Abstract
Aims
The secretion of enzymatically active heparanase (HepA) has been implicated as an essential metabolic adaptation in the heart following diabetes. However, the regulation and function of the enzymatically inactive heparanase (HepL) remain poorly understood. We hypothesized that in response to high glucose (HG) and secretion of HepL from the endothelial cell (EC), HepL uptake and function can protect the cardiomyocyte by modifying its cell death signature.
Methods and results
HG promoted both HepL and HepA secretion from microvascular (rat heart micro vessel endothelial cells, RHMEC) and macrovascular (rat aortic endothelial cells, RAOEC) EC. However, only RAOEC were capable of HepL reuptake. This occurred through a low-density lipoprotein receptor-related protein 1 (LRP1) dependent mechanism, as LRP1 inhibition using small interfering RNA (siRNA), receptor-associated protein, or an LRP1 neutralizing antibody significantly reduced uptake. In cardiomyocytes, which have a negligible amount of heparanase gene expression, LRP1 also participated in the uptake of HepL. Exogenous addition of HepL to rat cardiomyocytes produced a dramatically altered expression of apoptosis-related genes, and protection against HG and H2O2 induced cell death. Cardiomyocytes from acutely diabetic rats demonstrated a robust increase in LRP1 expression and levels of heparanase, a pro-survival gene signature, and limited evidence of cell death, observations that were not apparent following chronic and progressive diabetes.
Conclusion
Our results highlight EC-to-cardiomyocyte transfer of heparanase to modulate the cardiomyocyte cell death signature. This mechanism was observed in the acutely diabetic heart, and its interruption following chronic diabetes may contribute towards the development of diabetic cardiomyopathy.
Keywords: Hyperglycemia, Diabetes, LRP1, Endothelial cell, Cardiomyocyte, Cell death
1. Introduction
In the heart, where contracting cardiomyocytes are incapable of regeneration, intrinsic mechanisms are available within the endothelial cell (EC) to protect the cardiomyocyte against cellular demise.1–4 One conceivable cardioprotective protein, secreted exclusively from the EC in the heart, is heparanase.5,6 This endoglycosidase is initially synthesized as a latent (enzymatically inactive; HepL) 65 kDa proheparanase enzyme. HepL undergoes cellular secretion, which is followed by reuptake into lysosomes for proteolytic cleavage (removal of a 6 kDa linker peptide).5 Consequently, a 50 kDa polypeptide (enzymatically active; HepA) is formed that is ∼100-fold more active than HepL.
In cancer biology, HepA degradation of heparan sulphate proteoglycan (HSPG) is associated with extracellular matrix and basement membrane disruption, facilitating tumour cell invasion.5,7–9 Following its nuclear entry, HepA also influences transcription by cleaving nuclear HSPG, mitigating the suppressive effect of heparan sulphate on histone acetyltransferase.10–14 More recently, we established a novel role for HepA in modulating cardiac metabolism during diabetes.10,15 The above studies in cancer and diabetes fixated on the effects of HepA, incorrectly assuming that only the HSPG-hydrolyzing ability of heparanase was of importance. Intriguingly, HepL also has some remarkable properties, including its ability to activate signalling elements like Erk1/2, PI3K-AKT, RhoA, and Src, which in turn can contribute to changes in transcription.5 Cancer cells use secreted HepL to alter gene expression (either through its cell signalling properties, or by its conversion to HepA) in neighbouring cells, preventing their cellular demise and promoting tumour growth.5,16,17 In the heart, a similar paradigm would appear advantageous, with endothelial HepL protecting the cardiomyocyte against cell death. For this to happen, HepL needs to be secreted, followed by its subsequent binding and uptake into the cardiomyocyte. We hypothesized that, following its secretion from the EC, HepL uptake and function in the cardiomyocyte is protective against cell death. Results from this study suggest that HG increases heparanase secretion from EC, in addition to augmenting its uptake into the cardiomyocyte, where it has a favourable effect on the expression of apoptosis-related genes and limits the incidence of cell death. Occurrence of this EC-to-cardiomyocyte transfer of heparanase in the acutely diabetic heart, and the interruption of this process following chronic and progressive diabetes, may contribute towards the development of diabetic cardiomyopathy.18–20
2. Methods
2.1 Animal care
This investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and the University of British Columbia (Animal Care Certificate A13-0098).
2.2 Experimental animals
Streptozotocin (STZ) is a β-cell specific toxin used to induce diabetes.21 Male Wistar rats (240–260 g) were injected intravenously with 55 mg/kg STZ. With this dose, the animals become hyperglycemic within 24 h. These animals, used as a model of poorly controlled Type 1 diabetes, were kept for 4 days (acute) or 6 weeks (chronic) before heart isolation.
2.3 Isolation of cardiomyocytes
Rats were euthanized using a 100 mg/kg intraperitoneal injection of sodium pentobarbital. Once toe pinch and corneal reflexes were lost, a thoracotomy was performed prior to removal of the heart. Rat ventricular calcium-tolerant cardiomyocytes were prepared following previously described procedures.22 Isolated rat cardiomyocytes were plated on laminin-coated culture dishes and allowed to settle for 3 h. Unattached cells were washed away prior to different treatment protocols.
2.4 EC culture
Representative macrovascular (rat aortic endothelial cells, RAOEC) and microvascular (rat heart micro vessel endothelial cells, RHMEC) EC were cultured at 37 °C in a 5% CO2 humidified incubator. Cells from the fifth to the eighth passages of three different starting batches, for each cell line, were used.
2.5 Treatments
To promote the secretion of heparanase, EC were incubated with high glucose (25 mM, HG). To test whether exogenous heparanase can be taken up into EC and cardiomyocytes, cells were treated with 500 ng/mL recombinant myc-tagged HepL (myc-HepL) for different time intervals. To elucidate the contribution of cell surface lipoprotein receptor-related protein 1 (LRP1) towards heparanase uptake, we used receptor-associated protein (RAP, 200–400 nM, 1 h) or LRP1 neutralizing antibodies (20–40 mg/mL, 1 h) to inhibit LRP1. To inhibit LRP1 expression, small interfering RNA (siRNA) specific for LRP1 was used in RAOEC. SST0001 (125 μg/mL, 4 h) was used to inhibit heparanase activity. To induce apoptosis, cardiomyocytes were incubated in HG (30 mM) for 48 h, or H2O2 (10 μM) for 12 h.
2.6 Immunofluorescence
To visualize heparanase uptake into cardiomyocytes, cells were treated for 4 h with myc-HepL. Cells were washed with cold PBS and fixed with 4% formaldehyde solution. This was followed by permeabilization with 0.2% Triton X-100 for 10 min and incubation with blocking buffer containing 5% goat serum for 1 h at room temperature. Incubation with primary antibodies was at 4 °C overnight and secondary antibodies at room temperature for 1 h. To detect lysosome localization, LysoTracker was added 30 min before fixation. For determination of apoptosis, Annexin V (1:200) and propidium iodide (PI, 1:500) were used.
2.7 Nuclear isolation
Nuclear and cytosolic fractions were separated using the nuclear/cytosol fractionation kit from Thermo Fisher Scientific. To validate the purity of proteins, we used cytosolic (GAPDH) and nuclear (histone H3) protein markers to detect their predominance in cytosolic and nuclear fractions, respectively.
2.8 Western blot
Western blot was done as described previously.23 In some experiments using EC, cell culture media was concentrated with an Amicon centrifuge filter (Millipore) before the detection of heparanase protein.
2.9 Quantitative real-time PCR
Total RNA was isolated from EC, whole hearts, or cardiomyocytes using TRIzol (Invitrogen). This was followed by extraction using chloroform and isopropanol, washing with ethanol, and dissolving in RNase-free water. RNA was reverse transcribed into cDNA using a mixture of dNTPs, oligo-(dT), and SuperScript II Reverse Transcriptase. cDNA was amplified by TaqMan probes (β-actin, heparanase, lrp1, tnfrsf10b, tnfsf10, tnfrsf11b, cflar, bcl-2, tradd, tnfsf1b, bad, caspase 7, and caspase 8) in triplicate, using a StepOnePlus Real-Time (RT) PCR system (Applied Biosystems). Gene expression was calculated by the comparative cycle threshold (ΔΔCT) method.
2.10 Apoptosis PCR microarrays
For the apoptosis PCR array (Qiagen), 300–1000 ng RNA was isolated using an RNeasy Mini Kit and cDNA were transcribed using the RT2 First Strand Kit. The expression of 84 apoptosis-related genes was determined in control and HepL-treated rat cardiomyocytes.
2.11 Materials
RAOEC and RHMEC were obtained from cell applications and VEC technologies, respectively. STZ (S0130) and D-Mannitol (M4125) were obtained from Sigma-Aldrich. Anti-LRP1 antibody (ab92544) was purchased from Abcam. Purified HepL was prepared as described previously.24 LysoTracker (L-7528) was purchased from Life Technologies. For western blots that detect only HepL, we used the heparanase (N-Term) antibody (ABIN786265), which preferentially recognizes the 65 kDa HepL, from Aviva Systems Biology. For detection of HepA, we initially used mAb 130 (ANT-193), which can also detect HepL, from InSight (Rehovot, Israel). However, due to discontinuation of this antibody, we subsequently used HP3/17 (INS-26-0000), also from InSight (Rehovot, Israel). RAP (03-62221) and the LRP1 neutralizing antibody (8G1) were from American Research Products and Millipore, respectively. Antibodies for TNFRSF10B (sc-19529), CFLAR (sc-5276) that recognizes both the full length and short isoforms, and TRAIL (sc-6079) were obtained from Santa Cruz Biotechnology. TNFRSF11B (PA5-19841) was from Thermo Fisher Scientific. SST0001 was a kind gift from Sigma-Tau Research Switzerland S.A. Antibodies for PARP (9542), caspase-3 (9662), and cleaved caspase-3 (9664) were purchased from Cell Signalling.
2.12 Statistical analysis
Values are means ± SE. Wherever appropriate, a non-parametric Mann–Whitney test (for comparison between two groups) or one-way or two-way analysis of variance (ANOVA), followed by the Tukey test (for comparison between multiple groups) was used to determine differences between group mean values. The level of statistical significance was set at *P < 0.05, **P < 0.01, or ***P < 0.001.
3. Results
3.1 Macrovascular and microvascular EC secretion and reuptake of heparanase in response toHG
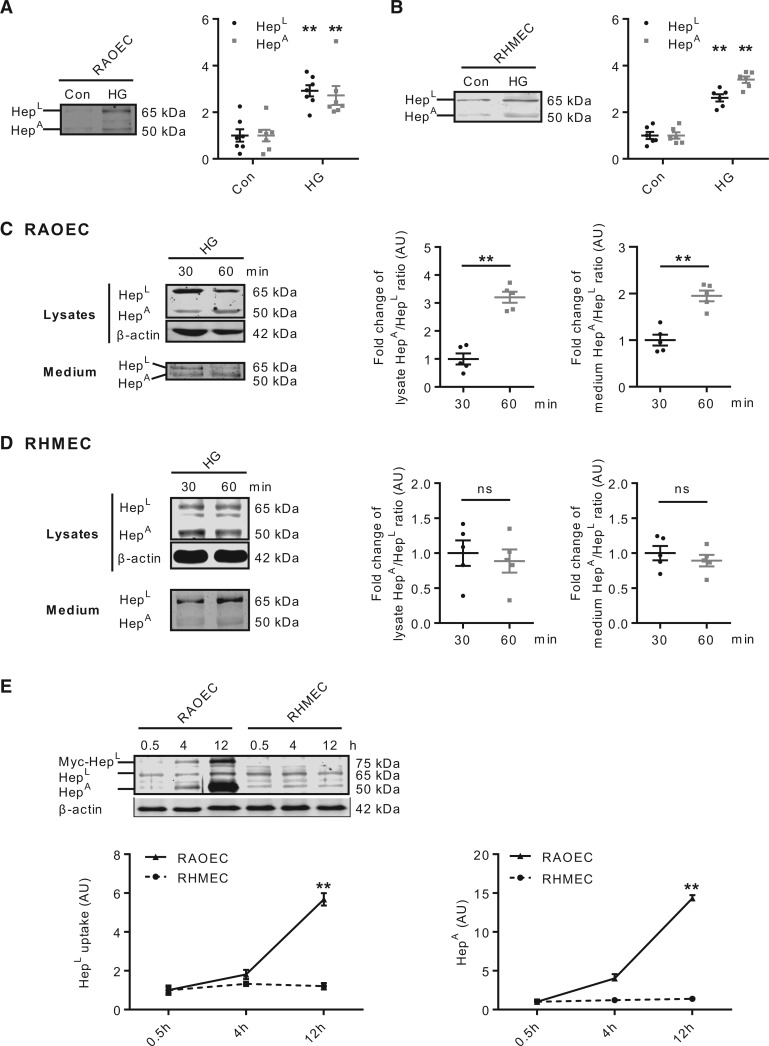
The concentration and activity of heparanase are elevated in the plasma and urine of diabetic patients.25 We have also reported that HG can stimulate the secretion of both latent and active forms of heparanase from EC.26 As EC behave differently based on their vessel type and environment,27 in this study, we compared the effects of HG on releasing HepL and HepA from macrovascular and microvascular EC. Incubation of RAOEC in HG promoted the release of both forms of heparanase into the incubation medium (Figure 1A); HepA by purinergic receptor activation and lysosomal secretion,28 and HepL by activation of the serine/threonine protein kinase D (PKD), an enzyme involved in the fission of proteins destined for the cell surface (see Supplementary material online, Figure S1). Similar results were observed when using RHMEC (Figure 1B). The osmolarity control, mannitol, had no influence on heparanase release in either cell type (data not shown). After its cellular release, HepL must be taken back up into EC5 for maturation into HepA. Hence, heparanase reuptake was also determined in EC subsequent to its release by HG. The decline in RAOEC lysate HepA at 30 min was followed by a substantial recovery at 60 min, resulting in an increase in the HepA/HepL ratio (Figure 1C, left panel). Measurement of heparanase in the medium also demonstrated a higher HepA/HepL ratio over time (Figure 1C, right panel), confirming the reuptake and processing of HepL into HepA, which was eventually secreted into the medium. Remarkably, unlike RAOEC, the reuptake and subsequent processing of HepL into HepA was not evident in RHMEC (Figure 1D). To substantiate that only macrovascular, but not microvascular, EC can take up HepL, we used EC incubated with recombinant myc-tagged latent heparanase (myc-HepL). In RAOEC, there was a robust time-dependent uptake of HepL and conversion into HepA, effects that were not apparent for RHMEC (Figure 1E), suggesting that microvascular EC have a limited capacity for HepL reuptake.
Figure 1.
Heparanase secretion and reuptake into ECs. RAOEC (passage 5–8) and RHMEC (passage 5–8) were incubated in either 5.5 (normal glucose control; Con) or 25 mM (HG) glucose for 30 min. Incubation medium was collected and used to determine latent (HepL) and active (HepA) heparanase secretion, n = 6–7 (A and B), **P < 0.01 compared to Con. RAOEC (C) and RHMEC (D) were incubated in 25 (HG) mM glucose for 30 or 60 min. Cell lysates and incubation medium were used to determine the intracellular and extracellular content of HepL and HepA, n = 5, **P < 0.01. RAOEC and RHMEC were incubated with normal glucose and 500 ng/mL myc-HepL. Cell lysates were collected at indicated time points to measure the uptake of myc-HepL and its conversion to HepA, n = 6 (E), **P < 0.01 compared to RHMEC.
3.2 LRP1 is important for HepL uptake
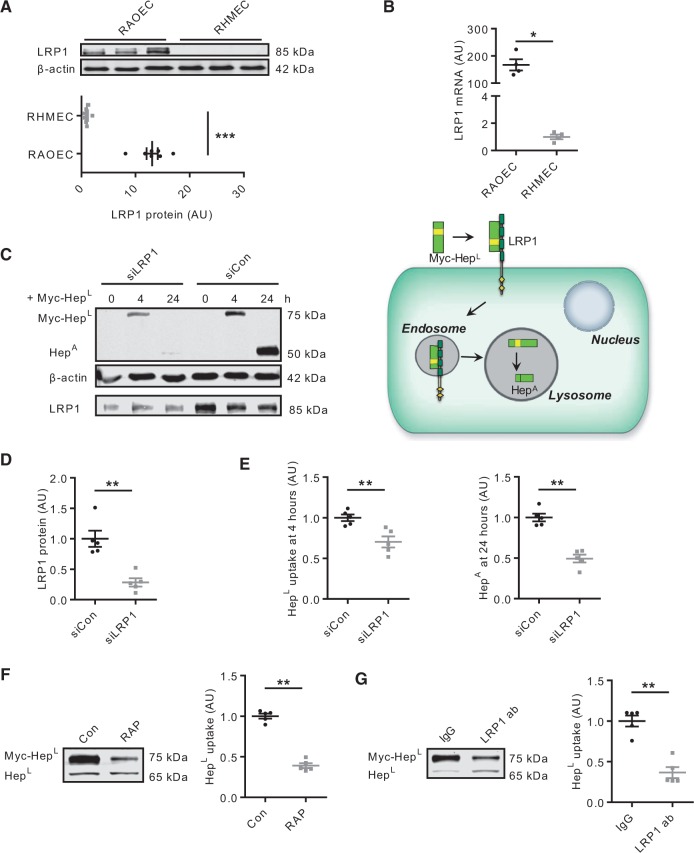
Multiple receptors have been implicated in facilitating the uptake of HepL, including the mannose-6-phosphate receptor, HSPG, and LDL receptor related protein (LRP1).29 We focused on LRP1 given its promiscuous role in the endocytosis of a number of different proteins.30,31 Of considerable interest was the observation that RAOEC demonstrated a robust expression of LRP1. This expression was not apparent in RHMEC (Figure 2A and B), and could explain the disparate abilities of these two cell types to take up HepL. Using siRNA, we effectively reduced LRP1 expression in RAOEC (Figure 2C bottom panel and 2D). As a consequence, myc-HepL uptake and conversion to HepA over 24 h was reduced in these cells compared to control (Figure 2C top panel and 2E), validating the contribution of LRP1 in HepL uptake (schematic). In spite of LRP1 knockdown, some HepL was still detected, albeit at a level much lower as compared to control, and likely as a consequence of non-specific binding of HepL to the EC surface. Simple binding to the cell surface exterior, with limited uptake, will fail to increase the amount of HepA, as shown in Figure 2C. The essential role of LRP1 was further substantiated using the specific blocker RAP (an LRP1 chaperone) (Figure 2F), and an LRP1 neutralizing antibody (Figure 2G), both of which reduced the uptake of HepL by RAOEC. Our data implicate LRP1 as an essential contributor in the endocytosis of HepL in EC.
Figure 2.
LRP1 is a key receptor for heparanase reuptake into ECs. RAOEC and RHMEC lysates were used to determine the expression of LRP1, n = 7 and n = 4 (A and B). In RAOEC, siRNA was used to silence LRP1, followed by determination of myc-HepL uptake and conversion to HepA, n = 5 (C–E). RAOEC were pre-treated with or without 200 nM RAP (F) or 20 μg/mL LRP1 neutralizing antibody (G) for 1 h prior to incubation with 500 ng/mL myc-HepL for 4 h. Cell lysates were collected to determine HepL uptake, n = 5. 20 μg/mL IgG was used as a control for the LRP1 neutralizing antibody experiment. *P < 0.05, **P < 0.01, ***P < 0.001.
3.3 Extracellular uptake determines presence of heparanase in cardiomyocytes
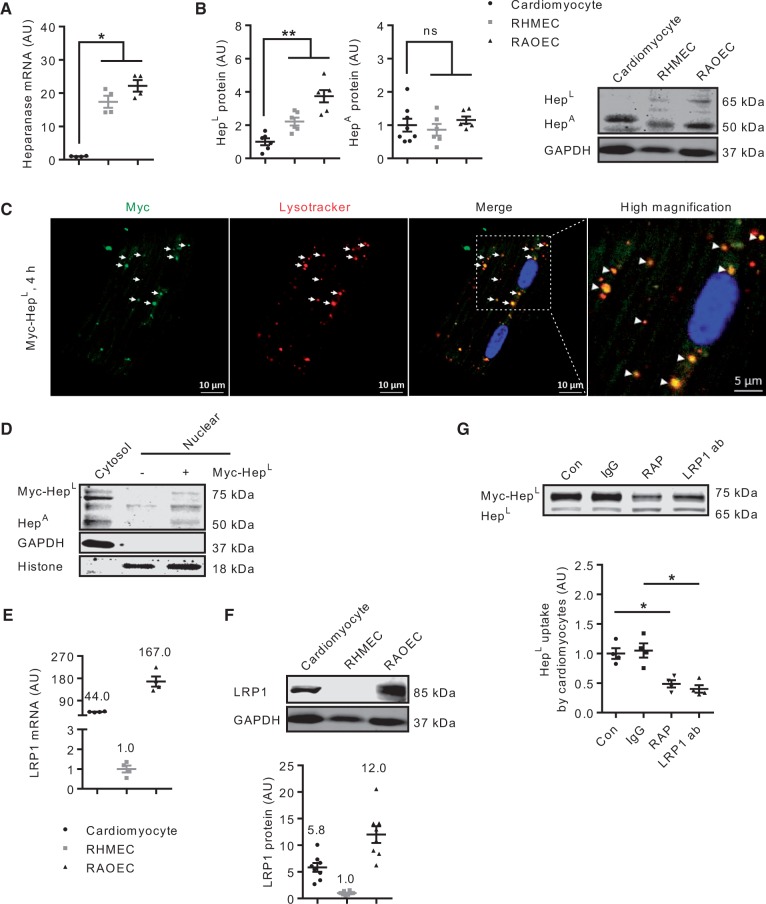
In the heart, EC outnumber cardiomyocytes by 3:12. Intriguingly, compared to EC, there is a negligible amount of heparanase gene expression in cardiomyocytes (Figure 3A). We reasoned that the absence of a reuptake machinery in microvascular EC would lead to HepL, secreted from these cells, to be taken up into cells that are in close proximity; for example, the cardiomyocytes. Indeed, our results indicate that cardiomyocytes contain a significant amount of heparanase protein (Figure 3B), suggesting that HepL, taken up from neighbouring microvascular EC, is converted to HepA in the cardiomyocyte lysosome. These results are supported by our previous work using EC co-cultured with cardiomyocytes.10 The uptake and lysosomal localization of myc-HepL were further confirmed using immunofluorescence (Figure 3C), whereas the nuclear presence of HepA was established using western blot (Figure 3D). Given the importance of LRP1 in EC HepL uptake, we determined and confirmed its expression in cardiomyocytes (Figure 3E and F). In addition, and analogous to RAOEC, administration of either RAP or an LRP1 neutralizing antibody reduced the cardiomyocyte uptake of myc-HepL (Figure 3G).
Figure 3.
Cardiomyocytes are also capable of HepL uptake. Cell lysates of primary rat cardiomyocytes, RAOEC or RHMEC were obtained for determination of heparanase mRNA (A) and protein (B), n = 4–8. Cardiomyocytes seeded on coverslips were placed in a 6-well plate, and treated with 500 ng/mL myc-HepL prior to immunofluorescence staining examined under a confocal microscope. The merged image of heparanase and lysosomes is described in the third (scale bar, 10 µm) and fourth (scale bar, 5 µm) panels from left (C) and are data from a representative experiment. Isolated myocytes were also treated with or without myc-HepL for 4 h. Following this incubation, nuclear and cytosolic fractions were isolated, and HepA protein levels determined by western blot (D). Cell lysates of primary rat cardiomyocytes, RAOEC, or RHMEC were obtained for determination of LRP1 mRNA (E) and protein (F), n = 4 and n = 8. In a different experiment, in cardiomyocytes incubated with HG, cells were pre-treated with or without 400 nM RAP or 40 μg/mL LRP1 neutralizing antibody for 1 h prior to incubation with 500 ng/mL myc-HepL for 4 h. Cell lysates were collected to determine HepL uptake, n = 4 (G). 40 μg/mL IgG was used as a control for the LRP1 neutralizing antibody experiment. *P < 0.05, **P < 0.01.
3.4 HepL modulates expression of apoptosis-related genes in cardiomyocytes
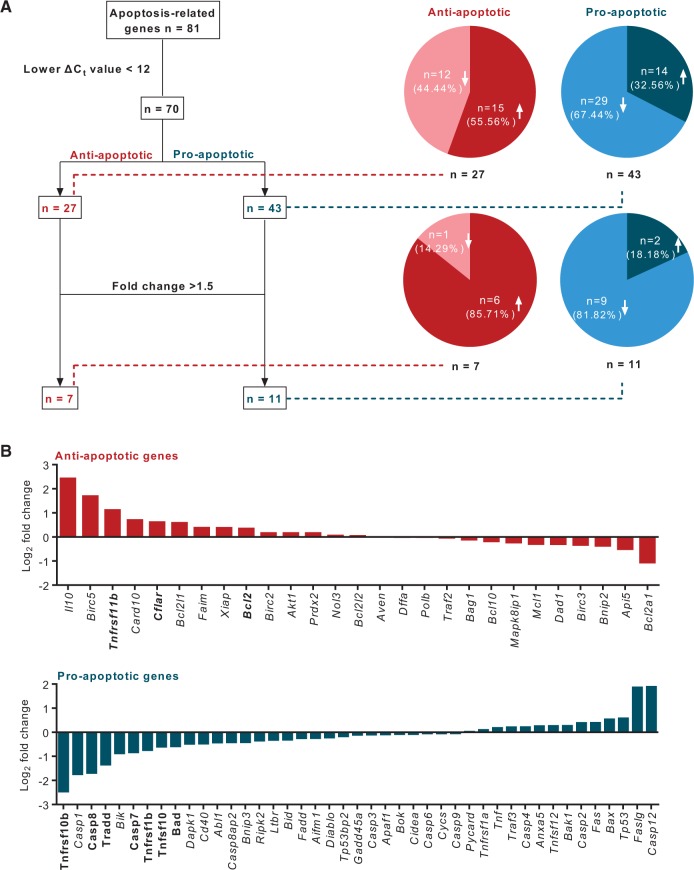
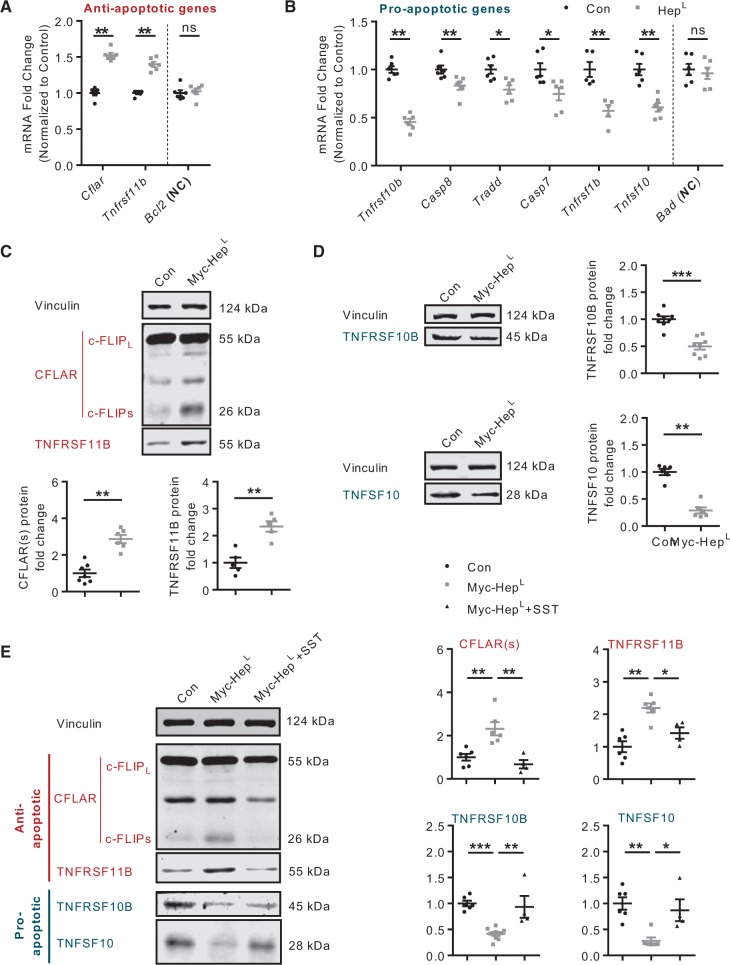
Entry of heparanase into the nucleus to regulate histone acetylation has been suggested as a mechanism modulating gene transcription and protection against apoptosis.11 We hypothesized that, following its uptake into the cardiomyocyte, HepL can protect against cell death by influencing apoptosis-related genes. Using a rat apoptosis gene array in cardiomyocytes incubated with myc-HepL we found that, among the 70 genes that had well-defined functions and significant levels of expression, 15 out of 27 anti-apoptotic genes were up-regulated, and 29 out of 43 pro-apoptotic genes were down-regulated (Figure 4A and B). Of the 18 genes that were significantly different (fold change >1.5) compared to control, 15 were in favour of cell survival (six anti-apoptotic genes were up-regulated; nine pro-apoptotic genes were down-regulated) (Figure 4A and B; Supplementary material online, Table S1). Further examining selective pro-apoptotic genes that were down-regulated, and anti-apoptotic genes that were up-regulated, results from the microarray were confirmed by quantitative RT–PCR (Figure 5A and B) and western blot (Figure 5C and D). As SST0001, a specific heparanase inhibitor, reversed the effects of heparanase (Figure 5E), our results suggest that heparanase can protect against apoptotic cell death.
Figure 4.
Expression of apoptosis-related genes in cardiomyocytes exposed to exogenous HepL. Primary cardiomyocytes isolated from the adult rat heart were treated with or without 500 ng/mL myc-HepL for 12 h prior to RNA isolation and subsequent determination of 81 apoptosis-related genes using a PCR array (Fig. 4A and B).
Figure 5.
Inhibition of HepA abrogates changes in gene expression. RT–PCR and western blot were employed to confirm our results from the gene array using selected pro- and anti- apoptosis genes, n = 5–8 (Fig. 5A–D). Vinculin was used as a loading control, NC-negative control. In a separate experiment, cardiomyocytes were pre-treated with or without 125 μg/mL SST0001 for 4 h, prior to incubation with 500 ng/mL myc-HepL for 12 h, and the expression of selected genes determined, n = 4–9 (Fig. 5E). *P < 0.05, **P < 0.01, ***P < 0.001.
3.5 Contrasting effects of diabetes on cardiomyocyte cell death signature
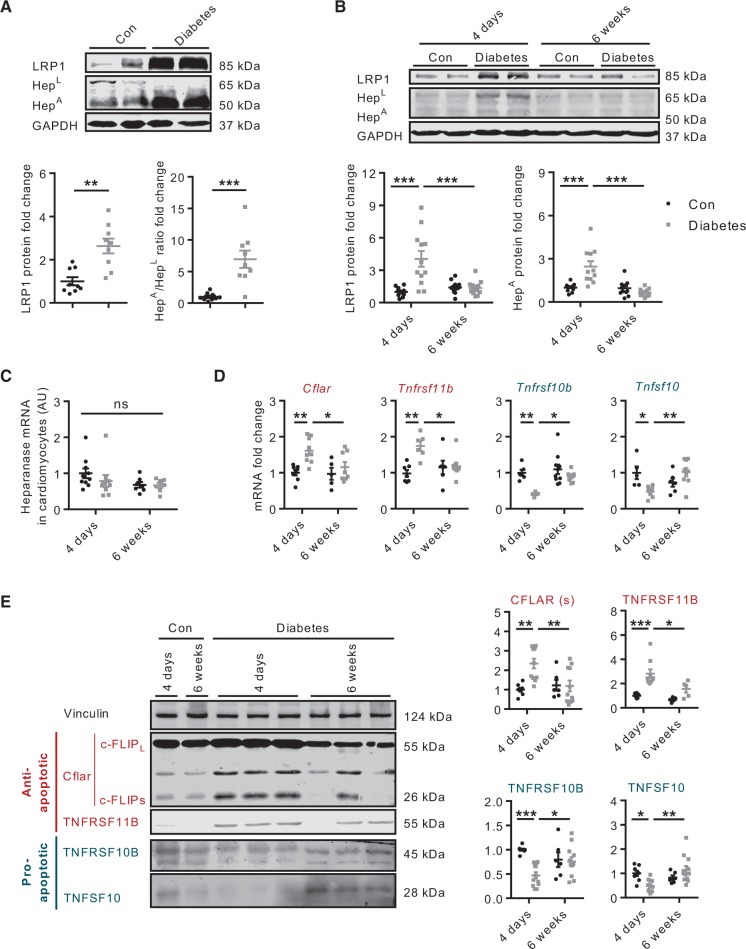
RAOEC incubated in HG demonstrate an increase in LRP1 expression (see Supplementary material online, Figure 2), emphasizing the importance of HG in mediating its expression. Using a model of acute (4 days) diabetes, we assessed the impact of HG on whole heart and cardiomyocyte LRP1. Hearts from acute diabetic animals demonstrated augmented LRP1 expression (Figure 6A). This effect likely contributed to a higher uptake of HepL and its subsequent conversion into HepA, which resulted in a higher HepA/HepL ratio (Figure 6A). Extending this observation, cardiomyocytes isolated from animals with acute diabetes also exhibited higher LRP1 expression and intracellular heparanase content (Figure 6B). The latter effect was unrelated to changes in heparanase gene expression (Figure 6C). It should be noted that, unlike EC, when cardiomyocytes were exposed to HG, no change in LRP1 expression was observed, up to 48 h after incubation (data not shown). Nevertheless, we observed an increased uptake and lysosomal localization of heparanase at 4 h in cardiomyocytes incubated in HG (see Supplementary material online, Figure 3A and B). As the inhibition of Src activation by PP2 abrogated this effect, this proto-oncogene, rather than augmented expression of LRP1, can be implicated in HG-mediated cardiomyocyte heparanase uptake in vitro (see Supplementary material online, Figure 3C and D). Whether Src activation also has a contributory effect in vivo is currently unclear because its activation by HG was detected within 30 min in vitro, whereas diabetic animals are euthanized after 4 days of STZ. Of considerable significance was the observation that these effects on cardiomyocyte LRP1 and heparanase were abolished upon extending the duration of diabetes to 6 weeks (Figure 6B), suggesting that cardiomyocyte LRP1 expression and heparanase uptake are affected in an opposite fashion depending on the duration of hyperglycemia. As apoptosis-related gene (Figure 6D) and protein (Figure 6E) expression and cleaved caspase 3 and PARP (see Supplementary material online, Figure S4) followed a similar pattern predicated on the duration of diabetes, our data suggest that chronic diabetes nullifies the favourable effects of heparanase in cardiomyocytes.
Figure 6.
Acute and chronic effects of diabetes on cardiomyocyte cell death signature. In animals made diabetic with STZ, hearts were obtained after 4 days of hyperglycemia and LRP1 protein and the HepA/HepL ratio determined, n = 9 (A). Cardiomyocytes from acute (diabetes-4 days) and chronic (diabetes-6 weeks) diabetic animals were isolated for determination of LRP1 and heparanase protein (B) and heparanase gene (C), n = 7–12. Selected pro- and anti-apoptosis genes (D) and protein (E) were also evaluated in acute and chronic diabetic cardiomyocytes, n = 5–12. *P < 0.05, **P < 0.01, ***P < 0.001.
3.6 HG and H2O2 induced cardiomyocyte cell death is attenuated by HepL
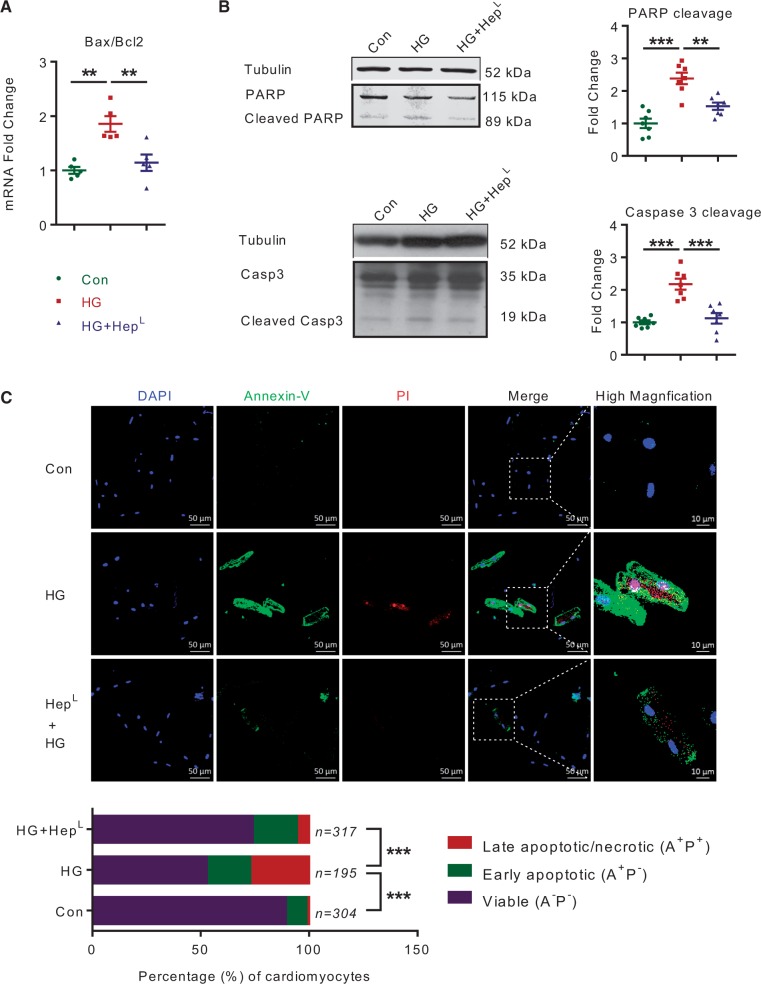
In HG, a greater production of reactive oxygen species (ROS) together with its disrupted detoxification causes cardiomyocyte cell death.32 Given the effects of ROS on gene expression in cells undergoing apoptosis, cardiomyocytes were incubated with HG in the presence or absence of heparanase. In HG, HepL caused a significant decrease in the Bax/Bcl-2 mRNA ratio, a marker of cellular apoptosis (Figure 7A). Cleaved PARP and caspase 3, apoptosis biomarkers that were augmented in cardiomyocytes treated with HG, were also significantly decreased upon heparanase addition (Figure 7B). Importantly, the HG-induced decrease in the number of viable cardiomyocytes, as determined by Annexin V/PI staining, was improved by HepL (Figure 7C). As these beneficial effects of HepL on apoptosis were reproduced in H2O2 induced oxidative stress (see Supplementary material online, Figure 5), our data suggest that heparanase modulates the cell death signature and is protective against cardiomyocyte cell death.
Figure 7.
HepL protects cardiomyocytes from HG induced apoptosis. Isolated rat cardiomyocytes were incubated with 30 mM glucose (HG) and/or 500 ng/mL myc-HepL for 12–48 h, n = 6. After 12 h, the Bax/Bcl2 mRNA ratio was determined (A). PARP and caspase 3 cleavage were evaluated after 48 h (B), n = 7. Annexin V/PI staining, as markers of apoptosis, were also determined after cardiomyocyte incubation with HG and/or myc-HepL (C), n = 195–317 myocytes pooled from four independent experiments. The merged image of Annexin V/PI staining is described in the fourth panel (scale bar 50 µm), whereas a higher magnification image (scale bar 10 µm) is described in the fifth panel. Data are from a representative experiment. **P < 0.01, ***P < 0.001.
4. Discussion
Under physiological conditions, the EC is responsible for secreting factors that support cardiomyocyte function.1–4 Heparanase is one such example, having a unique responsibility to release cardiomyocyte cell surface HSPG-bound lipoprotein lipase (LPL) to promote lipoprotein-TG breakdown. The resultant fatty acid (FA) generated is then transported to the cardiomyocyte for oxidative energy generation.15 In addition to liberating HSPG-bound proteins, heparanase, either by binding to putative cell-surface receptors, or subsequent to its internalization and nuclear entry, has also been suggested to affect gene transcription.5,11–14,33,34 In cancer cells, this property of secreted heparanase can induce cell signalling and gene expression in both parent and adjacent cells, maintaining their survival and delaying demise.11,16,17,35–37 Our data suggest, for the first time, that HG promotes both the secretion of heparanase from EC as well as its uptake into cardiomyocytes, initiating pro-survival mechanisms to temper the consequences of hyperglycemia in the diabetic heart.
In EC, HepA resides in lysosomes5 and hyperglycemia, a major complication of diabetes, is an effective stimulus for its secretion.28 We have previously described a mechanism for this process, which includes purinergic receptor activation, as well as cortical and stress actin reorganization.28 As EC are not all created equal and exhibit differences depending on their anatomical sites-such as arterial compared to venous architecture, or macro compared to their microvascular locations27—we compared the secretion of heparanase in RAOEC and RHMEC. Here we show that HG similarly affects the secretion of HepL from both EC cell types. Following its secretion, the EC has a capacity to reuptake HepL for lysosomal conversion to HepA. Interestingly, although both cell types had a similar capacity to secrete HepL in response to HG, only macrovascular EC were competent for its reuptake, an observation that was confirmed using myc-HepL. A receptor that has been implicated in HepL uptake is LRP1.38 Consistent with the differential uptake of HepL into the two cell types, only RAOEC showed a robust expression of LRP1. We further established that LRP1 is indispensable for HepL uptake into RAOEC by silencing the receptor using RAP or an LRP1 neutralizing antibody, both of which decreased the uptake of HepL. Our data imply that the reuptake of HepL by macrovascular EC is dependent on LRP1, an uptake mechanism that is missing in microvascular EC. At present, the mechanism behind the differential LRP1 expression observed in macrovascular and microvascular ECs is unclear, but could be related to shear stress, a stimulus that is known to change gene expression.39,40 The absence of this reuptake machinery in microvascular EC suggests that the HepL secreted from these cells is likely taken up, in the heart, by proximal cells. Given the proximity of cardiomyocytes (which do not express the heparanase gene) to microvascular EC, it is plausible to envision the exogenous uptake of EC-secreted heparanase into cardiomyocytes. In support of this theory, we detected both the latent and active forms of heparanase in isolated cardiomyocytes. This observation, coupled with the robust expression of LRP1 in cardiomyocytes, whose inhibition abrogates HepL uptake, indicates that transfer from exogenous sources determines the presence of heparanase in cardiomyocytes.
One implication of cardiomyocytes acquiring HepL is its subsequent intracellular conversion to HepA, followed by its nuclear entry to influence gene transcription. By cleaving nuclear HSPG, HepA mitigates the suppressive effect of heparan sulphate on histone acetyltransferase to activate gene expression.11 Using an apoptosis PCR array, which detects both pro- and anti-apoptotic genes, we discovered that cardiomyocytes incubated with HepL down-regulated pro-apoptotic genes (e.g. Tnfrsf10b, Tnfsf10), whereas anti-apoptotic genes (e.g. Cflar, Tnfrsf11b) were up-regulated. As cardiomyocytes isolated from heparanase transgenic mice also showed a similar trend in this gene expression pattern (unpublished data), our data imply that HepL displayed pro-survival effects on the cardiomyocyte by initiating a program that protects against apoptosis. This effect of heparanase on gene expression relies on its activity, as its inhibition by a specific heparanase inhibitor reversed its beneficial effects on gene expression. Additionally, the changes in gene expression induced by heparanase translated into protection against cardiomyocyte cell death, as confirmed by the reduction in the Bax/Bcl-2 mRNA ratio, cleaved PARP and caspase 3, and Annexin V/PI staining. In diabetes, hyperglycemia can provoke cardiomyocyte cell death and contribute to cardiomyopathy.18–20,41 However, it should be noted that it is the EC that is exposed to this metabolic alteration before the cardiomyocyte. As such, through their release of HepL, EC, as first responders to hyperglycemia, could pre-condition the cardiomyocyte against impending metabolic damage. For this to work, hyperglycemia also needs to increase HepL uptake into the cardiomyocyte. Indeed, we observed robustly increased LRP1 expression and levels of HepA, as well as a pro-survival gene signature in whole hearts and cardiomyocytes isolated from acutely diabetic animals. Hyperglycemia and its associated oxidative stress, which resembles hypoxia, and its attendant increase in HIF-1α, could be one explanation for LRP1 induction in short-term hyperglycemia. HIF-1α is a known factor that can induce LRP1 expression in cardiomyocytes42 and in other cell types.43–46 These effects were lost following chronic diabetes, and could contribute to the development of cardiomyopathy in these animals. The disappearance of LRP1, with prolonged duration of diabetes, may be related to a further attenuation of circulating insulin, as islets that escaped the initial insult by STZ are later lost due to the combined features of hyperglycemia and hyperlipidemia (gluco-lipotoxicity). Interestingly, several studies have reported that LRP1 is down-regulated in brains from chronically diabetic mice, an effect associated with sustained hyperglycemia and insulin deficiency in these animals.47,48 Confirmation of the beneficial effects of heparanase in the prevention of diabetic cardiomyopathy requires the induction of diabetes in mice that overexpress heparanase, experiments that are currently underway in our lab.
In summary, our data reveal a novel and complex role for EC in providing functional support to subjacent cardiomyocytes by communicating via soluble paracrine mediators. In this study, HG was a common stimulus for HepL secretion from the EC, in addition to promoting its uptake into the cardiomyocyte. The presence of heparanase in the cardiomyocyte dramatically changed the expression of apoptosis-related genes, providing an acute cardioprotective effect. Data obtained from these studies, suggesting a novel, favourable effect of HepL in the cardiomyocyte, will assist in devising novel therapeutic strategies to prevent or delay diabetic heart disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by an operating grant from the Canadian Institutes of Health Research to BR (CIHR-MOP-133547), and the Israel Science Foundation (SF601/14) to IV. A.P.-L.C. and D.Z. are the recipients of Doctoral Student Research Awards from the Canadian Diabetes Association. Funding to pay the open access publication charges for this article was provided by CIHR.
References
- 1.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem 2004;279:51141–51147. [DOI] [PubMed] [Google Scholar]
- 2.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation 2004;110:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol 2006;68:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation 2010;122:928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol 2006;38:2018–2039. [DOI] [PubMed] [Google Scholar]
- 6.Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse beta cell survival and autoimmune diabetes. J Clin Invest 2012;122:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao NB, Tang B, Wang GZ, Xie R, Hu CJ, Wang SM, Wu YY, Liu E, Xie X, Yang SM. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-kappaB signaling pathway for gastric cancer metastasis. Cancer Lett 2015;361:57–66. [DOI] [PubMed] [Google Scholar]
- 8.Hammond E, Khurana A, Shridhar V, Dredge K. The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front Oncol 2014;4:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood 2010;115:2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Pei-Ling Chiu A, Neumaier K, Wang F, Zhang D, Hussein B, Lal N, Wan A, Liu G, Vlodavsky I, Rodrigues B. Endothelial cell heparanase taken up by cardiomyocytes regulates lipoprotein lipase transfer to the coronary lumen following diabetes. Diabetes 2014;63:2643–2655. [DOI] [PubMed] [Google Scholar]
- 11.Purushothaman A, Hurst DR, Pisano C, Mizumoto S, Sugahara K, Sanderson RD. Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype. J Biol Chem 2011;286:30377–30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He YQ, Sutcliffe EL, Bunting KL, Li J, Goodall KJ, Poon IK, Hulett MD, Freeman C, Zafar A, McInnes RL, Taya T, Parish CR, Rao S. The endoglycosidase heparanase enters the nucleus of T lymphocytes and modulates H3 methylation at actively transcribed genes via the interplay with key chromatin modifying enzymes. Transcription 2012;3:130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobuhisa T, Naomoto Y, Okawa T, Takaoka M, Gunduz M, Motoki T, Nagatsuka H, Tsujigiwa H, Shirakawa Y, Yamatsuji T, Haisa M, Matsuoka J, Kurebayashi J, Nakajima M, Taniguchi S, Sagara J, Dong J, Tanaka N. Translocation of heparanase into nucleus results in cell differentiation. Cancer Sci 2007;98:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Wang Y, Zhang D, Puthanveetil P, Johnson JD, Rodrigues B. Fatty acid-induced nuclear translocation of heparanase uncouples glucose metabolism in endothelial cells. Arterioscler Thromb Vasc Biol 2012;32:406–414. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang D, Chiu AP, Wan A, Neumaier K, Vlodavsky I, Rodrigues B. Endothelial heparanase regulates heart metabolism by stimulating lipoprotein lipase secretion from cardiomyocytes. Arterioscler Thromb Vasc Biol 2013;33:894–902. [DOI] [PubMed] [Google Scholar]
- 16.Nadir Y, Brenner B, Zetser A, Ilan N, Shafat I, Zcharia E, Goldshmidt O, Vlodavsky I. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost 2006;4:2443–2451. [DOI] [PubMed] [Google Scholar]
- 17.Gingis-Velitski S, Zetser A, Flugelman MY, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem 2004;279:23536–23541. [DOI] [PubMed] [Google Scholar]
- 18.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223. [DOI] [PubMed] [Google Scholar]
- 19.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 2006;98:596–605. [DOI] [PubMed] [Google Scholar]
- 20.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004;25:543–567. [DOI] [PubMed] [Google Scholar]
- 21.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001;50:537–546. [PubMed] [Google Scholar]
- 22.Sambandam N, Chen XS, Cam MC, Rodrigues B. Cardiac lipoprotein lipase in the spontaneously hypertensive rat. Cardiovasc Res 1997;33:460–468. [DOI] [PubMed] [Google Scholar]
- 23.Pulinilkunnil T, An D, Ghosh S, Qi D, Kewalramani G, Yuen G, Virk N, Abrahani A, Rodrigues B. Lysophosphatidic acid-mediated augmentation of cardiomyocyte lipoprotein lipase involves actin cytoskeleton reorganization. Am J Physiol Heart Circ Physiol 2005;288:H2802–H2810. [DOI] [PubMed] [Google Scholar]
- 24.Zetser A, Bashenko Y, Miao HQ, Vlodavsky I, Ilan N. Heparanase affects adhesive and tumorigenic potential of human glioma cells. Cancer Res 2003;63:7733–7741. [PubMed] [Google Scholar]
- 25.Shafat I, Ilan N, Zoabi S, Vlodavsky I, Nakhoul F. Heparanase levels are elevated in the urine and plasma of type 2 diabetes patients and associate with blood glucose levels. PLoS One 2011;6:e17312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, Wan A, Chiu AP, Wang Y, Wang F, Neumaier K, Lal N, Bround MJ, Johnson JD, Vlodavsky I, Rodrigues B. Hyperglycemia-induced secretion of endothelial heparanase stimulates a vascular endothelial growth factor autocrine network in cardiomyocytes that promotes recruitment of lipoprotein lipase. Arterioscler Thromb Vasc Biol 2013;33:2830–2838. [DOI] [PubMed] [Google Scholar]
- 27.Zetter BR. The endothelial cells of large and small blood vessels. Diabetes 1981;30:24–28. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Wang Y, Kim MS, Puthanveetil P, Ghosh S, Luciani DS, Johnson JD, Abrahani A, Rodrigues B. Glucose-induced endothelial heparanase secretion requires cortical and stress actin reorganization. Cardiovasc Res 2010;87:127–136. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Zaken O, Shafat I, Gingis-Velitski S, Bangio H, Kelson IK, Alergand T, Amor Y, Maya RB, Vlodavsky I, Ilan N. Low and high affinity receptors mediate cellular uptake of heparanase. Int J Biochem Cell Biol 2008;40:530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 2001;108:779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 2008;88:887–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 1999;99:2934–2941. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Sanderson RD. Heparanase regulates levels of syndecan-1 in the nucleus. PLoS One 2009;4:e4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Gorzelanny C, Bauer AT, Halter N, Komljenovic D, Bauerle T, Borsig L, Roblek M, Schneider SW. Nuclear heparanase-1 activity suppresses melanoma progression via its DNA-binding affinity. Oncogene 2015;34:5832–5842. [DOI] [PubMed] [Google Scholar]
- 35.Purushothaman A, Babitz SK, Sanderson RD. Heparanase enhances the insulin receptor signaling pathway to activate extracellular signal-regulated kinase in multiple myeloma. J Biol Chem 2012;287:41288–41296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyango I, Barash U, Naroditsky I, Li JP, Hammond E, Ilan N, Vlodavsky I. Heparanase co-operates with Ras to drive breast and skin tumorigenesis. Cancer Res 2014;74:4504–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, Ilan N. Heparanase induces vascular endothelial growth factor expression: Correlation with p38 phosphorylation levels and Src activation. Cancer Res 2006;66:1455–1463. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharjee PS, Huq TS, Potter V, Young A, Davenport IR, Graves R, Mandal TK, Clement C, McFerrin HE, Muniruzzaman S, Ireland SK, Hill JM. High-glucose-induced endothelial cell injury is inhibited by a peptide derived from human Apolipoprotein E. PLoS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topper JN, Gimbrone MA. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today 1999;5:40–46. [DOI] [PubMed] [Google Scholar]
- 40.Chen BPC, Li YS, Zhao YH, Chen KD, Li S, Lao JM, Yuan SL, Shyy JYJ, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 2001;7:55–63. [DOI] [PubMed] [Google Scholar]
- 41.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol 2003;3:219–228. [DOI] [PubMed] [Google Scholar]
- 42.Gao QQ, Guan LN, Huc SS, Yao YW, Ren XL, Zhang ZW, Cheng CL, Liu Y, Zhang C, Huang JP, Su DM, Ma X. Study on the mechanism of HIF1a-SOX9 in glucose-induced cardiomyocyte hypertrophy. Biomed Pharmacother 2015;74:57–62. [DOI] [PubMed] [Google Scholar]
- 43.Chang ML, Chiu CJ, Shang F, Taylor A. High glucose activates ChREBP-mediated HIF-1 alpha and VEGF expression in human RPE Cells under Normoxia. Retin Degenerati Dis: Mech Exp Ther 2014;801:609–621. [DOI] [PubMed] [Google Scholar]
- 44.Kawata K, Kubota S, Eguchi T, Aoyama E, Moritani NH, Kondo S, Nishida T, Takigawa M. Role of LRP1 in transport of CCN2 protein in chondrocytes. J Cell Sci 2012;125:2965–2972. [DOI] [PubMed] [Google Scholar]
- 45.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1 alpha promoter via a functional NF kappa B site. Arterioscl Throm Vas 2007;27:755–761. [DOI] [PubMed] [Google Scholar]
- 46.Castellano J, Aledo R, Sendra J, Costales P, Juan-Babot O, Badimon L, Llorente-Cortes V. Hypoxia stimulates low-density lipoprotein receptor-related protein-1 expression through hypoxia-inducible factor-1 alpha in human vascular smooth muscle cells. Arterioscler Thromb Vas 2011;31:1411–1420. [DOI] [PubMed] [Google Scholar]
- 47.Liu CC, Hu J, Tsai CW, Yue M, Melrose HL, Kanekiyo T, Bu GJ. Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. J Neurosci 2015;35:5851–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong H, Liu LP, Liao JM, Wang TS, Ye FY, Wu J, Wang YY, Wang Y, Li YQ, Long Y, Xia YZ. Downregulation of LPR1 at the blood-brain barrier in streptozotocin-induced diabetic mice. Neuropharmacology 2009;56:1054–1059. [DOI] [PubMed] [Google Scholar]