Abstract
Background
Immune activation and exhaustion drive several co-morbidities and disease progression in HIV-infected adults; however, they are not well-studied in HIV-infected youth. Thus, this study sought to examine levels of immune activation and exhaustion in this population, investigate associated HIV- and non-HIV-related variables, and compare results with a matched healthy control group.
Methods
HIV-infected youth 8–25 years old on stable antiretroviral therapy with an HIV-1 RNA level <1000 copies/mL were enrolled, along with matched healthy controls. We measured T-cell and monocyte immune activation and exhaustion markers in cryopreserved PBMC and plasma samples.
Results
136 subjects (80 HIV+: 66% male; 91% black) were enrolled. Markers of CD4+ and CD8+ T-cell activation were higher in the HIV-infected group vs. controls [mean % CD4+CD38+HLA-DR+ and CD8+CD38+HLA-DR+ = 2.2 vs. 1.5 (P=0.002) and 4.9 vs. 2.2 (P<0.0001), respectively], as were exhausted CD4+ and CD8+ T-cells [mean % CD4+CD38+HLA-DR+PD-1+ and CD8+CD38+HLA-DR+PD-1+ = 1.0 vs. 0.5 (P<0.0001) and 1.6 vs. 0.7 (P<0.0001), respectively]. There were no differences in proportions of inflammatory or patrolling monocytes between groups (P>0.05); however, soluble CD14 was higher in HIV-infected compared with controls (1.6 vs. 1.4 μg/mL; P=0.01). Current CD4 count, low-density lipoprotein cholesterol, and age were the variables most associated with CD4+ and CD8+ T-cell activation.
Conclusions
CD4+ and CD8+ T-cell immune activation and exhaustion are higher in HIV-infected youth compared with matched controls, while monocyte sub-populations are not altered despite a high soluble CD14 level. The clinical significance of the increased immune activation and exhaustion should be further explored.
Keywords: HIV, immune activation, immune exhaustion, pediatrics
INTRODUCTION
HIV-infected individuals are living decades longer with the advent of combination antiretroviral therapy (cART). Despite virologic suppression, however, they are still at an increased risk of co-morbidities, such as cardiovascular disease (CVD) and osteoporosis [1, 2]. While the etiology of these co-morbidities is multi-factorial, heightened immune activation (IA) plays an important role in the adult population [3–5]. Moreover, IA is associated with HIV disease progression, mortality, and poorer CD4+ T-cell reconstitution after initiation of cART [6–9].
Data suggest that HIV-infected youth are also at an increased risk of HIV-related co-morbidities like their adult counterparts [10–15]. Extrapolating from adult data would suggest that heightened IA would be an essential component of chronic HIV infection here too; however, it has not been well-characterized. Previous studies have investigated IA in this younger population [16–24]; however, there is limited prior research comprehensively investigated HIV-related and non-HIV-related factors associated with IA in HIV-infected youth.
In addition to heightened IA, immune exhaustion (IE) is also increased in HIV-infected adults suppressed on cART [25]. One acceptable marker of IE is the increased expression of the inhibitory receptor, programmed cell death 1 (PD-1) on T-cells. Increased PD-1 expression levels predict the rate of HIV disease progression in adults [26, 27]. In addition, data suggest that IE could be critical in the viral persistence that characterizes chronic HIV infection [28]. To date, few studies have investigated IE in HIV-infected children [18, 23]. These studies both demonstrated an increase in IE compared to healthy controls, but only ART-naïve children were included.
Thus, the primary objective of this study was to comprehensively examine levels of IA and IE in HIV-infected youth on cART with virologic suppression or low-level viremia. We hypothesized that similar to what is seen in HIV-infected adults, HIV-infected youth on cART have increased levels of IA and IE. Secondary objectives included investigating HIV- and non-HIV-related variables associated with IA and IE in this population and comparing results to a matched healthy control group.
METHODS
Study Design/Population
This was a prospective, cross-sectional study investigating levels of IA and IE in HIV-infected youth on stable cART and age-, sex-, and race-matched healthy controls. HIV-infected youth were recruited from the HIV clinics of University Hospitals Case Medical Center, Cleveland, OH and Grady Health System, Atlanta, GA via electronic medical record system queries and case manager/provider referrals. Subjects were eligible if they were between 8–25 years of age with documented HIV-1 infection on cART for ≥12 weeks prior to enrollment and with ≥6 months cumulative duration of cART and an HIV-1 RNA level <1,000 copies/mL. Exclusion criteria included any acute illness or inflammatory condition unless complete resolution ≥30 days prior to enrollment, malignancy, or medication use (e.g. chemotherapy agents, systemic steroids) which could affect results.
Controls were 8–25 years of age and healthy and were selected so that the group matched the HIV-infected subjects in regards to sex, race, and age. Controls were recruited in multiple ways, including a) friends or family members of the HIV-infected subjects, b) physician referrals from local pediatric and adult clinics, c) extensive outreach to various local organizations, churches, and schools, and d) recruitment flyers in targeted locations throughout the two cities. Both HIV-infected subjects and healthy controls were excluded from the study if they were pregnant or lactating.
The study was reviewed and approved by the Institutional Review Boards of University Hospital Case Medical Center, Emory University and Grady Health System. All parents or legal guardians and subjects ≥18 years of age gave written informed consent to participate in the study. Subjects aged 17 years of age signed a written consent along with their parent or legal guardian. Subjects between the ages of 8–10 years gave verbal assent and those 11–16 years gave written assent.
Study Assessments
Clinical evaluations
For HIV-infected subjects and healthy controls, relevant data were obtained by questionnaire, including demographics, current and past medical history, alcohol intake, tobacco use, and drug habits. Further information was also collected from the HIV-infected subjects’ medical records including past and current medical diagnoses, CD4 nadir, detailed past and current antiretroviral (ARV) and non-ARV medication use, HIV diagnosis date, and acquisition method (perinatal or horizontal). Targeted physical examination, weight and height measurements were obtained in all subjects, and blood pressure was obtained with an automated, calibrated blood pressure machine.
Laboratory evaluations
After at least an 8-hour fast, blood was collected from all subjects for real-time measurements of insulin, glucose, and lipoprotein profile. Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR; [fasting glucose (mg/dl)× fasting insulin (mU/ml)]/405) [29]. Absolute CD4 count and plasma HIV-1 RNA level were concomitantly measured as markers of HIV disease activity.
Additional whole blood was collected in EDTA tubes for immediate plasma and peripheral blood mononuclear cells (PBMCs) isolation and cryopreservation without prior thawing until analysis. For all laboratory assessments, laboratory personnel were blinded to clinical information and HIV status.
CD4+ and CD8+ T-cell and monocyte subsets as well as their respective levels of activation and exhaustion (for T-cells only) were assessed via flow cytometry. All flow cytometry staining was performed on the cryopreserved PBMCs that were thawed in a 37°C water bath and used immediately. Multi-parametric flow cytometric analysis was performed on these cells according to standard procedures, and the following panel of fluorochrome-labeled antibodies was used: anti-CD3-Alexa 700 (clone SP34-2), anti-CD8-allophycocyanin (APC)-Cy7 (clone SK1), anti-CD16-APC (clone 3G8), anti-CD38-phycoerythrin (PE) (clone HIT2), anti-CD86-fluroescein isothiocyanate (FITC) (clone FUN-1), and anti-HLA-DR-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone G46-6) (BD Bioscience, San Jose, CA), anti-CD4-Pacific Blue (clone OKT4), and anti-CD20-Brilliant Violet 650 (clone 2H7) (Biolegend, San Diego, CA), anti-CD279 (PD-1)-PE-Cy7 (clone J105) (eBioscience, San Diego, CA), anti-CD14-ECD (RMO52) (Beckman Coulter, Brea, CA), and Live/Dead Fixable Aqua (Invitrogen, Carlsbad, CA).
Monocytes were identified by size and granularity, then as CD3−, CD20−, CD8−, CD4dim before being categorized into subsets by CD14 and CD16 expression: CD14+CD16− (classical), CD14+CD16+ (pro-inflammatory), and CD14dimCD16+ (non-classical/patrolling). T-cells were identified by size and granularity, as well as CD3+ and CD4+ or CD8+. T-cell activation and exhaustion levels were assessed by CD38, HLA-DR, and PD-1 expression, respectively. Flow cytometric acquisition was performed on an LSRII cytometer driven by FACS DiVa software and analyzed using FlowJo software (Treestar, Ashland, OR).
Monocyte activation was also assessed by measuring plasma concentrations of soluble CD14 (sCD14) with an enzyme-linked immunosorbent assay (ELISA) using Human CD14 DuoSet (R&D Systems, Minneapolis, MN). Median intra-assay and inter-assay coefficients of variance were <9%.
Statistical Considerations
Demographics and clinical and laboratory characteristics are described by study group, and HIV-related characteristics are described for the HIV-infected youth. Continuous measures are described using the appropriate measures of central tendency (mean or median) and dispersion (standard deviation (SD) or interquartile range (IQR)). Normally-distributed variables were compared using Student’s t-tests, and non-normally-distributed variables were compared using Wilcoxon rank sum tests.
Correlations between the IA markers and variables of interest within the HIV-infected group were assessed using Spearman correlation coefficients for continuous variables. Appropriate two-sample tests were used to assess marker differences in sub-groups for dichotomous variables (e.g. male vs. female). Multiple regression analyses applying a backward elimination selection method was used to determine variables independently associated with CD4 and CD8 activation. Variables were selected for inclusion in the regression analyses based on the results of the bivariate analyses.
Statistical analysis was conducted using SAS 9.2 (The SAS Institute, Cary, NC). Statistical significance was set at the 0.05 level.
RESULTS
Subject Characteristics
Study enrollment occurred from January 2012 to March 2014. A total of 136 subjects were enrolled, including 80 HIV-infected subjects and 56 healthy controls. By design, groups were well-matched for age, sex, and race (Table 1). The HIV-infected group had significantly more smokers, higher median systolic blood pressure and triglyceride level and a lower median high-density lipoprotein cholesterol level than the control group. None of the healthy controls were biologically-related to any of the HIV-infected subjects, and none had a history of in utero HIV/ARV exposure.
Table 1.
Subject Characteristics by Study Group
| Median (Q1, Q3), or no. (%) | HIV-infected N = 80 |
Controls N = 56 |
P |
|---|---|---|---|
| Age, years | 21 (17, 23) | 18 (13, 23) | 0.12 |
| Male sex | 53 (66) | 28 (50) | 0.08 |
| Black race | 73 (91) | 46 (82) | 0.12 |
| Body mass index, kg/m2 | 22.5 (19.6, 25.3) | 22.0 (19.1, 27.5) | 0.88 |
| Systolic BP, mmHg | 122 (114, 129) | 110 (103, 121) | <0.01 |
| Diastolic BP, mmHg | 73 (68, 80) | 69 (64, 77) | 0.05 |
| HDL cholesterol, mg/dL | 47 (40, 56) | 54 (45, 63) | <0.01 |
| LDL cholesterol, mg/dL | 87 (69, 112) | 92 (69, 113) | 0.96 |
| Triglycerides, mg/dL | 79 (64, 114) | 70 (47, 87) | 0.01 |
| HOMA-IR | 2.0 (1.4, 3.8) | 2.1 (1.5, 3.0) | 0.73 |
| Current smoking | 23 (29) | 4 (7) | <0.01 |
| HIV duration, years | 6.5 (2.2, 14.5) | N/A | N/A |
| Current CD4 count, cells/mm3 | 652 (421, 872) | N/A | N/A |
| CD4 nadir, cells/mm3 | 290 (154, 424) | N/A | N/A |
| Undetectable HIV-1 RNA | 63 (79%) | N/A | N/A |
| HIV-1 RNA level if not undetectable, copies/mL (N=17)* | 89 (31, 590) | N/A | N/A |
| HIV-1 RNA <80 copies/mL | 70 (88%) | N/A | N/A |
| HIV-1 RNA level if >80 copies/mL (N=10) | 605 (143, 674) | N/A | N/A |
| Current ART use | 80 (100%) | N/A | N/A |
| Current NNRTI use | 33 (41%) | N/A | N/A |
| Current PI use | 49 (61%) | N/A | N/A |
| Cumulative duration of ART, years | 2.8 (1.2, 10.0) | N/A | N/A |
| Cumulative duration of NRTI, years | 2.6 (1.2, 8.8) | N/A | N/A |
| Cumulative duration of NNRTI, years | 0.6 (0, 2.2) | N/A | N/A |
| Cumulative duration of PI, years | 1.8 (0.6, 6.6) | N/A | N/A |
| Perinatal transmission | 36 (46%) | N/A | N/A |
| Prior AIDS diagnosis** | 28 (35%) | N/A | N/A |
Because there were several different assays used in the clinical laboratories to measure HIV-1 RNA levels, there were varying lower limits of detection (<20, <40, <48, <79 and <80 copies/mL). <80 copies/mL was the highest cut-off that defined an undetectable HIV-1 RNA.
As classified by the CDC 2008 Revised Surveillance Case Definition [47]
Q, quartile; BP, blood pressure; HDL, high-density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; N/A, not applicable; ART, antiretroviral therapy; NRTI, nucleoside analogue reverse transcriptase inhibitor; NNRTI, non-nucleoside analogue reverse transcriptase inhibitor; PI, protease inhibitor; CDC, Centers for Disease Control
Levels of IA and IE
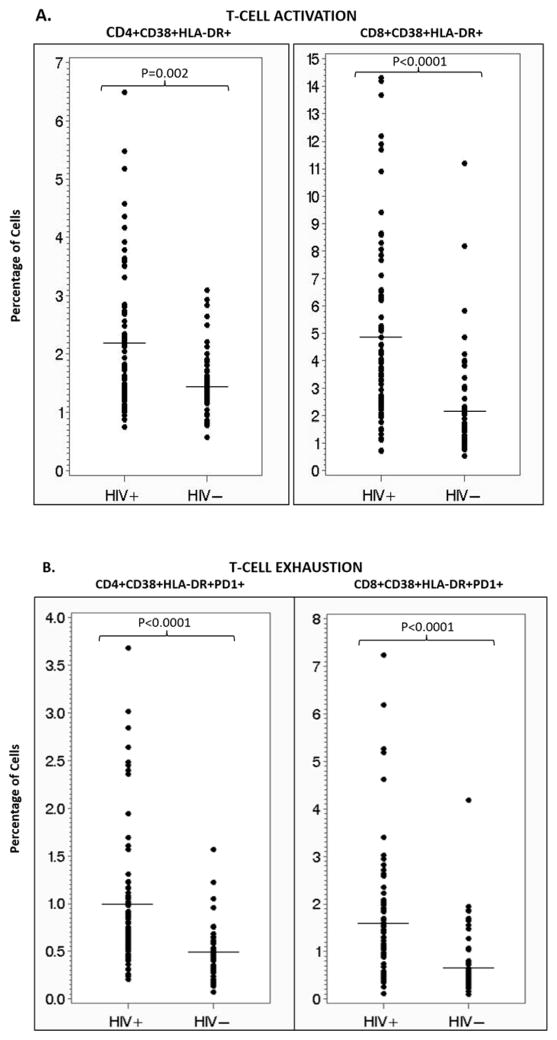
The mean (SD) of percent activated and exhausted CD4+ and CD8+ T-cells, as measured by co-expression of CD38 and HLA-DR and CD38, HLA-DR, and PD-1, respectively, was increased in the HIV-infected group compared to matched healthy controls (Figure 1A and 1B).
Figure 1. T-cell activation and exhaustion for HIV-infected subjects vs. controls.
These scatter plots show the percentage of activated (A) and exhausted (B) CD4+ and CD8+ T-cells for the HIV-infected subjects compared to matched healthy controls. In all cases, the HIV-infected subjects have a significantly higher percentage of activated and exhausted CD4+ and CD8+ T-cells. Horizontal lines denote means. Mean % (standard deviation (SD)) CD4+CD38+HLD-DR+ was 2.2 (1.2) and 1.5 (0.6) for HIV-infected and controls, respectively. Mean % (SD) CD8+CD38+HLA-DR+ was 4.9 (3.3) and 2.2% (1.9) for HIV-infected and controls, respectively. Mean % (SD) for CD4+CD38+HLD-DR+-PD1+ was 1.0% (0.8) and 0.5% (0.3) for HIV-infected and controls, respectively. Mean % (SD) for CD8+CD38+HLD-DR+-PD1+ was 1.6% (1.4) and 0.7% (0.7) for HIV-infected and controls, respectively.
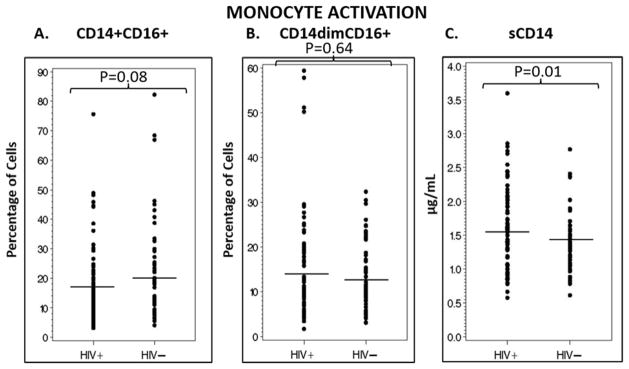
In contrast, the mean (SD) of percent CD14+CD16+ inflammatory and CD14dimCD16+ patrolling monocytes was similar between the HIV-infected and control groups (Figure 2A and 2B). However, the concentration of sCD14 was statistically higher in the HIV-infected group compared to the controls (Figure 2C).
Figure 2. Monocyte activation for HIV-infected subjects vs. controls.
These scatter plots show the percentage of inflammatory (A) and patrolling (B) monocytes, as well as the plasma concentration of sCD14 (C), a marker of monocyte activation, in HIV-infected subjects compared to healthy matched controls. Only sCD14 was significantly different between groups. Horizontal lines denote means. Mean % (standard deviation (SD)) CD14+CD16+ was 17.1% (13.1) vs. 21.6% (16.5), for HIV-infected and controls, respectively. Mean % (SD) CD14dimCD16+ was 14.3% (11.9) and 13.3% (7.6) for HIV-infected and control groups, respectively. sCD14 concentration was 1.6 (0.60) μg/mL and 1.4 (0.44) μg/mL for HIV-infected and control groups, respectively. sCD14, soluble CD14
Relationships between IA and Variables of Interest
Correlations between IA and variables of interest are described in Table 2. The most consistent non-HIV-related variable positively associated with both activated and exhausted CD4+ and CD8+ T-cells was age. The most consistent HIV-related variables negatively correlated with both CD4+ and CD8+ T-cell activation were ART and NRTI duration and current CD4 count. There were significant differences in both CD4 and CD8 activation and exhaustion markers when the following HIV-infected sub-groups were compared: male vs. female, HIV-1 RNA detectable vs. not detectable, and horizontal vs. perinatal transmission (Table 3).
Table 2.
Correlations Between Variables of Interest and Markers of Immune Activation and Exhaustion in the HIV-infected Subjects
| A. | % CD4+CD38+HLA-DR+1 | % CD8+CD38+HLA-DR+2 | |
|---|---|---|---|
| Age, years | R=0.36 P<0.01 |
R=0.39 <0.01 |
|
| LDL cholesterol, mg/dL | R=−0.29 P=0.01 |
R=−0.23 P=0.04 |
|
| HDL cholesterol, mg/dL | R=−0.42 P<0.01 |
R=−0.24 P=0.04 |
|
| HIV duration, years | NS | R=−0.27 P=0.02 |
|
| ART duration, years | R=−0.33 P<0.01 |
R=−0.34 P<0.01 |
|
| NRTI duration, years | R=−0.35 P<0.01 |
R=−0.34 P<0.01 |
|
| Current CD4 count, cells/mm3 | R=−0.33 P<0.01 |
R=−0.33 P<0.01 |
|
| % CD4+CD38+HLA-DR+ | N/A | R=0.71 P<0.01 |
|
| % CD4+CD38+HLA-DR+PD1+ | R=0.89 P<0.01 |
R=0.75 P<0.01 |
|
| % CD8+CD38+HLA-DR+PD1+ | R=0.46 P<0.01 |
R=0.76 P<0.01 |
|
| % CD14+CD16+ | R=0.26 P=0.03 |
NS | |
| % CD14dimCD16+ | R=−0.42 P<0.01 |
NS | |
| B. | % CD4+CD38+HLA-DR+PD1+3 | % CD8+CD38+HLA-DR+PD1+4 | |
| Age, years | R=0.48 P<0.01 |
R=0.24 P=0.03 |
|
| LDL cholesterol, mg/dL | R=−0.24 P=0.04 |
NS | |
| HDL cholesterol, mg/dL | R=−0.37 <0.01 |
NS | |
| ART duration, years | R=−0.38 P=0.04 |
NS | |
| NRTI duration, years | R=−0.39 P<0.01 |
NS | |
| Current CD4 count, cells/mm3 | R=−0.43 P<0.01 |
R=−0.3 P<0.01 |
|
| % CD8+CD38+HLA-DR+PD1+ | N/A | R=0.60 P<0.01 |
|
| % CD14+CD16+ | R=0.30 P=0.02 |
R=0.30 P<0.01 |
|
| % CD14dimCD16+ | R=−0.31 P<0.01 |
NS | |
| C. | % CD14+CD16+5 | % CD14dimCD16+6 | sCD147 |
| Waist-to-hip ratio | NS | R=−0.25 P=0.02 |
NS |
| NNRTI duration, years | NS | NS | R=0.33 P=0.03 |
| Total use of EFV, years | NS | NS | R=0.31 P=0.01 |
Mean % of CD4+ T-cells expressing CD38 and HLA-DR;
Mean % of CD8+ T-cells expressing CD38 and HLA-DR;
Mean % of CD4+ T-cells expressing CD38, HLA-DR, and PD-1;
Mean % of CD8+ T-cells expressing CD38, HLA-DR, and PD-1;
Mean % of monocytes expressing CD14+CD16+;
Mean % of monocytes expressing CD14dimCD16+;
Mean plasma concentration of sCD14
N.B. For continuous variables, Spearman Rank Correlation Coefficient was used to assess correlation. Number on top represents Spearman Correlation Coefficient and number on bottom represents P-value. CD4 nadir also tested but did not have any significant relationships. For each correlation between two immune activation/exhaustion markers, the inverse correlation is also true, but for simplicity, is listed only once.
NS, not significant; LDL, low density lipoprotein; HDL, high-density lipoprotein; ART, antiretroviral therapy; NRTI, nucleoside analogue reverse transcriptase inhibitor; N/A, not applicable; sCD14, soluble CD14; NNRTI, non-nucleoside analogue reverse transcriptase inhibitor; EFV, efavirenz
Table 3.
Differences in Markers of Immune Activation and Exhaustion in Sub-groups among HIV-infected Subjects
| % CD4+CD38+HLA-DR+1 | P* | % CD8+CD38+HLA-DR+2 | P | % CD4+CD38+HLA-DR+PD1+3 | P | % CD8+CD38+HLA-DR+PD1+4 | P | |
|---|---|---|---|---|---|---|---|---|
| Male sex | 2.3 (1.2) | 0.069 | 5.4 (3.3) | 0.02 | 1.1 (0.9) | 0.04 | 1.9 (1.5) | 0.01 |
| Female sex | 1.9 (1.2) | 3.8 (3.0) | 0.8 (0.6) | 1.1 (0.8) | ||||
| Detectable HIV-1 RNA | 2.6 (1.1) | 0.047 | 6.6 (3.6) | 0.02 | 1.4 (1.0) | 0.03 | 2.4 (2.0) | 0.04 |
| Undetectable HIV-1 RNA | 2.1 (1.2) | 4.4 (3.0) | 0.9 (0.7) | 1.4 (1.0) | ||||
| Horizontal Transmission | 2.3 (1.1) | 0.065 | 5.7 (3.3) | 0.003 | 1.2 (0.8) | 0.003 | 1.8 (1.5) | 0.22 |
| Perinatal transmission | 2.0 (1.2) | 3.9 (3.0) | 0.9 (0.8) | 1.5 (1.2) |
Mean % (SD) of CD4+ T-cells expressing CD38 and HLA-DR;
Mean % (SD) of CD8+ T-cells expressing CD38 and HLA-DR;
Mean % (SD) of CD4+ T-cells expressing CD38, HLA-DR, and PD-1;
Mean % (SD) of CD8+ T-cells expressing CD38, HLA-DR, and PD-1;
P-value represents the significance level for differences in mean % of T-cells expressing given phenotype for sub-group (e.g. male vs. female). Other sub-groups tested but were not significant include black race, current smoking, current marijuana use, current alcohol use, and previous AIDS diagnosis. There were no significant differences for % mean for CD14+CD16+ and CD14dimCD16+, or levels of soluble CD14 between any sub-group.
Multivariable Regression Models
Using stepwise, backward elimination regression models, the variables most associated with both activated CD4+ and CD8+ T-cells included age, LDL cholesterol, and current CD4 cell count (Table 4). In the final models, only age and LDL cholesterol were statistically associated with activated CD4+ T-cells, and only age was statistically associated with activated CD8+ T-cells.
Table 4.
Final Multiple Regression Models for Variables Associated with T-cell Activation in HIV-infected Subjects
| A. CD4 Activation1
| |||
|---|---|---|---|
| Variable | Beta | SE (Beta) | P |
| Age | 0.0631 | 0.0285 | 0.03 |
| LDL Cholesterol | −0.0081 | 0.0040 | 0.04 |
| Current CD4 count | −0.0007 | 0.0005 | 0.12 |
| R2 = 0.19 | |||
|
| |||
| B. CD8 Activation2 | |||
|
| |||
| Variable | Beta | SE (Beta) | P |
|
| |||
| Age | 0.1885 | 0.0764 | 0.02 |
| LDL Cholesterol | −0.0190 | 0.0108 | 0.08 |
| Current CD4 count | −0.0024 | 0.0012 | 0.06 |
|
| |||
| R2 = 0.20 | |||
Mean % of CD4+ T-cells expressing CD38 and HLA-DR;
Mean % of CD8+ T-cells expressing CD38 and HLA-DR
N.B. Other variables included in the initial models but eliminated using backward elimination selection method include sex, detectable HIV-1 RNA, ART duration, HIV duration
LDL, low-density lipoprotein; ART, antiretroviral therapy
Sub-Group Post-Hoc Analysis
After the initial results were known, a post-hoc analysis was conducted to try to account for the variability of time that subjects may have been virologically-suppressed prior to study enrollment. A sub-group of HIV-infected subjects was created who had known sustained virologic suppression, which was defined as an HIV-1 RNA level <80 copies/mL for at 6 months prior to enrollment. Less than 80 copies/mL was chosen because there were several different assays used in the clinical laboratories to measure HIV-1 RNA levels with varying lower limits of detection (<20, <40, <48, <79 and <80 copies/mL). Less than 80 copies/mL was the highest cut-off among these assays to define undetectable HIV-1 RNA. We compared marker values for this sub-group (N = 46) to those of the healthy controls. CD8+ activation, CD4+ exhaustion, CD8+ exhaustion, patrolling monocytes, and sCD14 followed a similar pattern and magnitude to the results from the entire cohort. However, there was no longer a statically significant difference between groups for CD4+ activation (P = 0.43), and inflammatory monocytes were statistically higher in the controls (P = 0.03). Sustained virologic suppression was added as a variable to the multivariable regression of CD4+ activation, and it was not significant in the model (data not shown).
DISCUSSION
To our knowledge, this is the first study that comprehensively investigated levels of activated and exhausted T-cells and monocyte activation in HIV-infected youth compared to matched healthy controls. Importantly, we enrolled a well-characterized group of HIV-infected subjects that were on cART with virologic suppression or low-level viremia to determine the effects of residual IA unrelated to ongoing active viral replication. We found that both activated and exhausted CD4+ and CD8+ T-cells were significantly higher in the HIV-infected group compared to the controls. Activated monocytes were more closely matched between groups, although sCD14 concentrations were statistically higher in the HIV-infected group compared to the levels in healthy controls.
In addition, an extensive investigation was undertaken to understand the variables most associated with IA and IE in this population. Arguably, current CD4 count was the most important HIV-related variable related to activated and exhausted T-cells. Age and LDL cholesterol appeared to be important non-HIV-related variables associated with activated CD4+ and CD8+ T-cells. There were fewer relevant variables associated with activated monocytes.
The increased percentage of activated CD4+ T-cells in HIV-infected children and young adults compared to healthy controls is consistent with other pediatric studies that investigated ART-treated, virologically-suppressed subjects [16, 17]. These previous reports conflicted about whether CD8+ T-cells are also increased in this population; however, the study Sainz, et al that did not show an increase, only evaluated 29 HIV-infected subjects and 9 controls. In our current study, with a larger sample size, ART-treated children and young adults have both an increased activated CD4+ and CD8+ T-cell population similar to adults [9, 30].
These findings may have serious implications for HIV-infected youth’s quality of life. While cART dramatically restores health, HIV-infected individuals have more age-related co-morbidities than individuals in the general population [31]. This is partly due to the increased IA that persists despite cART. In HIV-infected adults, T-cell activation is strongly predictive of disease progression and mortality in multiple studies [8, 9]. In addition, increased monocyte and T-cell activation is associated with HIV-related co-morbidities, such as CVD [3, 32]. It is generally unknown whether these relationships exist in HIV-infected youth, in whom chronic IA may be present for an entire lifetime (i.e., for perinatally-infected youth).
In our study, current CD4 count was negatively associated with both activated CD4+ and CD8+ T-cells and remained in both final multivariable regression models. This is not a surprising finding given that in adult studies on-going IA is associated with poorer recovery of CD4 count despite effective cART [9, 30, 33], and a higher frequency of activated CD8+ T-cells predicts a faster decrease of CD4 cells [34]. Thus, this association is an important finding for HIV-infected youth, as they will likely live with chronic HIV infection for many decades. Interventions aimed at decreasing residual IA should be investigated in this population. Statins, for example, have been shown to decrease levels of T-cell activation in HIV-infected adults [35].
We also saw an inverse relationship between LDL cholesterol and CD4+ and CD8+ T-cell activation (although only CD4+ activation was statistically significant in the multivariable regression model), where increased cell activation was associated with lower LDL cholesterol. This finding is consistent with previous studies showing that cytokines influence lipid and lipoprotein metabolism. For example, interferons decrease LDL cholesterol in non-HIV populations [36–38]. Thus, this inverse relationship likely represents levels of endogenous interferon activity (and other cytokines) which would be higher when CD4+ and CD8+ T-cell activation are increased.
Immune exhaustion is another feature of chronic HIV infection and is characterized by the expression of PD-1 on CD4+ and CD8+ T-cells. It is defined by loss of effector functions and proliferative capacity in memory T-cells [39]. The percent of IE was also increased in our study in the HIV-infected group compared to the healthy controls. This is consistent with adult studies [25] and one published pediatric study that investigated IE in solely ART-naïve subjects [18]. T-cell exhaustion was also associated with T-cell activation similar to the work in HIV-infected adults by the Pahwa group [40]. As with T-cell activation, T-cell exhaustion is predictive of HIV disease progression in HIV-infected adults [26, 27], and likewise should be further investigated in HIV-infected youth.
Interestingly, there was no statistically significant difference between groups for the percent of cells expressing the phenotype of activated monocytes. This may, however, be due to inadequate power, as concentrations of sCD14 were statistically higher in the HIV-infected group compared to the controls. The sCD14 concentrations found in this study among the HIV-infected youth were similar to other studies in both adults and pediatrics [16, 41].
Soluble CD14 is a marker of monocyte activation and independently predicts mortality in HIV-infected adults [7, 42, 43]. It is the receptor for lipopolysaccharide, a component of the cell wall of gram-negative bacteria, which circulates in the plasma after microbial translocation from a compromised intestinal mucosal barrier. These subsequent microbial products bind membrane or soluble CD14, contributing to IA [44, 45]. While this relationship has been clearly demonstrated in adults with HIV [43], only two studies have evaluated the relationship between sCD14 and gut microbial translocation in HIV-infected children. In both studies, the authors demonstrated a significant bivariate relationship between sCD14 and lipopolysaccharide levels [16, 18]. However, studies are needed in this younger population to investigate whether increased levels of sCD14 are predictive of mortality and/or disease progression in HIV.
Interestingly, sCD14 was positively correlated with cumulative duration of NNRTI and efavirenz use. This finding is consistent with a recent study that evaluated changes in plasma markers of inflammation and IA in ART-naïve HIV-infected adults initiating therapy with either elvitegravir/cobicistat/emtricitabine/tenofovir (EVG/c/FTC/TDF) or efavirenz/emtricitabine/tenofovir (EFV/FTC/TDF). In subjects who were in the EFV/FTC/TDF group, percent increases in sCD14 were seen at the 24- and 48-week time points, where both within-group and between-group changes were statistically significant [46]. The reason that EFV would cause an increase in sCD14 is unclear, however, and should be investigated further. Another interesting finding was that CD8+ activation and CD4+ exhaustion was higher in subjects who acquired HIV via horizontal transmission. These results follow the same pattern as ARV and HIV duration for CD8+ activation and ARV duration for CD4+ exhaustion, where the shorter the duration, the higher the marker. Thus, the significant relationship between horizontal transmission and CD8+ activation and CD4+ exhaustion is likely due to ARV and HIV duration as a reflection of virologic status, rather than mode of transmission per se. It should be pointed out, however, that neither ARV nor HIV duration was significant in the multivariable regression model with CD4+ activation.
There are limitations to this study. As a cross-sectional study, the associations demonstrated cannot prove causality, despite the potentially important findings. In addition, the subjects spanned a wide age range, including some younger and perinatally-infected subjects. However, this may be the population who would benefit the most from interventions which would decrease IA and IE, given the number of decades that they will live with HIV. Likewise, some of the HIV-infected subjects had low-level viremia, which may have affected the results. We tried to account for this possibility by conducting a sub-analysis including only subjects with sustained virologic suppression for at least 6 months prior to study enrollment. Results were similar for the majority of the markers with the exception of CD4+ T-cell activation and inflammatory monocytes. However, when sustained virologic suppression was included in the multivariable regression model with CD4+ T-cell activation, it was not significant. Thus, the lack of significance between the sub-group and the controls for CD4+ T-cell activation may be related to a smaller HIV-infected sample size vs. decreased (but not normal) CD4+ T-cell activation after 6 months of virologic suppression. It is unclear why the level of inflammatory monocytes in the HIV-infected sub-group was statistically lower than in the controls; however, there may be unmeasured confounders in this sub-group which would have to be further explored before drawing any conclusions. Finally, this study was not powered and investigated a number of associations between IA and IE and variables of interest, which increases the risk of Type I errors. Nevertheless, these exploratory data provide a substrate from which to design future trials.
This study offers insight into IA and IE in HIV-infected youth. These results are novel as no previous pediatric study has comprehensively studied both IA and IE in cART-treated subjects. Given the association with HIV disease progression, mortality, and the risk of HIV-related co-morbidities, such as CVD, in HIV-infected adults, it is imperative that we investigate this important topic in HIV-infected youth. Additional studies, particularly longitudinal trials, are needed to determine the long-term effect of increased IA and IE in this younger population, and serious consideration should be given to conducting randomized, placebo-controlled trials of potential interventions to minimize the negative effects of chronic IA and IE.
Acknowledgments
Sources of Support: This work was made possible by the National Institute of Child Health and Development at the National Institutes of Health [K23 HD069199 to ARE; R01 HD070490 to GAM; K12 HD072245 to AC], Case Western Reserve University’s Center for AIDS Research (P30 AI36219), Emory University’s Center for AIDS Research (P30 AI050409), Emory+Children’s Pediatric Research Center (Biomarkers, Immunology and Flow Cytometry Cores), and the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ARE and AC contributed to study design, data acquisition, analysis and interpretation, and manuscript preparation; JCR contributed to data acquisition and manuscript preparation; STL contributed to data acquisition and interpretation; MAO contributed to data analysis and interpretation and manuscript preparation; JGH, JED, BK, and DL contributed to data acquisition; AC contributed to study design, data acquisition, analysis and interpretation, and manuscript preparation. GAM obtained study funding, contributed to study design, data acquisition, data analysis and interpretation, and manuscript preparation.
Footnotes
Previous Publication: Data were presented at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington D.C., Sept 2014. Abstract G-987.
Conflicts of Interest: ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor and speaker for Gilead. GAM serves as a consultant for Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Gilead, Pfizer, and ICON, and has received grant funding from Bristol-Myers Squibb, ViiV/GlaxoSmithKline, and Gilead. All other authors declare no conflicts of interest.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 3.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: a primer. Neuropsychol Rev. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalayjian RC, Machekano RN, Rizk N, Robbins GK, Gandhi RT, Rodriguez BA, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–1805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 9.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 10.Ross AC, Storer N, O’Riordan MA, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J. 2010;29:634–638. doi: 10.1097/inf.0b013e3181d770c4. [DOI] [PubMed] [Google Scholar]
- 11.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Vigano A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 12.Uban KA, Herting MM, Williams PL, Ajmera T, Gautam P, Huo Y, et al. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS. 2015;29:1035–1044. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsuran D, Bhimma R, Ramdial PK, Naicker E, Adhikari M, Deonarain J, et al. The spectrum of HIV-related nephropathy in children. Pediatr Nephrol. 2012;27:821–827. doi: 10.1007/s00467-011-2074-8. [DOI] [PubMed] [Google Scholar]
- 14.DiMeglio LA, Wang J, Siberry GK, Miller TL, Geffner ME, Hazra R, et al. Bone mineral density in children and adolescents with perinatal HIV infection. AIDS. 2013;27:211–220. doi: 10.1097/QAD.0b013e32835a9b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McComsey GA, O’Riordan M, Hazen SL, El-Bejjani D, Bhatt S, Brennan ML, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21:921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 16.Madrid L, Noguera-Julian A, Falcon-Neyra L, Fortuny C, De Felipe B, Torrebadell M, et al. Microbial translocation and T cell activation are not associated in chronic HIV-infected children. AIDS. 2014;28:1989–1992. doi: 10.1097/QAD.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 17.Sainz T, Alvarez-Fuente M, Navarro ML, Diaz L, Rojo P, Blazquez D, et al. Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: the CaroVIH Study. J Acquir Immune Defic Syndr. 2014;65:42–49. doi: 10.1097/QAI.0b013e3182a9466a. [DOI] [PubMed] [Google Scholar]
- 18.Pilakka-Kanthikeel S, Kris A, Selvaraj A, Swaminathan S, Pahwa S. Immune activation is associated with increased gut microbial translocation in treatment-naive, HIV-infected children in a resource-limited setting. J Acquir Immune Defic Syndr. 2014;66:16–24. doi: 10.1097/QAI.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz L, Mendez-Lagares G, Correa-Rocha R, Pacheco YM, Ferrando-Martinez S, Ruiz-Mateos E, et al. Detectable viral load aggravates immunosenescence features of CD8 T-cell subsets in vertically HIV-infected children. J Acquir Immune Defic Syndr. 2012;60:447–454. doi: 10.1097/QAI.0b013e318259254f. [DOI] [PubMed] [Google Scholar]
- 20.Jin CZ, Feng L, Xie TS, Wu LJ, Fang MX, Zhang FJ, et al. Expression of CD38 and HLA-DR on CD8+ T cells in pediatric AIDS patients receiving highly active antiretroviral therapy (HAART) Zhonghua Er Ke Za Zhi. 2011;49:49–52. [PubMed] [Google Scholar]
- 21.Romeiro JR, Pinto JA, Silva ML, Eloi-Santos SM. Further evidence that the expression of CD38 and HLA-DR(+) in CD8(+) lymphocytes does not correlate to disease progression in HIV-1 vertically infected children. J Int Assoc Physicians AIDS Care (Chic) 2012;11:164–168. doi: 10.1177/1545109711421642. [DOI] [PubMed] [Google Scholar]
- 22.Rainwater-Lovett K, Nkamba H, Mubiana-Mbewe M, Moore CB, Margolick J, Moss WJ. Changes in cellular immune activation and memory T-cell subsets in HIV-infected Zambian children receiving HAART. J Acquir Immune Defic Syndr. 2014;67:455–462. doi: 10.1097/QAI.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prendergast A, O’Callaghan M, Menson E, Hamadache D, Walters S, Klein N, et al. Factors influencing T cell activation and programmed death 1 expression in HIV-infected children. AIDS Res Hum Retroviruses. 2012;28:465–468. doi: 10.1089/AID.2011.0113. [DOI] [PubMed] [Google Scholar]
- 24.Falcon-Neyra L, Benmarzouk-Hidalgo OJ, Madrid L, Noguera-Julian A, Fortuny C, Neth O, et al. No Differences of Immune Activation and Microbial Translocation Among HIV-infected Children Receiving Combined Antiretroviral Therapy or Protease Inhibitor Monotherapy. Medicine (Baltimore) 2015;94:e521. doi: 10.1097/MD.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breton G, Chomont N, Takata H, Fromentin R, Ahlers J, Filali-Mouhim A, et al. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol. 2013;191:2194–2204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 27.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 28.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 31.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 32.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006;120:163–170. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 34.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans RD, Argiles JM, Williamson DH. Metabolic effects of tumour necrosis factor-alpha (cachectin) and interleukin-1. Clin Sci (Lond) 1989;77:357–364. doi: 10.1042/cs0770357. [DOI] [PubMed] [Google Scholar]
- 37.Schectman G, Kaul S, Mueller RA, Borden EC, Kissebah AH. The effect of interferon on the metabolism of LDLs. Arterioscler Thromb. 1992;12:1053–1062. doi: 10.1161/01.atv.12.9.1053. [DOI] [PubMed] [Google Scholar]
- 38.Massaro ER, Borden EC, Hawkins MJ, Wiebe DA, Shrago E. Effects of recombinant interferon-alpha 2 treatment upon lipid concentrations and lipoprotein composition. J Interferon Res. 1986;6:655–662. doi: 10.1089/jir.1986.6.655. [DOI] [PubMed] [Google Scholar]
- 39.Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep. 2011;8:4–11. doi: 10.1007/s11904-010-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastard JP, Fellahi S, Couffignal C, Raffi F, Gras G, Hardel L, et al. Increased systemic immune activation and inflammatory profile of long-term HIV-infected ART-controlled patients is related to personal factors, but not to markers of HIV infection severity. J Antimicrob Chemother. 2015 doi: 10.1093/jac/dkv036. [DOI] [PubMed]
- 42.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210:1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, et al. Differential Reduction in Monocyte Activation and Vascular Inflammation With Integrase Inhibitor-Based Initial Antiretroviral Therapy Among HIV-Infected Individuals. J Infect Dis. 2015;212:345–354. doi: 10.1093/infdis/jiv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years--United States, 2008. MMWR Recomm Rep. 2008;57:1–12. [PubMed] [Google Scholar]