-naïve (A) and anterior vlBNST (C) is present within regions that are densely innervated by DbH-positive NA fibers (brown label). PVN immunolabeling in (A) is from a rat with a history of cocaine self-administration, vlBNST immunolabeling in (C) is from a rat with a history of control handling. Summary data (B, PVN; D, BNST) show the average number of activated neurons per section after acute saline vehicle (VEH; white bars) or cocaine (COC; black bars) injections in rats with a history of control handling (CTRL), cocaine self-administration (COC SA), or self-administration and extinction (EXT). In panel B, * indicates cocaine-induced cFos that is significantly greater than cFos after saline vehicle in control handled rats. In panel D, * indicates cocaine-induced cFos that is significantly greater than cFos after saline vehicle in all three drug history groups.
2.8 Statistical analysis
The number of infusions, active lever responses during extinction, and cell count data are presented as group means ± SEM. The number of cocaine infusions received by rats during the last 2 days of self-administration was analyzed using two-way ANOVA to ensure equivalent drug exposure in rats subsequently divided into different extinction groups. Terminal responding during the last 2 days of extinction was compared using a t-test to ensure equivalent levels of responding in rats that received a final injection of cocaine vs. vehicle before perfusion fixation. Cell count data from the NTS and VLM were initially analyzed using three-way ANOVAs to determine effects of drug history, acute injection, and rostral-caudal level on NA activation. Since the NTS and VLM caudal to the AP displayed minimal cFos and DbH double-labeling (see Results), a subsequent analysis eliminated the most caudal NTS and VLM counts (i.e., caudal to the AP) and collapsed the data for sections through and rostral to the AP. Two-way ANOVAs were then performed to further examine main effects of (and interactions between) drug injection and history on cFos activation within the NTS and VLM. Cell counts in the PVN and anterior vlBNST were similarly analyzed using two-way ANOVA. Significant main effects and interactions were further investigated using Bonferroni post-hoc tests. Correlational analyses were performed to evaluate potential within-subjects relationships between cocaine-induced cFos activation in the NTS, VLM, PVN, and BNST, and whether these relationships were altered by cocaine history. Differences were considered statistically significant when p<0.05. Tissue sections from one rat were excluded from analysis because count data from this rat were >3 standard deviations from the group mean.
3.0 Results
3.1 Self-administration and extinction
All animals readily acquired cocaine self-administration and maintained stable levels of responding throughout the maintenance phase of the experiment. The number of infusions received by rats during maintenance of self-administration was statistically similar between groups that subsequently were or were not exposed to extinction training (F(1,24)=0.53, p>0.05), and final acute cocaine/saline injections (F(1,24)=2.23, p>0.05), with no interactions (F(1,24)=0.03, p>0.05). Thus, cocaine intake was similar between groups before extinction and/or acute cocaine or saline injections (Table 1).
Table 1.
Infusions during self-administration (top rows) and Active Lever Responding (bottom rows) in rats that received saline (left column) or cocaine (middle column). There were no significant differences between groups as noted by p-values (right column).
| SELF-ADMINISTRATION INFUSIONS (Days 11-12) | SALINE | COCAINE | |
|---|---|---|---|
| Drug Naïve | n/a | n/a | |
| Self-administration | 42.7±9.8 | 48.9±7.6 | P=0.4 |
| Self-administration and extinction | 39.5±7.8 | 47.4±7.4 | P=0.3 |
| EXTINCTION ACTIVE LEVER RESPONDING (Days 7-8) | |||
| Drug Naive | n/a | n/a | |
| Self-administration | n/a | n/a | |
| Self-administration and extinction | 8.8±2.7 | 7.4±0.9 | P=0.6 |
Rats decreased active lever responding during extinction, when cocaine infusion and cue presentations were removed. Comparison of active lever responding prior to the final acute injection of cocaine or saline (i.e., before perfusion) revealed no significant differences between groups (t(11)=0.53, p>0.05), evidence that animals were drug-seeking to a similar degree before terminal injection and perfusion (Table 1).
3.2 Central cFos immunolabeling
DbH-positive NA neurons at different rostrocaudal levels of the NTS and VLM were differentially activated to express cFos, as evidenced by a main effect of rostrocaudal level (F(2,37)=34.63, p<0.0001). NA neurons located within the most caudal levels of the NTS and VLM displayed minimal activation, regardless of final acute injection or cocaine/extinction history. Conversely, acute cocaine treatment significantly increased NA neuronal activation within the NTS and VLM at the rostrocaudal level of the AP and just rostral to it (Fig. 1A, C). Thus, subsequent analyses were conducted on collapsed data obtained from tissue sections through the AP and rostral to it, eliminating the more caudal sections.
Figure 1.
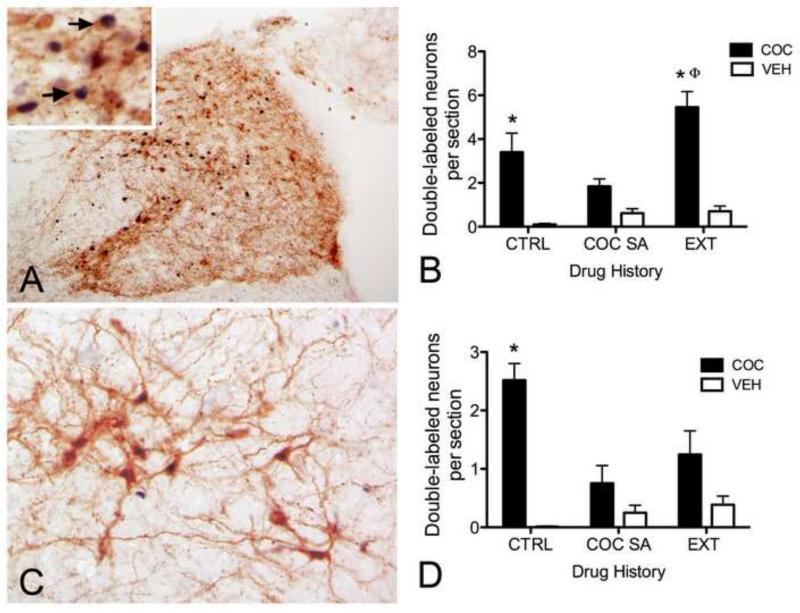
Photographic images (A, C) and summary data (B, D) demonstrating treatment-induced cFos (black nuclear label) in NA neurons (brown cytoplasmic label) within the NTS (A) and VLM (C) just rostral to the level of the AP. NTS immunolabeling (A) is from a representative cocaine-injected rat (20 mg/kg, i.p.) with a history of cocaine self-administration and extinction. VLM immunolabeling (C) is from a control handled rat after acute cocaine injection (20 mg/kg, i.p.). Inset in A: arrows indicate a few activated DbH-positive NTS neurons. Activated DbH-positive VLM neurons are evident in C. Summary data (B, NTS; D, VLM) show the average number of activated neurons per section after acute saline vehicle (VEH; white bars) or cocaine (COC; black bars) injections in rats with a history of control handling (CTRL), cocaine self-administration (COC SA), or self-administration and extinction (EXT). In panels B and D, * indicates cocaine-induced cFos that is significantly greater than cFos after saline vehicle in control handled (B and D) or extinguished rats (D); Φ indicates cocaine-induced cFos that is significantly greater in extinguished rats than cocaine-induced cFos in control handled rats.
3.2.1 NTS
There was a significant interaction between prior self-administration/extinction history and acute cocaine/saline injection on activation of NA neurons within the NTS (F(2,36)=4.45, p<0.02). There was a significant main effect of acute treatment, such that cocaine significantly increased the number of NTS neurons that were double-labeled for cFos and DbH (F(1,36)=46.85, p<0.0001). There also was a significant main effect of prior history, with significantly more NTS NA neurons in extinguished rats expressing cFos than the other two groups (F(2,36)=6.13, p<0.005). Bonferroni post-hoc tests revealed that, compared to acute saline, cocaine increased the number of cFos-positive NA neurons in the NTS only in control cocaine naïve (p<0.01) and extinguished (p<0.001) groups (Figure 1B). Further, cocaine-induced activation of NTS NA neurons was greater in rats that experienced extinction training compared to activation in handled controls (p<0.01). There was no significant effect of history and/or injection on the total number of DbH-positive NA neurons counted within the NTS (data not shown).
3.2.2 VLM
There was a significant interaction between prior history and acute cocaine/vehicle injection on NA activation within the VLM (F(2,36)=7.17, p<0.002), as well as a significant main effect of acute cocaine treatment to increase the number of VLM neurons that were double-labeled for cFos and DbH (F(1,36)=28.93, p<0.0001). However, there was no significant main effect of prior history on VLM neuronal activation (F(2,36)=3.10, p=0.056). Bonferroni post-hoc tests revealed that, compared to the effects of saline injection, cocaine increased the number of cFos-positive NA neurons within the VLM only in cocaine naïve rats (p<0.001, Figure 1D). Further, after acute cocaine treatment, significantly more NA VLM neurons were activated to express cFos in naïve rats compared to rats with a history of self-administration (p<0.001) or extinction training (p<0.05). There was no significant effect of history and/or injection on the total number of DbH-positive NA neurons counted within the VLM (data not shown).
3.3.3 LC
No cFos labeling was observed within the LC, regardless of rats’ prior history or final acute treatment. The lack of cFos labeling included immunoperoxidase-labeled DbH-positive LC neurons (A6 cell group) as well as the much smaller number of non-DbH-positive neurons. The LC comprises a densely-packed population of DbH-positive NA neurons, which impairs cFos visualization in tissue sections reacted for dual immunoperoxidase localizations. To ensure that this did not contribute to the perceived absence of double-labeled LC neurons, an alternate set of tissue sections was processed for cFos immunoperoxidase followed by immunofluorescent localization of TH. Microscopic analysis of these tissue sets confirmed the virtual absence of cFos immunolabeling among TH-positive LC neurons in rats from all experimental treatment groups (not shown).
3.3.4 PVN
There was a significant interaction between prior history and acute injection on cFos activation within the PVN (F(2,37)=3.46, p<0.05) (Fig. 2A,B). There also was a significant main effect of acute injection, such that cocaine increased cFos immunolabeling in the PVN above levels measured in rats after acute saline (F(1,37)=6.19, p<0.02). The interaction effect was due to cocaine-induced PVN cFos activation only in control rats that were cocaine-naïve (Fig. 2B). Rats with a history of self-administration plus extinction displayed a trend towards more cocaine and saline-activated PVN neurons compared to rats in other groups, but this effect did not reach significance (F(2,37)=3.46, p=0.08).
3.3.5 Anterior vlBNST
There was no significant interaction between prior history and acute injection on cFos activation within the BNST (F(2,37)=0.36, p>0.05) (Fig. 2C). Compared to acute saline injection, acute cocaine produced a significant increase in cFos immunolabeling within the anterior vlBNST (F(1,37)=14.81, p<0.001), with no main effect of prior self-administration/extinction history (F(2,37)=1.0, p>0.05) (Fig. 2D).
3.3.6 Correlational analyses
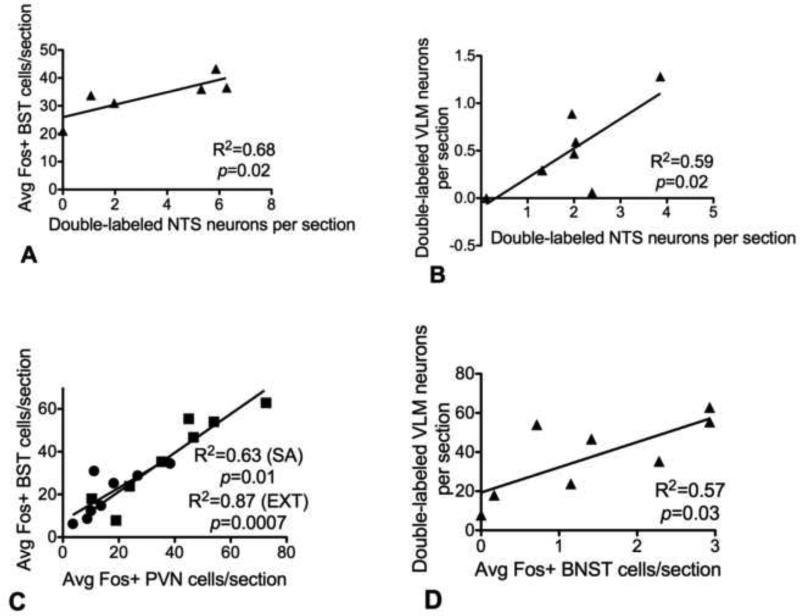
Correlational analyses of cocaine-induced cFos across analyzed brain regions in previously cocaine-naïve rats revealed a significant positive correlation between activation of DbH-positive NTS neurons and activation of anterior vlBNST neurons (R2=0.68, p<0.05, Table 2), with no other significant correlations. In rats with a history of cocaine self-administration, there was a significant positive correlation between activation of DbH-positive NTS and VLM (R2=0.59, p<0.05), and between cFos activation within the PVN and anterior vlBST (R2=0.63, p<0.01). Activation of DbH-positive NTS neurons was no longer correlated with cFos activation in any other region in rats with a history of cocaine self-administration and extinction. However, activation within the PVN and anterior vlBNST remained significantly positively correlated (R2=0.93, p<0.001). Activation of DbH-positive VLM neurons was correlated with cFos activation within the anterior vlBNST (R2=0.57, p<0.05). This group also displayed a non-significant trend for correlated cFos activation between the VLM and PVN (R2=0.48, p=0.058).
Table 2.
Correlations (R2 - top number, p values – bottom number) between cocaine-induced cFos activation of DbH-positive NA neurons (NTS, VLM) or activation in NA projection targets (PVN, anterior vlBNST).
| COCAINE-NAIVE | SELF-ADMIN | EXTINCTION | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NTS | VLM | PVN | NTS | VLM | PVN | NTS | VLM | PVN | |
| NTS | n/a | 0.50 0.07 |
0.004 0.21 |
n/a |
0.59
0.02* |
0.01 0.78 |
n/a | 0.009 0.82 |
0.002 0.91 |
| VLM | 0.50 0.07 |
n/a | 0.04 0.67 |
0.59
0.02* |
n/a | 0.02 0.73 |
0.009 0.82 |
n/a | 0.48 0.06 |
| PVN | 0.004 0.21 |
0.04 0.67 |
n/a | 0.01 0.78 |
0.02 0.73 |
n/a | 0.002 0.91 |
0.48 0.06 |
n/a |
| BST |
0.68
0.02* |
0.54 0.09 |
0.47 0.13 |
0.02 0.73 |
0.04 0.62 |
0.63
0.01* |
0.019 0.74 |
0.57
0.03* |
0.87
0.0007* |
(bold) indicates statistically significant positive correlation
4.0 Discussion
Previous studies have demonstrated that acute cocaine increases neuronal expression of cFos in multiple brain regions, including those examined here (17, 24, 32). However, those experiments did not examine cFos in neurochemically identified cell populations or in their primary projection targets. This is the first study, to our knowledge, to report cocaine-induced cFos in NA hindbrain neurons. Further, while data exist regarding how the route of administration (i.e., self- or experimenter-administered) alters neuronal responses to cocaine (18), the present study is unique in several respects. First, we used a stimulus (i.e., acute noncontingent cocaine injection) that elicits reinstatement in cocaine-trained rats, and we kept that stimulus consistent across groups. Second, we examined the ability of this stimulus to increase cFos expression in rats after extinction training, which is the period most relevant to reinstatement. While many studies have examined central induction of cFos protein in multiple brain regions in rats during reinstatement (33-36), cFos expression in those studies could be due to the presented stimulus (stress, drug-paired cues, or drug prime), or to the drug-paired context in which the animals lever press, or to the lever press behavior itself. In order to avoid these confounds, we examined cFos in response to the reinstatement stimulus alone (i.e., acute cocaine injection) in the absence of other factors.
Our results demonstrate that acute cocaine activates regions of the brain most often associated with stress-related behavior in cocaine-naïve rats, specifically NA neurons of the caudal brainstem, the PVN, and the anterior vlBNST. Cocaine-induced activation of NA neurons was specific to the NTS and VLM. The most caudal levels of the NTS and VLM (i.e., the caudal A2 and A1 cell groups, respectively) were less sensitive to acute cocaine compared to more rostral levels (i.e., the A2/C2 and A1/C1 cell groups), regardless of cocaine history. The A6 NA cell group within the LC was not activated to express cFos in rats after cocaine treatment, regardless of drug history, consistent with prior evidence that direct manipulation of NA neurons within the LC has no effect on reinstatement of cocaine-seeking behavior (37).
4.1 NTS and VLM
The ability of cocaine to activate NA neurons within the NTS and VLM was altered by a prior history of cocaine self-administration and extinction. A history of self-administration without extinction suppressed the ability of acute cocaine injection to activate NA neurons within both the NTS and VLM, whereas extinction training increased the ability of acute cocaine to activate NA neurons within the NTS but not VLM above levels measured in drug-naïve controls. These findings suggest a previously unidentified participation of hindbrain NA cell groups in the ability of prior drug history to alter sensitivity to acute cocaine administration, with potential relevance to cocaine-induced reinstatement behavior.
4.2 PVN and BNST
The ability of acute cocaine to activate cFos within the PVN was attenuated in rats with a prior history of cocaine self-administration, with or without extinction, whereas prior cocaine history did not alter acute cocaine-induced cFos within the anterior vlBNST. Thus, enhanced activation of NTS neurons after extinction, which may play a role in reinstatement, is likely not due to enhanced NTS recruitment by activated PVN or BNST neurons, as the latter regions did not display enhanced activation after extinction. This is consistent with previous reports that the BNST and PVN are unnecessary for cocaine-induced reinstatement (38, 39).
4.3 Correlational analyses
The relationship between cocaine-induced cFos activation across the four brain regions of interest (NTS, VLM, PVN, and BNST) was altered by previous history with cocaine self-administration and extinction training. In cocaine-naïve rats, the only significant relationship was a positive correlation between activation of NA neurons in the NTS and neurons in the anterior vlBNST. Thus, in cocaine-naïve rats, NTS activation might contribute to activation of the anterior vlBNST, which could drive the acute effects of cocaine to increase aversive responses and anxiety-like behavior (40). However, this relationship was no longer significant in rats with a history of cocaine self-administration, consistent with a previous report that rats with a history of cocaine self-administration display less cocaine-induced anxiety (41). Conversely, rats with a history of cocaine self-administration displayed a positive correlation between activation of NA neurons within the NTS and VLM. This may be driven, in part, by the diminished cocaine-induced activation of NA neurons in both regions after self-administration. Finally, in rats with a history of cocaine self-administration followed by extinction, activation of NA neurons within the NTS was no longer correlated with cFos activation in the other brain regions examined, consistent with the hypothesis that extinction training differentially and uniquely increases NTS neural activation compared to activation in other stress-related brain regions.
4.4 Functional implications of history-dependent recruitment of hindbrain NA neurons
NA neurons within the caudal NTS and VLM have been implicated in behavioral state regulation, modulation of stress responses, and affective learning [for review, see (42)]. NA signaling from the NTS and VLM to the extended amygdala, including the BNST, appears to be critical for generating negative affective states associated with opiate withdrawal (21, 43). Reduction of NA signaling via NA autoreceptor stimulation by systemic guanfacine, an alpha-2a receptor agonist, reduces anxiety-like behavior during early withdrawal from cocaine, and can attenuate subsequent reinstatement of cocaine-seeking behavior (10). Further, alpha-2 receptor agonists given on reinstatement test day can reduce stress- and cue-induced reinstatement (44), and NA signaling within the BNST participates in stress-induced reinstatement of cocaine-seeking (14). Lesions of ascending NA fibers also block stress-induced reinstatement of heroin-seeking (37). While a specific role for NA NTS or VLM neurons in reinstatement triggered by cocaine priming injections has not yet been demonstrated, systemic blockade of alpha-1 receptors blocks cocaine primed reinstatement (15), and footshock stress (which increases central NA signaling) potentiates cocaine-induced reinstatement of drug-seeking (45). Our results suggest that NA neurons within the NTS play a critical role in cocaine-primed reinstatement, given their uniquely enhanced sensitivity to cocaine injection after extinction training. Additional studies employing direct manipulation of NA signaling from the NTS will be necessary to confirm a functional role in cocaine reinstatement.
Previous studies have reported differential activation of caudal and rostral portions of NTS by psychological and physical stressors as well as rewarding stimuli (sweetened milk) (30, 31). While physiological stressors such as immune challenge or hemorrhage preferentially increase cFos activation in more rostral NA neurons of the NTS (i.e., A2/C2 neurons), psychological stressors such as restraint or forced swim preferentially activate NA neurons in more caudal NTS regions (i.e., caudal A2 cell group) (31). Our regional analysis revealed that the effect of acute cocaine treatment was more pronounced in rostral vs. caudal levels of the NTS and VLM, similar to activation patterns associated with other physiological stressors. Thus, this activation may be related to the physiological stress associated with acute cocaine administration.
Cocaine-induced activation of the endocrine hypothalamic-pituitary-adrenal axis (HPA) stress axis has been reported in rats (46, 47) and humans (48), consistent with the significant cocaine-induced activation of PVN cFos expression in previously cocaine-naïve rats observed in the present study. Interestingly, however, a prior history of cocaine self-administration followed by extinction significantly attenuated the ability of acute cocaine treatment to activate PVN neurons, consistent with other reports that reinstatement behavior does not depend on the release of stress hormones (49). Tolerance to cocaine may contribute to the reduced sensitivity of VLM and PVN neurons in rats with a history of self-administration, similar to a previous report for the PVN (18). At the behavioral and neuronal level, both tolerance and sensitization to cocaine have been reported depending on cocaine dose, route of administration, and treatment regimen (18, 50-52). In the current study, a prior history of cocaine self-administration followed by extinction led to a nearly significant increase in “baseline” PVN cFos activation in rats injected acutely with saline (p=0.07). Indeed, previous findings demonstrate an increase in basal corticosterone levels in rats during cocaine withdrawal (53, 54). Although the effect of prior cocaine self-administration and extinction on baseline PVN cFos activation did not reach statistical significance, it might have masked an effect of acute cocaine treatment to further increase PVN cFos in extinguished rats. Similarly, clinical studies demonstrate that basal cortisol levels are elevated in alcohol-addicted human subjects, which may contribute to more rapid stress-induced relapse (55).
4.5 Conclusions
In summary, results from the present study demonstrate that a history of cocaine self-administration and extinction can alter the ability of a reinstatement stimulus (i.e., acute noncontingent cocaine injection) to elicit cFos in isolation from placement in the self-administration context or performance of the response behavior. As reinstatement aims to model relapse, and relapse is the largest challenge to the successful treatment of addiction, our new findings may offer insight into brain regions that play a potentially critical role in relapse. Further, these data suggest that NA projection pathways arising from neurons within the caudal medulla, rather than the pontine LC, may be an appropriate experimental target for the continued development of novel pharmacotherapies for addiction treatment.
Highlights.
Cocaine activates NA neurons of the nucleus of the solitary tract and ventrolateral medulla
Cocaine activation of NA neurons is altered by cocaine self-administration and extinction history
NA neurons of the nucleus of the solitary tract show enhanced activation after cocaine self-administration and extinction.
Figure 3.
Significant correlations between cocaine-induced cFos activation of DbH-positive NA neurons within the NTS (A, B) or VLM (D) and activation within NA projection targets in the PVN (C) or anterior vlBNST (A,C,D). A significant correlation between cocaine-induced activation of NA NTS neurons and neurons within the anterior vlBNST existed only in cocaine-naïve rats (A), while a significant correlation between cocaine-induced activation of NA neurons within the NTS and VLM was only present in rats after cocaine self-administration (B). A significant correlation between cocaine-induced activation of neurons within the PVN and anterior vlBNST was observed in rats with a history of cocaine self-administration (○) and/or extinction (□) (C). A significant correlation between cocaine-induced activation of NA VLM neurons and neurons within the anterior vlBNST also was present in rats after extinction (D).
Acknowledgments
Funding source
This work was supported by National Institutes of Health grant MH59911 (Linda Rinaman) and DA031787 (Deanne Buffalari).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pifl C, Drobny H, Reither H, Hornykiewicz O, Singer EA. Mechanism of the dopamine-releasing actions of amphetamine and cocaine: plasmalemmal dopamine transporter versus vesicular monoamine transporter. Mol Pharmacol. 1995;47:368–373. [PubMed] [Google Scholar]
- 2.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 3.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 4.Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 5.Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP. Critical role of alpha1- adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- 7.Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, et al. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- 8.Freeman KB, Verendeev A, Riley AL. Noradrenergic antagonism enhances the conditioned aversive effects of cocaine. Pharmacol Biochem Behav. 2008;88:523–532. doi: 10.1016/j.pbb.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- 10.Buffalari DM, Baldwin CK, See RE. Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2705-1. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder JP, Alisha Epps S, Grice TW, Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha BA. Stimulant and reinforcing effects of cocaine in monoamine transporter knockout mice. Eur J Pharmacol. 2003;479:107–115. doi: 10.1016/j.ejphar.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 13.Brown ZJ, Nobrega JN, Erb S. Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behav Brain Res. 217:472–476. doi: 10.1016/j.bbr.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XY, Kosten TA. Prazosin, an alpha-1 Adrenergic Antagonist, Reduces Cocaine-Induced Reinstatement of Drug-Seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69:266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Grabus SD, Glowa JR, Riley AL. Morphine- and cocaine-induced c-Fos levels in Lewis and Fischer rat strains. Brain Res. 2004;998:20–28. doi: 10.1016/j.brainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, et al. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology. 2010;35:445–463. doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKellar S, Loewy AD. Efferent projections of the A1 catecholamine cell group in the rat: an autoradiographic study. Brain Res. 1982;241:11–29. doi: 10.1016/0006-8993(82)91224-0. [DOI] [PubMed] [Google Scholar]
- 20.Woulfe JM, Flumerfelt BA, Hrycyshyn AW. Efferent connections of the A1 noradrenergic cell group: a DBH immunohistochemical and PHA-L anterograde tracing study. Exp Neurol. 1990;109:308–322. doi: 10.1016/s0014-4886(05)80022-6. [DOI] [PubMed] [Google Scholar]
- 21.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 22.Terenzi MG, Ingram CD. A combined immunocytochemical and retrograde tracing study of noradrenergic connections between the caudal medulla and bed nuclei of the stria terminalis. Brain Res. 1995;672:289–297. doi: 10.1016/0006-8993(94)01453-o. [DOI] [PubMed] [Google Scholar]
- 23.Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- 24.Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, et al. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology. 35:445–463. doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestler EJ. Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 28.Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Academic Press; New York: 1997. [Google Scholar]
- 30.Gaykema RP, Daniels TE, Shapiro NJ, Thacker GC, Park SM, Goehler LE. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res. 2009;1294:61–79. doi: 10.1016/j.brainres.2009.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 32.Brown RM, Short JL, Lawrence AJ. Identification of brain nuclei implicated in cocaine- primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PLoS ONE. 5:e15889. doi: 10.1371/journal.pone.0015889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, et al. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience. 171:1187–1196. doi: 10.1016/j.neuroscience.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress- induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 38.Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- 39.Mantsch JR, Goeders NE. Ketoconazole does not block cocaine discrimination or the cocaine-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:65–73. doi: 10.1016/s0091-3057(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 40.Wenzel JM, Cotten SW, Dominguez HM, Lane JE, Shelton K, Su ZI, et al. Noradrenergic beta-receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci. 34:3467–3474. doi: 10.1523/JNEUROSCI.3861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Shahar O, Posthumus EJ, Waldroup SA, Ettenberg A. Heightened drug- seeking motivation following extended daily access to self-administered cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 300:R222–235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 44.Buffalari DM, See RE. Guanfacine blockade of stress-induced and conditioend cue-induced cocaine-seeking in an animal model of relapse. Soc Neurosci Abstr. 2009:387, 385. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000;387:59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- 47.Mantsch JR, Goeders NE. Effects of cocaine self-administration on plasma corticosterone in rats: relationship to hippocampal type II glucocorticoid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:633–646. doi: 10.1016/s0278-5846(00)00098-1. [DOI] [PubMed] [Google Scholar]
- 48.Heesch CM, Negus BH, Bost JE, Keffer JH, Snyder RW, 2nd, Eichhorn EJ. Effects of cocaine on anterior pituitary and gonadal hormones. J Pharmacol Exp Ther. 1996;278:1195–1200. [PubMed] [Google Scholar]
- 49.Shalev U, Finnie PS, Quinn T, Tobin S, Wahi P. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2006;187:376–384. doi: 10.1007/s00213-006-0427-y. [DOI] [PubMed] [Google Scholar]
- 50.Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal Pattern of Cocaine Intake Determines Tolerance vs Sensitization of Cocaine Effects at the Dopamine Transporter. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- 52.Bardo MT, Neisewander JL, Miller JS. Repeated testing attenuates conditioned place preference with cocaine. Psychopharmacology (Berl) 1986;89:239–243. doi: 10.1007/BF00310636. [DOI] [PubMed] [Google Scholar]
- 53.Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like- immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 54.Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, et al. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]