Abstract
Cysteine is a highly reactive amino acid and is subject to a variety of reversible post-translational modifications (PTMs), including nitrosylation, glutathionylation, palmitoylation, as well as formation of sulfenic acid and disulfides. These modifications are not only involved in normal biological activities, such as enzymatic catalysis, redox signaling and cellular homeostasis, but can also be the result of oxidative damage. Especially in aging and neurodegenerative diseases, oxidative stress leads to aberrant cysteine oxidations that affect protein structure and function leading to neurodegeneration as well as other detrimental effects. Methods that can identify cysteine modifications by type, including the site of modification, as well as the relative stoichiometry of the modification can be very helpful for understanding the role of the thiol proteome and redox homeostasis in the context of disease. Cysteine reversible modifications however, are challenging to investigate as they are low abundant, diverse, and labile especially under endogenous conditions. Thanks to the development of redox proteomic approaches, large-scale quantification of cysteine reversible modifications is possible. These approaches cover a range of strategies to enrich, identify, and quantify cysteine reversible modifications from biological samples. This review will focus on nongel-based redox proteomics workflows that give quantitative information about cysteine PTMs and highlight how these strategies have been useful for investigating the redox thiol proteome in aging and neurodegenerative diseases.
Keywords: cysteine, reversible PTMs, oxidation, mass spectrometry, quantitative proteomics, redox proteomics, aging, neurodegenerative diseases, Alzheimer’s disease, review
1. Introduction
Aging is a complex process accompanied by decline in biological and physiological functions of many organs [1]. Aging is also one of the most significant risk factors for neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [2]. An imbalance of reactive oxygen/nitrogen species (ROS/RNS) with antioxidant defense systems in aging and neurodegenerative diseases is well-known [3], and oxidative stress has been reported in many organs [4, 5], tissues and fluids [6, 7] from animal models and human subjects. ROS/RNS while detrimental in some cases, serve as important regulatory mechanisms for many cellular activities [8]. Thus oxidative post-translational modifications (PTMs) on various biomolecules, including DNA, RNA and proteins [9] can be the result of helpful cellular signaling processes, the result of oxidative damage, or a transient used to prevent oxidative damage from occurring in a stressful cellular environment.
One of the most susceptible amino acids to oxidative PTMs is cysteine. Cysteine is a rare amino acid with a natural occurrence of 2.3% among all amino acids in the mammalian proteome [10]. The percentage of cysteinyl peptides is only ~15% after in silico digestion of the whole human proteome [10]. Cysteine is highly nucleophilic and redox sensitive compared with other amino acid side chains. It is involved in redox homeostasis, enzymatic catalysis, signal conduction, metal binding and structural stabilization [11, 12]. The pKa value of the cysteine thiol is ~ 8.0 but can be as low as 3.5 in some proteins due to electrostatic interactions and hydrogen bonding [13]. The low pKa results in spontaneous in vivo reactions of cysteine with electrophilic and/or oxidizing molecules [14], such as lipid derived electrophiles.
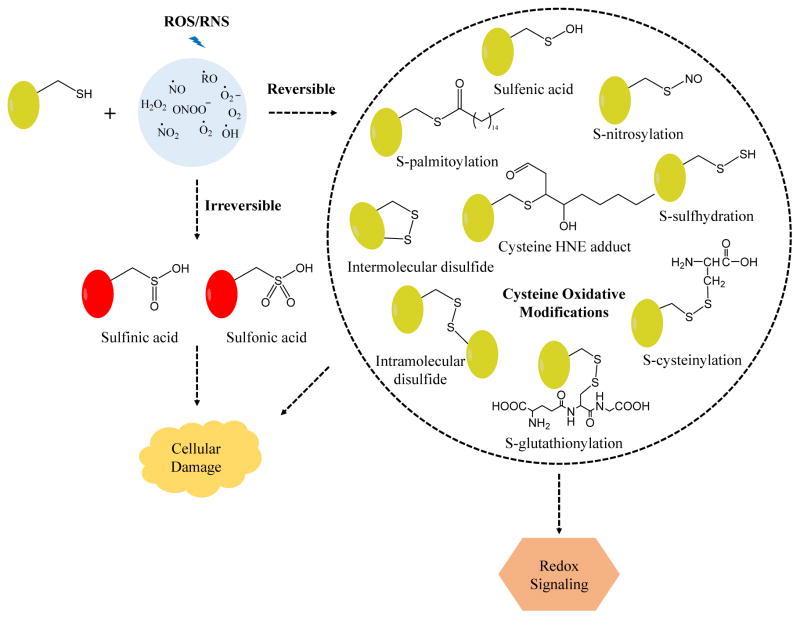
Common reversible modifications of cysteine include formation of sulfenic acid (SOH), S-nitrosylation (SNO), S-glutathionylation (SSG), S-palmitoylation, and disulfide bonds [15] (Figure 1). In vivo it is estimated that ~ 10% of cysteine residues are reversibly oxidized [16]. These reversible PTMs have important biological roles and help maintain homeostasis by preventing the formation of irreversible oxidative modifications [e.g., sulfinic (SO2H) and sulfonic acid (SO3H)] [17]. Furthermore, these modifications are critical for cellular signaling. Dyshomeostasis resulting from reversible or irreversible cysteine PTMs can lead to cellular damage. SOH can be an intermediate state of cysteines often located in the active site and occur during enzymatic catalysis. For example, Cys96 of peroxiredoxin-5 switches between free form and SOH to catalyze the degradation of peroxides [18]. SNO results from the attack of endogenous nitric oxide (NO) on free cysteines, and is believed to be important for cellular signal transduction pathways, similar to phosphorylation [19]. SSG is the reversible formation of protein disulfides with glutathione (GSH) and can modulate protein activity. SOH and SNO derivatives can be reduced by glutaredoxin to SSG, which is an important mechanism to maintain thiol homeostasis [20]. S-Palmitoylation is the covalent lipid modification of cysteine with the 16-carbon fatty acid palmitate (CH3(CH2)14COOH), and this PTM helps to regulate protein trafficking and subcellular localization [21]. Disulfide bonds are important for maintaining protein tertiary structure and regulating protein function [22]. Finally, cysteine can be modified by electrophiles such as lipid adducts where common PTMs are lipid peroxidation products such as hydroxynonenal (HNE). HNE-modified cysteines can occur through both enzymatic and nonenzymatic reactions [15] and have been reported to inhibit protein disulfide isomerase in rat liver mitochondrion [23].
Figure 1.
Representative oxidative modifications of cysteine by ROS/RNS. Low levels of ROS/RNS lead to reversible cysteine modifications, including S-glutathionylation, S-nitrosylation, S-palmitoylation, S-sulfhydration, sulfenic acid and disulfides formation. These modifications have important roles in various normal cellular activities but aberrant cysteine reversible modifications can result in detrimental effects. ROS/RNS can also oxidize cysteine irreversibly, and result in loss of protein function and cellular damage.
Thiol-based redox regulation is important in metabolism. Dysregulated redox homeostasis of thiols has been implicated in aging and diseases, such as cancer, diabetes, cardiovascular and neurodegenerative diseases [14]. Better understanding of the landscape of the thiol redox proteome can give insight to biochemical events that occur in disease, and may lead to potential biomarkers for disease diagnosis and therapy [24]. Redox proteomics can detect hundreds to thousands of oxidized proteins in a single experiment and this is attractive for understanding redox status of proteins [7]. Designing strategies to study cysteine PTMs can be challenging however, as: 1) cysteine PTMs are in low abundance and highly diverse, 2) the modifications are labile and dynamic, and 3) potentially small changes in oxidative PTM levels may occur between different biological conditions [25]. Redox proteomics approaches have sought to address these challenges through enrichment, identification and quantification of cysteine oxidative PTMs with gel-based or nongel-based [25] strategies. Gel-based methods offer direct detection of cysteine modifications via electrophoretic gel separation and immunoblotting [26]. In recent years, nongel-based methods that incorporate multi-dimensional chromatographic separations and high-throughput protein quantification by mass spectrometry (MS) have become more popular [27–29].
In this review, different proteomic methods to study and quantify cysteine modifications will be discussed with more attention on MS-based quantitative profiling of cysteine PTMs using nongel-based approaches. The application of redox proteomics to understand aging and neurodegenerative diseases will also be presented.
2. Redox proteomic approaches to quantify cysteine reversible modifications
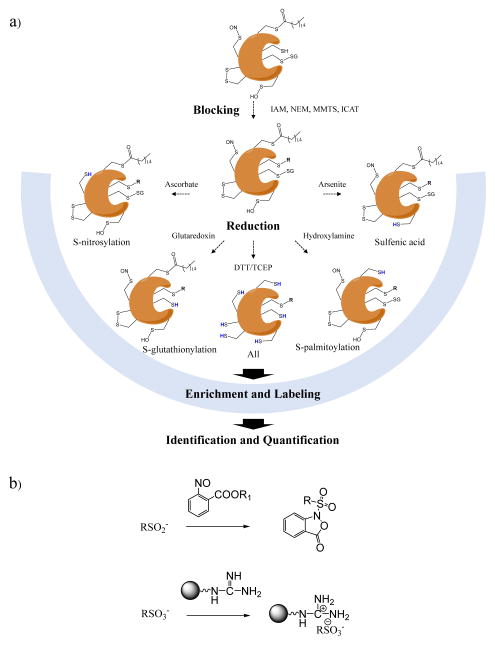
Proteomics is able to simultaneously identify and quantify nearly the whole proteome in a single experiment [30], making it a desirable technique to gain insights into the redox status of proteins. Different chemical or biological probes can make the methods selective to cysteine oxidized PTMs [31]. Cysteine PTMs are chemically diverse (Figure 1) and can be reduced enzymatically but are also susceptible to further oxidation by ROS/RNS during sample preparation [32]. Considering the diverse, labile and dynamic nature of cysteine PTMs, differential thiol blocking and selective reduction is frequently used [7, 25, 33]. The primary steps of such workflows are summarized in Figure 2a. The general strategy of blocking free thiols and using selective reduction with ascorbate to capture SNO modification sites [34] was later adapted to study a variety of cysteine reversible modifications in gel-based and nongel-based approaches. Regardless of the cysteine PTM of interest, the general sample processing steps are similar. First, free thiols are blocked by using N-ethylmaleimide (NEM), iodoacetamide (IAM) or methyl methanethiosulfonate (MMTS) [32] with typical concentrations from 10 mM to 200 mM [35–54]. In addition, cysteine-reactive mass tags can also be used, such as isotope-coded affinity tag (ICAT) [37] and iodoacetyl tandem mass tag (iodoTMT) [55]. Thiol blocking is recommended at the earliest stage of sample processing such as cell lysis or tissue homogenization, in order to minimize artificial cysteine oxidation [25]. Second, after removal of excess blocking reagents, substrate-specific reductants are added to the sample. Widely-used reducing reagents, including ascorbate, arsenite, glutaredoxin, and hydroxylamine can be used to selectively reduce SNO, SOH, SSG, and S-palmitoylation, respectively [56]. Dithiothreitol (DTT) or tris(2-carboxyethyl)phosphine (TCEP) are strong reductive reagents that can target total reversible modifications. Next, the reduced thiols react with cysteine-reactive isotopic or isobaric mass tags [55], affinity resins [56], biotin-based tags [37] or fluorophores [57]. The following steps for protein identification and quantification are highly diverse, and may include gel- or nongel-based separation, Western blot, proteolytic digestion, mass tagging, affinity purification and MS analysis. The overall objective is to discover redox-sensitive proteins (i.e., proteins that are most susceptible to oxidation) and quantify the differences in relative concentration of site-specific cysteine PTMs across different conditions/treatments or diseases. Cysteine reversible PTMs can also be detected by direct approaches, in which the functional groups of interest are retained. For example, dimedone-based chemical probes linked with affinity tags have been used for capturing SOH [58, 59], and SNO identification by MS can be achieved using phosphine-based probes [60, 61] or organic mercury resin [62, 63]. Finally, irreversible cysteine modifications (e.g., SOOH and SO3H) can be isolated and detected by molecular probes, including aryl-nitroso compounds [64] and poly-arginine-coated nanodiamonds [65] (Figure 2b).
Figure 2.
a) Schematic summary of the principles of differential alkylation for identifying and quantifying cysteine reversible modifications in redox proteomics. Endogenously reduced cysteine thiols are first blocked by IAM, NEM, MMTS or ICAT (see text). Subsequently, different reducing reagents are used to selectively reduce targeted cysteine modifications. S-nitrosylation, S-glutathionylation, S-palmitoylation and sulfenic acid can be reduced by ascorbate, glutaredoxin, hydroxylamine and arsenite, respectively. A strong reductant such as DTT and TCEP (see text) reduces all reversible modifications. Next, nascent thiols are labeled with a variety of reagents for different purposes. Finally, samples are further processed using different tools for identification and quantification of cysteine modifications. b) Representative probes for detecting cysteine irreversible modifications.
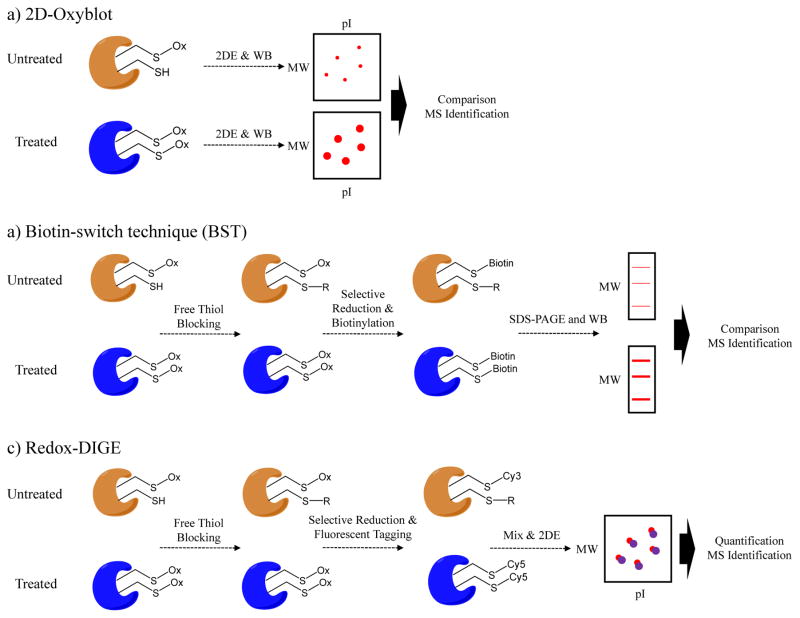
In gel-based methods, proteins are electrophoretically separated on a gel, transferred to an immunoblot, and probed with cysteine PTM specific antibody. For gel spots of interest, the gel band is digested and peptides detected by MS are used to identify the proteins [1]. Examples of gel-based redox proteomics approaches, such as 2D-Oxyblot, biotin-switch technique (BST) and redox difference gel electrophoresis (Redox-DIGE), are shown in Figure 3. 2D-Oxybot is a widely used gel-based technique to detect oxidative PTMs, e.g., protein carbonyls [66], 3-nitrotyrosine [67], 4-HNE [68], SNO [69], SSG [70] (Figure 3a). This is a powerful technique for simplifying the complex proteome to only those proteins that have the given oxidative PTM; however it can be relatively low throughput and may result in unambiguous and false-positive identification of oxidized proteins due to the co-existence of multiple proteins in the same spot. 2D-Oxyblot is also limited by artificial oxidation and thiol exchange reactions that occur during the sample processing [29]. To overcome these limitations, the biotin-switch technique (BST) was developed [34], in which free thiols are first blocked, selectively reduced for SNO modifications, newly-formed thiols are labeled with pyridyldithiol-biotin (biotin-HPDP), and finally enriched using an avidin affinity medium (Figure 3b). Avidin-conjugated horseradish peroxidase (Avidin-HRP) antibody is the probe used to detect total levels of SNO-modified proteins by Western blot, while another antibody against a protein of interest can be used to detect an individual SNO-modified protein (Figure 3b). LC-MS/MS analysis of the avidin-enriched mixtures allows the site-specific identification of oxidized cysteines without gel separation [39].
Figure 3.
Gel-based approaches for quantification of cysteine reversible modifications. a) In 2D-Oxyblot method first samples are separated by 2D SDS-PAGE. Protein bands are transferred to a membrane and the blots are probed using antibodies that recognize the particular cysteine PTM, such as S-nitrosylation or S-glutathionylation. Differentially-expressed protein spots are excised and identified by MS. b) Biotin-switch technique (BST) employs differential thiol blocking followed by selective reduction of the cysteine modification. The nascent thiols are labeled with biotin-HPDP. The oxidized proteins are then enriched by avidin affinity medium and analyzed by SDS-PAGE and Western blot. c) In Redox-DIGE method, different fluorescent tags are used to label samples after differential thiol blocking and selective reduction of cysteine modifications. Two samples are combined and analyzed on a single gel. The protein spots with differential fluorescent signals are excised and analyzed by MS.
Both 2D-Oxyblot and BST approaches can suffer from poor reproducibility because samples are analyzed and quantified separately. An alternative strategy is redox difference gel electrophoresis (Redox-DIGE) [71]. This method employs a differential sample labeling step that uses two fluorescent dyes (e.g., Cy3 and Cy5) for untreated and treated samples. Both samples are mixed and separated on the same 2D gel (Figure 3c). Fluorescence scanning of the gel reveals the oxidized proteins with different levels between untreated and treated samples, which can be subsequently identified by MS. By using this approach, 13 mitochondrial SNO-proteins were identified upon treatment of rat mitochondrion with MitoSNO (mitochondria targeted S-nitrosothiol), and they were related with inhibition of energy-related metabolic enzymes, including aconitase, mitochondrial aldehyde dehydrogenase and α-ketoglutarate dehydrogenase [72].
There are some gel-based workflows using the stable isotopic labeling technique for MS quantification of cysteine redox status. One method is called d-Switch [73, 74], in which light and heavy NEM (d5-NEM) are used to label endogenously reduced and oxidized cysteine, respectively. The sample is separated by gel electrophoresis, and the region containing the target protein is excised and analyzed by MS to obtain the quantitative information. Acrylamide matrix can also be used to lower sample loss, which is demonstrated in the gel-based stable isotope labeling of oxidized cysteine (GELSILOX) approach [75]. In GELSILOX sample preparation can be simplified, which is beneficial for reliable quantification and better recovery. Differential O16/O18 labeling of control and treated samples allows the MS quantification of oxidized thiols in a single experiment.
The general limitations of the gel-based quantitative approaches include lower sensitivity when analyzing proteins with high or low molecular weight, acidity or basicity, and hydrophobicity. Proteome coverage can be lower compared to nongel-based MS methods and these approaches heavily rely on good and specific antibodies. The ability to easily separate and visualize oxidized protein spots however, makes these approaches attractive to many researchers.
An effective strategy to overcome the limitations associated with gel-based methods and to probe deeper into the redox proteome is to employ nongel-based redox proteomics. Nongel-based approaches may identify and quantify hundreds to thousands of redox-sensitive cysteine residues from complex samples using an integrated shot-gun proteomic workflow. To date, numerous approaches have been developed and applied in biological studies, e.g., oxidized isotope-coded affinity tag [37], oxidized isobaric tag for relative and absolute quantitation [45], SNO analysis by resin-assisted capture [36], oxidized multiple reaction monitoring [40], cysteine tandem mass tags and isobaric tag for relative and absolute quantification [48], and two approaches developed by our laboratory: oxidized cysteine-selective dimethylation (OxcysDML) [76] and oxidized cysteine-selective cPILOT (OxcyscPILOT) [77]. Compared with global protein quantification, more variables have to be considered for cysteine redox quantification, such as the modification type investigated, the number of sample multiplexing channels required, the incorporation of chemical mass tags, the enrichment method, amount of starting material, and method for stoichiometric quantitation. Three general steps are common across all of the aforementioned methods: 1) differential thiol alkylation and selective reduction (Figure 2), 2) affinity purification of nascent thiols, and 3) protein/peptide quantification using labeled or label-free approaches by MS.
Affinity purification plays an important role in nongel-based methods due to the low occurrence rate (~ 0.1%) of endogenously oxidized cysteine [10, 16]. Enrichment methods reduce the complexity of the sample mixture and can dramatically improve the signal to noise ratios of modified peptides in the mass spectrometer. Affinity purification methods have high efficiencies, little to no non-specific binding, simple workflows and are MS compatible. The most common enrichment approaches to measure oxidative cysteine PTMs are: 1) biotin-avidin interaction, 2) thiol-affinity solid phase resin and 3) immunoaffinity capture.
Protein/peptide quantification involved in cysteine redox methods is similar to those techniques used in expression quantitative proteomics. For example, samples are differentially labeled with specific mass tags that can be recognized in MS analysis for relative quantification. These mass tags can be incorporated metabolically or chemically [27, 28]. An alternative to labeling approaches is “label-free”. Label-free approaches rely on ion intensity or spectrum counting to report on the abundance differences of redox-sensitive cysteine sites. Label-free can generally provide higher dynamic range compared to labeling methods, especially with multiple reaction monitoring (MRM) mode on a triple quadrupole MS analyzer [28].
The following discussion gives more details of different enrichment techniques currently utilized.
2.1 Biotin-avidin interaction
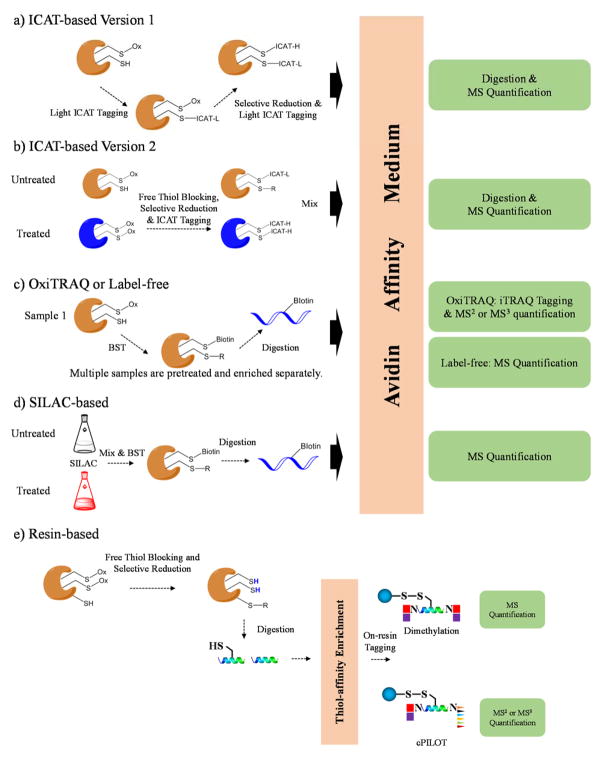
The strong binding affinity and specificity of biotin and avidin interactions is often exploited to pulldown cysteine-containing peptides. For example isotope-coded affinity tag (ICAT) in its initial version consisted of a thiol-reactive group (iodoacetyl), a deuterium-coded light or heavy linker, and biotin affinity group for capturing tagged peptides [78]. ICAT was originally developed for quantifying protein expression in two different complex samples. Because only a small portion of the tryptic peptides has cysteine residues, the ICAT technique dramatically simplifies complex mixtures and enriches proteins with low abundance. ICAT was readily adapted into the characterization of reversibly oxidized thiol proteomes. Based on how the differential alkylation is performed, ICAT-based redox methods can either quantify the absolute oxidation status of each cysteine site in a single sample (termed OxICAT, Figure 4a) [79–82], or quantify the relative abundance ratio of oxidized cysteine sites from two different samples (Figure 4b) [37, 83–87].
Figure 4.
Representative workflows using biotin and thiol-affinity resin as the purification technique. a) In an ICAT-based approach, only one sample is employed. The free thiols are blocked by light ICAT, and the nascent thiols after selective reduction are labeled with heavy ICAT. The sample is isolated by avidin affinity medium, digested and analyzed by MS. b) In another type of ICAT-based method, free thiol blocking, selective reduction and differential labeling of nascent thiols by light and heavy ICAT are performed for two different samples separately. After mixing, oxidized proteins are further processed, including enrichment, digestion and LC-MS/MS analysis. c) OxiTRAQ or label-free approaches use the biotin-switch technique (BST) to label oxidized cysteine sites followed by digestion and affinity purification for each sample. Enriched peptides are further tagged by iTRAQ and analyzed by MS, or analyzed by MS directly. d) In SILAC-based workflows, cells are grown in medium culture with light or heavy amino acids. Two samples are combined and processed using BST. Oxidized peptides are enriched and analyzed by MS. e) In resin-based approaches, proteins samples are treated so the newly-formed thiols are enriched by solid phase resin. On-resin dimethylation or cPILOT tagging techniques can be performed so two or twelve samples can be compared in one experiment, respectively.
Biotin-based affinity purification has also been coupled with other quantification methods, such as label-free, isobaric tag for relative and absolute quantitation (iTRAQ) and stable isotope labeling by amino acids in cell culture (SILAC). Palmitoylated proteins (S-acylated proteins) are isolated and quantified after free thiol blocking by NEM, selective reduction by hydroxylamine and label-free quantitation. This method is based on the classic biotin-switch technique [41], and can be expanded to study other types of cysteine modifications (Figure 4c). ITRAQ has been utilized in biotin-based workflow (termed OxiTRAQ) [45] (Figure 4c), in which up to eight samples can be compared in a single experiment. In SILAC, cell samples are labeled by either light or heavy arginine and/or lysine in cell culture medium (Figure 4d) [43]. Tryptic peptides carry at least one labeled amino acid resulting in a mass shift equivalent to the number of heavy isotope atoms incorporated. SILAC-based redox methods combine two different samples at the level of intact cells before affinity purification, which is ideal for minimizing sample error.
Generally, biotin-based affinity is a robust tool for enrichment of cysteine modification with a great deal of specificity, however it is known that there are often nonspecific interactions that can occur. Additionally, the bulkiness of the biotin tag can prevent buried cysteines from being captured. In vivo this bulkiness prevents the tags from being fully permeable across cells and thus may not fully represent all of the modifications occurring endogenously. Biotin tags can generate additional fragment peaks in the MS/MS spectra but this does not significantly interfere with the peptide identification.
2.2 Thiol-affinity solid phase resin
Thiol-affinity resin was initially used to enrich cysteine-containing peptides from complex mixtures to improve identification of low abundant proteins [88, 89]. The most widely used affinity resin is Thiopropyl sepharose® 6B, in which a reactive 2-thiopyridyl disulfide group is attached to sepharose through a chemically stable ether linkage. When mixing the affinity resin with peptide digests, cysteinyl peptides are covalently captured through the disulfide bond exchange reaction. The unbound, non-cysteinyl peptides and the released 2-thiopyridone are removed by washing. The captured peptides can be released by incubating the resin with a reducing reagent (e.g., DTT). This enrichment is quantitative and the specificity is ~98–99% [5, 76, 90].
Recently thiol-affinity resin has gained popularity for isolating and quantifying cysteine reversible modifications in complex mixtures. Different quantitative MS methods, e.g., isotopic labels [36, 90], isobaric tags [35, 51, 52, 56, 91], label-free methods [92–94], have been coupled with resin-based enrichment, to quantify SNO [36, 52, 90, 92, 93], SSG [51, 56, 95], S-palmitoylation [35, 56], and all oxidative cysteine PTMs [56, 91, 94] (Figure 4e). Resin-assisted approaches have several advantages. First, it is a simple workflow in which peptides are directly captured on the thiol-affinity resin without pre-derivatization. Second, enriched peptides do not have fragmentable tags that will be generated in MS/MS, and no side reaction is expected in the reversible capture and release reaction. Third, enriched peptides are linked to the resin through stable covalent bonding, so stringent washing steps can be applied to remove non-specific binders. Fourth, resin matrix can serve as a sample sorbent to facilitate the on-resin peptide labeling reaction by using different amine-reactive tags, e.g., acetylation [36], dimethylation [90], TMT [56, 90, 91] and iTRAQ [35, 51, 52]. This is very attractive, as no more sample cleanup is needed between steps, which is beneficial for minimizing sample loss.
Our laboratory developed an inexpensive stable-isotope dimethyl labeling, termed OxcysDML (Figure 4e), that also takes advantage of the thiol-affinity resin. This technique achieved efficient peptide on-resin dimethylation using different isotopomers of formaldehyde and cyanoborohydride. More importantly, because the dimethylation reaction was performed on the solid phase enrichment, the entire sample preparation workflow was shortened and the sample recovery was high. Also because isobaric tags are not used the average costs for each sample in a duplex experiment is relatively low. This simple method is suitable for comparing cysteine modifications from two samples with desirable proteome coverage, and can reach up to 5-plex comparison using other formaldehyde/cyanoborohydride isotopomers [96]. OxcysDML has been applied to study the redox proteome of liver tissues from an AD mouse model [76], as discussed below.
Sample multiplexing capabilities offer several advantages for studying reversible cysteine PTMs. One advantage is the ability to use a multiplexing channel to report on total cysteinyl peptide levels. The measurement of total cysteinyl peptide levels along with the modified cysteine levels in the same experiment, makes for more straightforward normalization of the data and provides site occupancy information about the cysteine PTM. Another advantage is that sample channels can be used to incorporate biological replication into a single experiment. This incorporation can improve the confidence of cysteine PTM identification and quantification and has the practical advantages of decreasing instrument acquisition time and minimizing sample variance. We recently extended the sample multiplexing capabilities for measuring oxidative cysteine PTMs with a method termed OxcyscPILOT (Figure 4e). Inspired by our previous cPILOT methodologies [4, 5, 97], OxcyscPILOT integrates peptide N-terminus dimethylation and C-terminus TMT tagging of lysine while modified peptides are captured on the thiol-affinity resin. In a single run twelve or potentially 24 samples, using different isobaric tagging reagents, can be analyzed. We were able to use this approach to study endogenous SNO levels of brain proteins in an AD mouse model [90].
2.3 Immunoaffinity Capture
Immunoaffinity capture has emerged in recent years with cysteine-reactive tandem mass tag (cysTMT) or iodoacetyl tandem mass tag (iodoTMT). CysTMT contains a mass reporter group, a mass normalizer moiety and a pyridyldithiol cysteine-reactive group. CysTMT-tagged peptides are enriched by anti-TMT resin immobilized with an antibody recognizing the mass reporter structure of the mass tag. During the LC-MS/MS step, cysTMT-tagged peptides can generate up to six reporter ions between 126 and 131 Da, the intensities of which are used for relative quantification. IodoTMT works similar to CysTMT, except it has a desired irreversible reaction with sulfhydryl groups. CysTMT and iodoTMT-based approaches have been successfully applied in studying the redox proteome alteration of human pulmonary arterial endothelial cells treated with S-nitrosoglutathione [47], the NO-mediated cardioprotection processes [55], and the SNO sites responding to lipopolysaccharide (LPS) stimulation in microglial cells [49]. Recently, a novel iodoTMT-based workflow, termed SNO/SOH TMT strategy, has been developed to provide quantitative profiling of SNO and SOH changes simultaneously [54]. The SNO/SOH TMT strategy uses two channels to isolate total cysteinyl peptides for correcting protein abundance changes during sample treatment and preparation and thus is very attractive.
Although not frequently reported, conventional protein immunoaffinity purification - using an immobilized antibody to pull down the targeted protein - can also be coupled with differential thiol blocking to quantify the redox status of cysteine residues in specific proteins [40]. In one study, diamide treated human breast cancer cells are differentially alkylated with d0 and d5 NEM for reduced and oxidized cysteine, respectively. The target protein p53 and protein tyrosine phosphatase-1B (PTP1B) are then serially immunoaffinity-purified and analyzed by MRM. This method, termed OxMRM, indicated that Cys182 and Cys215 are the redox-sensitive sites in p53 and PTP1B, respectively.
2.4 Non-enrichment Approaches
When comparing reversibly oxidized cysteine PTMs across multiple samples in one experiment, one has to consider the effects of total protein-level changes to the cysteine PTM changes. Generally a separate experiment is involved to address the protein turnover issue in the course of experiments [87]. In order to obtain the protein expression and cysteine redox information by using a single run, a possible strategy, although not widely used, is to retain the non-redox portion of the sample. Cysteinyl peptides help determine the redox changes, while the non-cysteinyl peptides are responsible for quantifying protein-level changes. In these cases, affinity purifications are normally not involved. The previously discussed GELSILOX approach is one of the relevant examples [75]. Another good example is cysTMTRAQ (cysTMT and iTRAQ combined) [48], which uses cysTMT to label protein thiols responsive to a treatment, and uses iTRAQ to label peptide amines for analysis of protein-levels changes between different treatments. Finally, cysteine Michael adducts (e.g., with 4-HNE) can be analyzed with or without enrichment [98–100]. One of the major limitations of non-enriched methods is lack of sensitivity as only a small portion of peptides carry oxidized cysteine in physiological conditions.
2.5 Bioinformatics Tools
Bioinformatics tools are valuable for studying the thiol redox proteome. First, the interpretation of datasets from LC-MS/MS experiments requires search engines to identify and quantify proteins as well as oxidized cysteine sites. Generic proteomic search engines such as MASCOT [101] and SEQUEST [102] can provide desirable identification and quantification results for samples that have been treated using thiol differential blocking and selective reduction, as mentioned before. One exception is disulfide bonds, which can be directly analyzed from MS but require special algorithms for spectral interpretation. Peptides containing disulfide bonds generate specific fragmentation patterns in tandem mass spectra such as cysteine thioaldehyde (−2 Da), cysteine persulfide (+ 32 Da) and dehydroalanine (− 34 Da) [103], which can be processed by bioinformatics tools including MassMatrix [104] and DBond [105]. Second, bioinformatics enrichment analysis is often performed to understand the impact of cysteine modifications on biological processes. These analyses categorize identified proteins based on cellular compartments, cellular processes and pathways. Some common tools include Gene Ontology (GO), Ingenuity Pathway Analysis (IPA), STRING, KEGG, REACTOME and DAVID [106]. Third, bioinformatics approaches can predict different functional categories of cysteine using existing protein databases and simulations. For example, PROSITE [107] and Support Vector Machine [108] can be used to predict the location of disulfide bonds and metal binding sites. The determination of disulfide bonds is mostly achieved by calculating the distance between two cysteine residues while the metal binding sites often come with conserved motifs. The algorithms search these characteristics from the query protein sequence, compare to information in external databases (e.g., Protein Data Bank) and make predictions about the modification sites. Some other cysteine modifications, such as SOH, SNO, SSG and S-palmitoylation, can also be predicted. SOH is often found to be close to Thr, which donates a proton to lower the pKa of cysteine making it more susceptible to ROS [109]. Cysteine modification by NO comes with the redistribution of charges on the side chain, and the calculation agrees with the experimental results [110]. SSG modifications can be predicted by PGluS [111], which is a prediction model using experimental SSG data in the dbGSH database [112] and there are strategies to predict S-palmitoylation [113, 114]. Cysteine modification predictions can be compared with experimentally verified oxidative modification data in RedoxDB, which includes 2157 redox proteins containing 2203 modified cysteine sites [115].
3. Applications of cysteine-selective redox proteomics in aging and neurodegenerative diseases
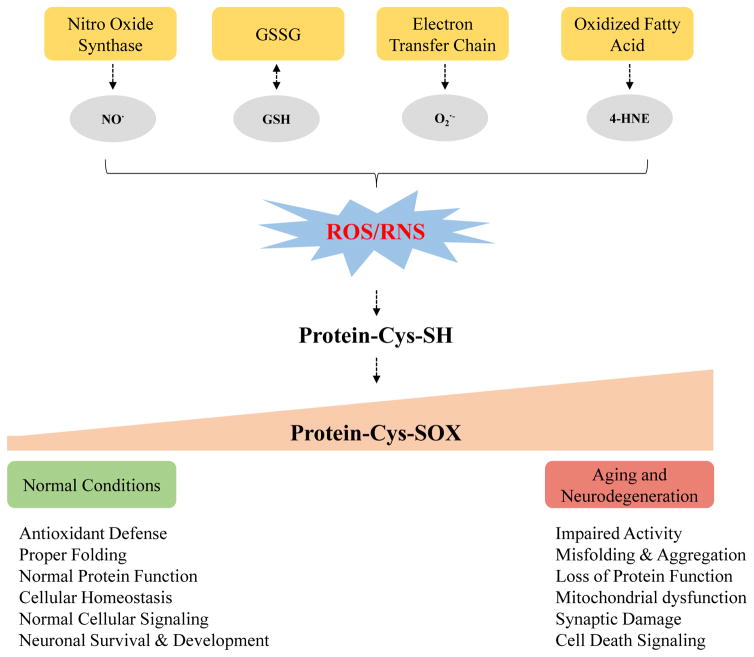
In aging and neurodegenerative diseases (e.g., AD and PD), increased cellular levels of ROS/RNS can imbalance the thiol homeostasis resulting in protein oxidative damage [116] (Figure 5). In the past two decades much effort has been made to better elucidate the mechanism of oxidative damage in aging and neurodegenerative diseases by using cysteine-selective redox proteomics [11, 25, 113, 114, 117]. Cysteine is involved in the modulation of many cellular processes via reversible modifications. Redox-sensitive proteins and highly susceptible cysteine sites to oxidation have been identified in human tissues such as brain and in animal models [69, 118] of neurodegenerative diseases. Cysteine reversible modifications have also recently been demonstrated as effective therapeutic targets for neurodegenerative diseases [119]. Below we report on more than 20 studies (Table 1) that have measured cysteine modifications in the context of aging and neurodegenerative diseases (i.e., AD and PD) and across brain and other tissues.
Figure 5.
Simplified schematic showing the generation of different cysteine reversible modification and their roles in aging and neurodegenerative diseases.
Table 1.
Representative redox proteomics approaches to quantify various types of cysteine modifications in aging and neurodegenerative diseases.
| Year | Sample | Disease | Method | Cysteine Modification | Results |
|---|---|---|---|---|---|
| 2004 | Human brain tissues | AD & PD | 2D gel, immunostaining and MS | Sulfonic acid | Cys220 of UCH-L1 is oxidized to cysteic acid [161]. |
| 2007 | Inferior parietal lobule from patients | AD | 2D-Oxyblots and MS | S-glutathionylation | Deoxyhemoglobin, α-crystallin B, glyceraldehyde phosphate dehydrogenase (GAPDH), and α-enolase were significantly S-glutathionylated in AD [155]. |
| 2008 | Fetal, aged normal and advanced nuclear cataract lenses from human | Aging | Gel-free switch assay, label-free quantification, no enrichment | Total oxidized cysteine | Quantified relative amount of reduced form and oxidized form of each cysteine site within the same biological condition. αA, Cys142; βA1/3, Cys52; βB3, Cys39 and Cys45 had higher oxidized form in nuclear cataract and aged normal lenses compared with fetal [127]. |
| 2009 | Entorhinal cortex from patients | Aging | 2D Western blots and MS | S-nitrosylation | Glial fibrillary proteins were nitrosylated in a brain tissue from a 78-year-old female, 13 h postmortem delay without neurological disease [143]. |
| 2010 | Liver tissues from mouse at 4–6 and 26–28 months | Aging | Fluorescence-based 2D gel and MS | Total oxidized cysteine | Global protein disulfide levels increased significantly with age in liver cytosolic proteins, and 11 proteins showed a more than twofold increase in disulfide content with age [120]. |
| 2011 | Complex I from PD mouse | PD | OxMRM | Total oxidized cysteine | Six out of 34 oxidized cysteine from 130 proteins had increased oxidation in mice undergoing in vivo glutathione depletion. [162] |
| 2011 | Brain cerebrum tissue from 5-month old transgenic mice (B6Cg-Tg) and WT controls | AD | CE-Laser induced fluorescence, switch assay | S-nitrosylation | Transgenic mice brain had higher SNO than control [166]. |
| 2012 | Brain from 11-month old transgenic mice (B6Cg-Tg) and WT controls | AD | 2D micro-electrophoresis | S-nitrosylation | 2D profiling of nitrosylated proteins in AD and WT brains. AD brain proteins with a MW between 35 kDa and 65 kDa were most susceptible to SNO [167]. |
| 2012 | Brain tissues and blood samples from 1-mon, 5-mon and 11-mon TG mice and controls | AD | CE-Laser induced fluorescence, switch assay, PCA analysis | S-glutathionylation | AD and controls could be differentiated (> 90% sensitivity and specificity) based on SSG electrophoretic profiling [168]. |
| 2012 | C. elegans wild-type strains, short-lived mutants and long-lived mutants | Aging | OxICAT | Total oxidized cysteine | The redox status of 137 different C. elegans proteins were determined. Short-lived mutants failed to restore the redox homeostasis and had a higher oxidant levels throughout the mature life in comparison with short-lived mutants [81]. |
| 2013 | Yeast cells | Aging | OxICAT | Total oxidized cysteine | 286 proteins were identified in all samples. Decreased cellular NADPH is associated with increased protein thiol oxidation in aging. Caloric restriction can increase NADPH levels and delay protein oxidation [125]. |
| 2014 | Autopsied brain specimens | AD | 2D-Oxyblot and MS | S-nitrosylation | Superoxide dismutase (SOD2) [Mn], fructose-bisphosphate aldolase C (ALDOC) and voltage-dependent anion-selective channel protein 2 (VDAC2) showed differential S-nitrosylation signal [69]. |
| 2014 | Synaptosomes of transgenic mouse (14–15 mon) | AD | BST, avidin enrichment, label-free quantification, gel-free | S-nitrosylation | 138 S-nitrosylated proteins were involved in various cellular pathways, including: glycolysis, gluconeogenesis, calcium homeostasis, ion and vesicle transport [118]. |
| 2014 | Mouse skeletal muscles | Aging | Labeling reduced and oxidized cysteine using light and heavy NEM, respectively. Gel-free, non-enriched | Total oxidized cysteine | The reversible redox state of specific cysteine residues within individual muscle samples was obtained [123]. |
| 2015 | Hippocampus from patients | AD | 2D-Oxyblots and MS | Sulfenic acid, sulfinic acid and sulfonic acid | Pin1 was identified to be oxidized on Cys113. This modification was elevated in human AD brain [157, 158]. |
| 2015 | Human cortex and cerebellum samples | Human prion diseases (neurodegenerative disorders) | BST, avidin enrichment, iTRAQ, gel-free | S-nitrosylation | 1509 S-nitrosylated proteins (SNO-proteins) were identified with differential expressions in many pathways [164]. |
| 2015 | Amyloid-activated BV2 cells | AD | BST, avidin enrichment, gel-free | Total oxidized cysteine | 60 proteins changed the redox status upon treatment with the amyloidogenic Aβ25–35 peptide [134]. |
| 2015 | Drosophila melanogaster (heads and thoraces) | Aging | OxICAT, gel-free | Total oxidized cysteine | Aging had no impact on cysteine-residue redox state. In contrast, fasting dramatically affected cysteine redox status [124]. |
| 2015 | Old and young Human eye tissues, glutathione depleted LEGSKO mouse lens | Aging | 2D gel and OxICAT | Total oxidized cysteine | Shift of intramolecular disulfides to intermolecular disulfides during the aging process was observed. Several disulfide formation sites necessitated prior conformational changes in γ-crystallin [128]. |
| 2015 | Heart tissues of wild type and catalase transgenic mice | Aging | iodoTMT | Total oxidized cysteine | Catalase overexpressed mice had lower protein glutathione adducts as well as hydrogen peroxide production from mitochondrial complex I and II [169]. |
| 2016 | Liver tissue from AD model mouse | AD | Gel-free, dimethylation | Total oxidized cysteine | More than 1000 oxidized cysteines were identified. The most dysregulated pathway was metabolism. Oxidized proteins involved in lipid metabolism could be linked with oxidative stress in AD liver [90]. |
| 2016 | Brain tissue from AD model mouse | AD | Gel-free, cPILOT | S-nitrosylation | 135 SNO-modified proteins were identified, and the majority of them were involved in metabolism and signal transduction. Statistical analysis indicated 11 SNO-modified peptides had differential levels in AD compared with WT [90]. |
3.1 Aging
Surprisingly, most of the aging-related redox proteomics studies are focused on total cysteine oxidation, whereby the specific PTM is not identified. For example, an analysis of liver cytosolic proteins from young (4–6 months) and old mice (26–28 months) revealed 11 proteins (such as GAPDH, regucalcin and peroxiredoxin 1) showing a more than two-fold increase in cysteine oxidation content with aging [120]. This is consistent with elevated levels of carbonylated proteins in aging [121, 122], and supports total cysteine oxidation as a potential biomarker for oxidative stress. Metabolic proteins (e.g., phosphofructokinase, glucose 6-phosphate isomerase, glycogen phosphorylase, phophoglycerate mutase 1, and phosphoglucomutase 2) have age-dependent cysteine oxidation in skeletal muscle from young adult (12 months) mice [123]. Older mice (25 months) have age-dependent changes also, whereby aconitase, an enzyme involved in the tricarboxylic acid cycle has altered oxidation for the four cysteine residues that coordinate the metal iron. Cysteine oxidation of aconitase is associated with decreased catalytic activity. In other models of aging such as Drosophila melanogaster, aging was found to have no impact on cysteine-residue redox status [124] except for after a 24-hour fasting period [124]. OxICAT studies of thiol redox status in aging using C. elegans and yeast cells had been reported. The C. elegans study found short-lived mutants failed to restore the redox homeostasis and had higher oxidant levels throughout the mature life in comparison with short-lived mutants [81]. The study of yeast cells indicated the association of decreased cellular NADPH with increased protein thiol oxidation in aging [125]. Age-related nuclear cataract (ARNC), a human eye disease, is associated with the loss of protein thiols [126] and results in site-specific oxidation of 10 cysteine residues that are not redox sensitive with normal lens aging [127]. γ-Crystallin is a redox sensitive protein in the context of both aging and ARNC and cysteine oxidation to disulfides facilitates necessary protein conformational changes [127, 128].
3.2 Alzheimer’s Disease (AD)
Alzheimer’s disease is a neurodegenerative disorder characterized by neurofibrillary tangles, senile plaques, loss of synapses [129] amongst other pathological hallmarks and has well-known oxidative stress in brain [130], plasma [131], heart [132], spleen [133] and liver [90] tissues. Similar to aging, cysteine reversible modifications are also implicated in AD pathogenesis. For example, the microglial proteome of BV2 cells after treatment of Aβ25–35 results in 60 proteins with different cysteine redox status [134]. In other tissues such as liver, we were able to identify 1129 reversibly-oxidized cysteine sites, among which 19 showed significant differences between AD and controls liver proteins from transgenic AD mouse [76]. Notably, oxidized proteins were involved in lipid metabolism and these changes were correlated with oxidative stress in AD liver tissue.
Although cysteine can be oxidized into a variety of PTMs, SNO has been investigated the most in AD. In physiological conditions NO is maintained at a moderate level, and SNO modification of some proteins are important for synaptic plasticity and neuronal development [135, 136]. For example, SNO of N-methyl-D-aspartate receptor (NMDAR) decreases enzymatic activity to negatively regulate NO production [137], and NO can activate the cAMP response element-binding protein (CREB) pathway for neuroprotection [138]. In AD, NO is overproduced via hyperactivation of NMDAR and attacks proteins, resulting in a series of detrimental consequences such as protein misfolding, mitochondrial fragmentation and neuronal loss [139–142]. Blocking site-specific SNO modification on proteins has been considered as a therapeutic approach for neurodegenerative diseases [119]. Four proteomic studies have focused on SNO in CNS using different tissues and redox proteomics techniques [69, 90, 118, 143]. These studies identified 39 [143], 45 [69], 138 [118] and 135 [90] SNO-modified proteins in tissues such as entorhinal cortex, entire brain, and synaptosomes from AD mice and humans. While a large number of SNO-modification sites were observed in these studies, only three report on proteins that have different SNO-modification levels in AD ([143],[69],[118] [90]). Additionally, these studies collectively identify the involvement of SNO in metabolism and signaling processes. Many SNO-modified proteins were also reported as significantly oxidized (e.g., carbonylation) in previous AD studies [1] and suggest that to some extent the SNO modification may be a result of oxidative stress. Specifically, an early study of entorhinal cortex from AD patients did not discover significantly changed SNO-proteins in AD [143], which was probably due to the insufficient sensitivity of the method and the complexity of the samples. Later, SNO-modified proteins of autopsied brain specimens, including hippocampus, substantia nigra and cortex from AD patients were investigated [69] and three differentially SNO-modified proteins in AD were observed. These proteins are superoxide dismutase [Mn], fructose-bisphosphate aldolase C and voltage-dependent anion-selective channel protein 2, which are associated with altered detoxification, glycolytic metabolism and ATP production [139], and SNO-modification is consistent with increased oxidative stress [1] and decreased glucose metabolism [144] in AD brain. Interestingly, these three proteins have also frequently been reported as modified by HNE, nitration and carbonylation in AD [66, 68, 145, 146]. SNO of brain synaptosomal proteins were also quantified using wild type and transgenic mice overexpressing mutated human amyloid precursor protein (hAPP) at 14 month old, a widely used animal model for AD [118]. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was significantly SNO-modified. GAPDH is a catalytic enzyme involved in glycolysis/gluconeogenesis, but also has roles in non-metabolic processes. SNO-modified GAPDH is likely binds with E3 ubiquitin-protein ligase SIAH1 (Siah1) and translocates into the nucleus, resulting in apoptotic signaling and cell death [119, 147]. Inhibition of SNO modification of GAPDH has been developed as a treatment for PD [148]. Recently, we measured SNO of brain tissues from an APP/PS1 AD mouse model using OxcyscPILOT [149], and observed 79 new SNO-modified proteins that have not been previously reported in AD [69, 118]. A number of SNO-modified proteins were only present in wild type or AD animals, indicating both the neuroprotective and neurodestructive roles of SNO in CNS [135]. Because total cysteine-containing peptides were isolated and quantified, this work was able to determine SNO site occupancy levels. Most SNO sites had relative low occupancy levels (i.e., < 1%), however isoforms of 14-3-3 proteins were SNO modified by as much as ~ 50%. That 14-3-3 proteins were heavily SNO modified could be related with it being an easy target for oxidation by ROS as it has been frequently reported as modified by others [150–152]. Glutamine synthetase and citrate synthase were significantly SNO-modified in AD brain. The former is important for controlling ammonia level, as hyperammonia can induce enhanced neurotoxicity and was observed in AD brain [153]. The latter is important for TCA cycle, and SNO-modified citrate synthase may be linked with dysregulated metabolism in AD brain [144]. In total we observed 135 modified sites and similar to the other studies, metabolism and cellular signaling were the most represented pathways for SNO-modified proteins.
SSG is also an important cysteine reversible PTM in neurodegenerative diseases [154]. SSG was probed in inferior parietal lobule (IPL) and hippocampus from AD patients [155]. This work found deoxyhemoglobin, α-crystallin B, GAPDH, and α-enolase were significantly modified by SSG in AD. GAPDH and α-enolase had reduced activity in the AD IPL, which may link to the impaired glucose metabolism in AD. Moreover, GAPDH and α-enolase were reported as modified by SNO and carbonylation in AD [1, 118].
Our knowledge of redox-modified proteins involved in AD is consistently being updated. Proline isomerase, Pin1, is significantly carbonylated in AD hippocampus with decreased activity both in vivo and in vitro [156]. Recent studies showed the oxidation of Pin1 Cys113 was significantly elevated in human AD brain and in AD mouse models [157]. Pin1 oxidation on Cys113 causes enzyme inactivation, but the oxidative inhibition of Pin1 could be partially reversed by treatment with DTT [158]. Cys113 of Pin1 in AD brain tissue was probably oxidized into sulfenic acid, sufinic acid or sulfonic acid. It is challenging to determine the relative abundances of the three PTMs in vivo, which may be solved by using chemical derivatization coupled with MS [159].
3.3 Other Neurodegenerative Diseases
PD is the second most prevalent degenerative disease of the nervous system with the accumulation of insoluble proteinaceous deposits such as Lewy bodies [1]. Ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1) is associated with familial forms of PD [160], and oxidation of Cys220, as well as Met124 and Met179 were discovered in both AD and PD [161]. The oxidative states of proteins involved in electron transport increased in a PD mouse model [162]. Human prion diseases, fatal neurodegenerative disorders characterized by neuronal damage in brain and accumulation of misfolded protein deposits in the CNS [163], have also been investigated by redox proteomics. SNO-modified proteins of human cortex and cerebellum tissues from normal controls, sporadic Creutzfeldt-Jakob disease (sCJD), fatal familial insomnia (FFI), and genetic CJD with a substitution of valine for glycine at codon 114 of the prion protein gene (G114V gCJD) were compared [164]. A total of 1509 endogenous SNO-proteins were identified, making it one of the most important cysteine oxidation proteomic studies as it provides the highest SNO-proteome coverage to-date. Differentially expressed SNO-proteins were mainly involved in metabolism, cell cytoskeleton/structure, immune system, cell-cell communication and miscellaneous function protein. Proteomic studies of cysteine PTMs in other neurodegenerative diseases, such as Huntington disease, amyotrophic lateral sclerosis and Down syndrome, are very limited [165].
4. Conclusions
Oxidative protein modifications to cysteine have been shown to be ubiquitously and dynamically involved in aging and aging-related neurodegenerative diseases. The growth of MS-based proteomics, including gel-based and nongel-based approaches, has led to discovery of new cysteine PTMs and insights into aging and neurodegenerative diseases. Continued advances in redox proteomics will further the understanding of aging and aging-related neurodegenerative diseases, especially the redox molecular mechanism, the roles of oxidative stress and redox signaling in cellular processes.
Acknowledgments
We acknowledge financial support of this work by NIH grant R01 GM 117191-01 awarded to R.A.S.R.
References
- 1.Butterfield DA, Gu L, Di Domenico F, Robinson RA. Mass spectrometry and redox proteomics: applications in disease. Mass spectrometry reviews. 2014;33:277–301. doi: 10.1002/mas.21374. [DOI] [PubMed] [Google Scholar]
- 2.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stadtman ER. Protein modification in aging. Journal of gerontology. 1988;43:B112–120. doi: 10.1093/geronj/43.5.b112. [DOI] [PubMed] [Google Scholar]
- 4.Evans AR, Gu L, Guerrero R, Jr, Robinson RA. Global cPILOT analysis of the APP/PS-1 mouse liver proteome. Proteomics. Clinical applications. 2015;9:872–884. doi: 10.1002/prca.201400149. [DOI] [PubMed] [Google Scholar]
- 5.Gu L, Evans AR, Robinson RA. Sample Multiplexing with Cysteine-Selective Approaches: cysDML and cPILOT. Journal of the American Society for Mass Spectrometry. 2015;26:615–630. doi: 10.1007/s13361-014-1059-9. [DOI] [PubMed] [Google Scholar]
- 6.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Annals of neurology. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield DA, Dalle-Donne I. Redox proteomics: from protein modifications to cellular dysfunction and disease. Mass spectrometry reviews. 2014;33:1–6. doi: 10.1002/mas.21404. [DOI] [PubMed] [Google Scholar]
- 8.Finkel T, Holbrook NJ. Nature. 2000:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 9.Verrastro I, Pasha S, Jensen KT, Pitt AR, Spickett CM. Mass spectrometry-based methods for identifying oxidized proteins in disease: advances and challenges. Biomolecules. 2015;5:378–411. doi: 10.3390/biom5020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giron P, Dayon L, Sanchez JC. Cysteine tagging for MS-based proteomics. Mass spectrometry reviews. 2011;30:366–395. doi: 10.1002/mas.20285. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Yang J, Yi J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxidants & redox signaling. 2012;16:649–657. doi: 10.1089/ars.2011.4313. [DOI] [PubMed] [Google Scholar]
- 12.Ratnayake S, Dias IH, Lattman E, Griffiths HR. Stabilising cysteinyl thiol oxidation and nitrosation for proteomic analysis. Journal of proteomics. 2013;92:160–170. doi: 10.1016/j.jprot.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Mossner E, Iwai H, Glockshuber R. Influence of the pK(a) value of the buried, active-site cysteine on the redox properties of thioredoxin-like oxidoreductases. FEBS letters. 2000;477:21–26. doi: 10.1016/s0014-5793(00)01738-5. [DOI] [PubMed] [Google Scholar]
- 14.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circulation research. 2013;112:382–392. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couvertier SM, Zhou Y, Weerapana E. Chemical-proteomic strategies to investigate cysteine posttranslational modifications. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbapap.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Santamarina S, Boronat S, Hidalgo E. Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. Biochemistry. 2014;53:2560–2580. doi: 10.1021/bi401700f. [DOI] [PubMed] [Google Scholar]
- 17.Boronat S, Garcia-Santamarina S, Hidalgo E. Gel-free proteomic methodologies to study reversible cysteine oxidation and irreversible protein carbonyl formation. Free radical research. 2015;49:494–510. doi: 10.3109/10715762.2015.1009053. [DOI] [PubMed] [Google Scholar]
- 18.Gupta V, Carroll KS. Sulfenic acid chemistry, detection and cellular lifetime. Biochimica et biophysica acta. 2014;1840:847–875. doi: 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends in molecular medicine. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxidants & redox signaling. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochimica et biophysica acta. 2011;1808:2981–2994. doi: 10.1016/j.bbamem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Hogg PJ. Disulfide bonds as switches for protein function. Trends Biochem Sci. 2003;28:210–214. doi: 10.1016/S0968-0004(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 23.Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chemical research in toxicology. 2005;18:1324–1331. doi: 10.1021/tx050078z. [DOI] [PubMed] [Google Scholar]
- 24.Cai Z, Yan LJ. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. Journal of biochemical and pharmacological research. 2013;1:15–26. [PMC free article] [PubMed] [Google Scholar]
- 25.Lennicke C, Rahn J, Heimer N, Lichtenfels R, et al. Redox proteomics: Methods for the identification and enrichment of redox-modified proteins and their applications. Proteomics. 2016;16:197–213. doi: 10.1002/pmic.201500268. [DOI] [PubMed] [Google Scholar]
- 26.Charles R, Jayawardhana T, Eaton P. Gel-based methods in redox proteomics. Biochimica et biophysica acta. 2014;1840:830–837. doi: 10.1016/j.bbagen.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Bantscheff M, Lemeer S, Savitski MM, Kuster B. Quantitative mass spectrometry in proteomics: critical review update from 2007 to the present. Analytical and bioanalytical chemistry. 2012;404:939–965. doi: 10.1007/s00216-012-6203-4. [DOI] [PubMed] [Google Scholar]
- 28.Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Analytical and bioanalytical chemistry. 2007;389:1017–1031. doi: 10.1007/s00216-007-1486-6. [DOI] [PubMed] [Google Scholar]
- 29.Held JM, Gibson BW. Regulatory control or oxidative damage? Proteomic approaches to interrogate the role of cysteine oxidation status in biological processes. Molecular & cellular proteomics : MCP. 2012;11:R111 013037. doi: 10.1074/mcp.R111.013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Fonslow BR, Shan B, Baek MC, Yates JR., 3rd Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113:2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leonard SE, Carroll KS. Chemical ‘omics’ approaches for understanding protein cysteine oxidation in biology. Current opinion in chemical biology. 2011;15:88–102. doi: 10.1016/j.cbpa.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Wojdyla K, Rogowska-Wrzesinska A. Differential alkylation-based redox proteomics - Lessons learnt. Redox biology. 2015;6:240–252. doi: 10.1016/j.redox.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couvertier SM, Zhou Y, Weerapana E. Chemical-proteomic strategies to investigate cysteine posttranslational modifications. Bba-Proteins Proteom. 2014;1844:2315–2330. doi: 10.1016/j.bbapap.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Forrester MT, Foster MW, Benhar M, Stamler JS. Detection of protein S-nitrosylation with the biotin-switch technique. Free radical biology & medicine. 2009;46:119–126. doi: 10.1016/j.freeradbiomed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrester MT, Hess DT, Thompson JW, Hultman R, et al. Site-specific analysis of protein S-acylation by resin-assisted capture. Journal of lipid research. 2011;52:393–398. doi: 10.1194/jlr.D011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forrester MT, Thompson JW, Foster MW, Nogueira L, et al. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nature biotechnology. 2009;27:557–559. doi: 10.1038/nbt.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Santamarina S, Boronat S, Espadas G, Ayte J, et al. The oxidized thiol proteome in fission yeast--optimization of an ICAT-based method to identify H2O2-oxidized proteins. Journal of proteomics. 2011;74:2476–2486. doi: 10.1016/j.jprot.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 38.Greco TM, Hodara R, Parastatidis I, Heijnen HF, et al. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7420–7425. doi: 10.1073/pnas.0600729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao G, Derakhshan B, Shi L, Campagne F, Gross SS. SNOSID a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Held JM, Danielson SR, Behring JB, Atsriku C, et al. Targeted Quantitation of Site-Specific Cysteine Oxidation in Endogenous Proteins Using a Differential Alkylation and Multiple Reaction Monitoring Mass Spectrometry Approach. Molecular & Cellular Proteomics. 2010;9:1400–1410. doi: 10.1074/mcp.M900643-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nature cell biology. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Science signaling. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li R, Huang JQ, Kast J. Identification of Total Reversible Cysteine Oxidation in an Atherosclerosis Model Using a Modified Biotin Switch Assay. Journal of Proteome Research. 2015;14:2026–2035. doi: 10.1021/acs.jproteome.5b00133. [DOI] [PubMed] [Google Scholar]
- 44.Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, et al. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Archives of biochemistry and biophysics. 2002;406:229–240. doi: 10.1016/s0003-9861(02)00468-x. [DOI] [PubMed] [Google Scholar]
- 45.Liu P, Zhang H, Wang H, Xia Y. Identification of redox-sensitive cysteines in the Arabidopsis proteome using OxiTRAQ, a quantitative redox proteomics method. Proteomics. 2014;14:750–762. doi: 10.1002/pmic.201300307. [DOI] [PubMed] [Google Scholar]
- 46.Lu XM, Lu M, Tompkins RG, Fischman AJ. Site-specific detection of S-nitrosylated PKB alpha/Akt1 from rat soleus muscle using CapLC-Q-TOF(micro) mass spectrometry. Journal of mass spectrometry : JMS. 2005;40:1140–1148. doi: 10.1002/jms.885. [DOI] [PubMed] [Google Scholar]
- 47.Murray CI, Uhrigshardt H, O’Meally RN, Cole RN, Van Eyk JE. Identification and quantification of S-nitrosylation by cysteine reactive tandem mass tag switch assay. Molecular & cellular proteomics : MCP. 2012;11:M111 013441. doi: 10.1074/mcp.M111.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker J, Balmant K, Zhu F, Zhu N, Chen S. cysTMTRAQ-an integrative method for unbiased thiol-based redox proteomics. Molecular & cellular proteomics : MCP. 2014;14:237–242. doi: 10.1074/mcp.O114.041772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu Z, Meng F, Bomgarden RD, Viner RI, et al. Proteomic Quantification and Site-Mapping of S-Nitrosylated Proteins Using Isobaric iodoTMT Reagents. J Proteome Res. 2014;13:3200–3211. doi: 10.1021/pr401179v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saurin AT, Neubert H, Brennan JP, Eaton P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17982–17987. doi: 10.1073/pnas.0404762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su D, Gaffrey MJ, Guo J, Hatchell KE, et al. Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling. Free radical biology & medicine. 2013 doi: 10.1016/j.freeradbiomed.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su D, Shukla AK, Chen B, Kim JS, et al. Quantitative site-specific reactivity profiling of S-nitrosylation in mouse skeletal muscle using cysteinyl peptide enrichment coupled with mass spectrometry. Free radical biology & medicine. 2013;57:68–78. doi: 10.1016/j.freeradbiomed.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nature protocols. 2007;2:1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 54.Wojdyla K, Williamson J, Roepstorff P, Rogowska-Wrzesinska A. The SNO/SOH TMT strategy for combinatorial analysis of reversible cysteine oxidations. Journal of proteomics. 2015;113:415–434. doi: 10.1016/j.jprot.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Pan KT, Chen YY, Pu TH, Chao YS, et al. Mass spectrometry-based quantitative proteomics for dissecting multiplexed redox cysteine modifications in nitric oxide-protected cardiomyocyte under hypoxia. Antioxidants & redox signaling. 2014;20:1365–1381. doi: 10.1089/ars.2013.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo J, Gaffrey MJ, Su D, Liu T, et al. Resin-assisted enrichment of thiols as a general strategy for proteomic profiling of cysteine-based reversible modifications. Nature protocols. 2014;9:64–75. doi: 10.1038/nprot.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y, Ho SO, Gassman NR, Korlann Y, et al. Efficient site-specific labeling of proteins via cysteines. Bioconjugate chemistry. 2008;19:786–791. doi: 10.1021/bc7002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, Gupta V, Carroll KS, Liebler DC. Site-specific mapping and quantification of protein S-sulphenylation in cells. Nature communications. 2014;5:4776. doi: 10.1038/ncomms5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klomsiri C, Nelson KJ, Bechtold E, Soito L, et al. Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods in enzymology. 2010;473:77–94. doi: 10.1016/S0076-6879(10)73003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bechtold E, King SB. Chemical methods for the direct detection and labeling of S-nitrosothiols. Antioxidants & redox signaling. 2012;17:981–991. doi: 10.1089/ars.2012.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Xian M. Chemical methods to detect S-nitrosation. Current opinion in chemical biology. 2011;15:32–37. doi: 10.1016/j.cbpa.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doulias PT, Greene JL, Greco TM, Tenopoulou M, et al. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16958–16963. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Science signaling. 2013;6:rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lo Conte M, Carroll KS. Chemoselective ligation of sulfinic acids with aryl-nitroso compounds. Angewandte Chemie. 2012;51:6502–6505. doi: 10.1002/anie.201201812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang YC, Huang CN, Lin CH, Chang HC, Wu CC. Mapping protein cysteine sulfonic acid modifications with specific enrichment and mass spectrometry: an integrated approach to explore the cysteine oxidation. Proteomics. 2010;10:2961–2971. doi: 10.1002/pmic.200900850. [DOI] [PubMed] [Google Scholar]
- 66.Sultana R, Perluigi M, Newman SF, Pierce WM, et al. Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer’s disease. Antioxidants & redox signaling. 2010;12:327–336. doi: 10.1089/ars.2009.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson RA, Lange MB, Sultana R, Galvan V, et al. Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: effects of the amyloid-beta peptide of amyloid precursor protein. Neuroscience. 2011;177:207–222. doi: 10.1016/j.neuroscience.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: Insights into the role of lipid peroxidation in the progression of AD. Brain research. 2009;1274:66–76. doi: 10.1016/j.brainres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Zahid S, Khan R, Oellerich M, Ahmed N, Asif AR. Differential S-nitrosylation of proteins in Alzheimer’s disease. Neuroscience. 2014;256:126–136. doi: 10.1016/j.neuroscience.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 70.Di Domenico F, Cenini G, Sultana R, Perluigi M, et al. Glutathionylation of the pro-apoptotic protein p53 in Alzheimer’s disease brain: implications for AD pathogenesis. Neurochemical research. 2009;34:727–733. doi: 10.1007/s11064-009-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurd TR, Prime TA, Harbour ME, Lilley KS, Murphy MP. Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis: implications for mitochondrial redox signaling. The Journal of biological chemistry. 2007;282:22040–22051. doi: 10.1074/jbc.M703591200. [DOI] [PubMed] [Google Scholar]
- 72.Chouchani ET, Hurd TR, Nadtochiy SM, Brookes PS, et al. Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem J. 2010;430:49–59. doi: 10.1042/BJ20100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinha V, Wijewickrama GT, Chandrasena RE, Xu H, et al. Proteomic and mass spectroscopic quantitation of protein S-nitrosation differentiates NO-donors. ACS chemical biology. 2010;5:667–680. doi: 10.1021/cb100054m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang YT, Piyankarage SC, Williams DL, Thatcher GR. Proteomic profiling of nitrosative stress: protein S-oxidation accompanies S-nitrosylation. ACS chemical biology. 2014;9:821–830. doi: 10.1021/cb400547u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez-Acedo P, Nunez E, Gomez FJ, Moreno M, et al. A novel strategy for global analysis of the dynamic thiol redox proteome. Molecular & cellular proteomics : MCP. 2012;11:800–813. doi: 10.1074/mcp.M111.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu L, Robinson RA. A simple isotopic labeling method to study cysteine oxidation in Alzheimer’s disease: oxidized cysteine-selective dimethylation (OxcysDML) Analytical and bioanalytical chemistry. 2016;408:2993–3004. doi: 10.1007/s00216-016-9307-4. [DOI] [PubMed] [Google Scholar]
- 77.Gu L, Robinson RA. High-throughput endogenous measurement of S-nitrosylation in Alzheimer’s disease using oxidized cysteine-selective cPILOT. The Analyst. 2016;141:3904–3915. doi: 10.1039/c6an00417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gygi SP, Rist B, Gerber SA, Turecek F, et al. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nature biotechnology. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 79.Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, et al. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumsta C, Thamsen M, Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antioxidants & redox signaling. 2011;14:1023–1037. doi: 10.1089/ars.2010.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knoefler D, Thamsen M, Koniczek M, Niemuth NJ, et al. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Molecular cell. 2012;47:767–776. doi: 10.1016/j.molcel.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brandes N, Reichmann D, Tienson H, Leichere LI, Jakob U. Using Quantitative Redox Proteomics to Dissect the Yeast Redoxome. Journal of Biological Chemistry. 2011;286:41893–41903. doi: 10.1074/jbc.M111.296236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fares A, Rossignol M, Peltier JB. Proteomics investigation of endogenous S-nitrosylation in Arabidopsis. Biochem Biophys Res Commun. 2011;416:331–336. doi: 10.1016/j.bbrc.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 84.Fares A, Nespoulous C, Rossignol M, Peltier JB. Simultaneous identification and quantification of nitrosylation sites by combination of biotin switch and ICAT labeling. Methods in molecular biology. 2014;1072:609–620. doi: 10.1007/978-1-62703-631-3_41. [DOI] [PubMed] [Google Scholar]
- 85.Wu C, Parrott AM, Liu T, Beuve A, Li H. Functional proteomics approaches for the identification of transnitrosylase and denitrosylase targets. Methods. 2013;62:151–160. doi: 10.1016/j.ymeth.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu C, Parrott AM, Liu T, Jain MR, et al. Distinction of thioredoxin transnitrosylation and denitrosylation target proteins by the ICAT quantitative approach. Journal of proteomics. 2011;74:2498–2509. doi: 10.1016/j.jprot.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia-Santamarina S, Boronat S, Domenech A, Ayte J, et al. Monitoring in vivo reversible cysteine oxidation in proteins using ICAT and mass spectrometry. Nature protocols. 2014;9:1131–1145. doi: 10.1038/nprot.2014.065. [DOI] [PubMed] [Google Scholar]
- 88.Liu T, Qian WJ, Strittmatter EF, Camp DG, 2nd, et al. High-throughput comparative proteome analysis using a quantitative cysteinyl-peptide enrichment technology. Analytical chemistry. 2004;76:5345–5353. doi: 10.1021/ac049485q. [DOI] [PubMed] [Google Scholar]
- 89.Liu T, Qian WJ, Chen WN, Jacobs JM, et al. Improved proteome coverage by using high efficiency cysteinyl peptide enrichment: the human mammary epithelial cell proteome. Proteomics. 2005;5:1263–1273. doi: 10.1002/pmic.200401055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gu L, Robinson RAS. High-throughput endogenous measurement of S-nitrosylation in Alzheimer’s disease using oxidized cysteine-selective cPILOT. Analyst. 2016 doi: 10.1039/c6an00417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo J, Nguyen AY, Dai Z, Su D, et al. Proteome-wide light/dark modulation of thiol oxidation in cyanobacteria revealed by quantitative site-specific redox proteomics. Molecular & cellular proteomics : MCP. 2014;13:3270–3285. doi: 10.1074/mcp.M114.041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kohr MJ, Aponte AM, Sun J, Wang G, et al. Characterization of potential S-nitrosylation sites in the myocardium. American journal of physiology. Heart and circulatory physiology. 2011;300:H1327–1335. doi: 10.1152/ajpheart.00997.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohr MJ, Sun J, Aponte A, Wang G, et al. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circulation research. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paulech J, Solis N, Edwards AV, Puckeridge M, et al. Large-scale capture of peptides containing reversibly oxidized cysteines by thiol-disulfide exchange applied to the myocardial redox proteome. Analytical chemistry. 2013;85:3774–3780. doi: 10.1021/ac400166e. [DOI] [PubMed] [Google Scholar]
- 95.Duan J, Kodali VK, Gaffrey MJ, Guo J, et al. Quantitative Profiling of Protein S-Glutathionylation Reveals Redox-Dependent Regulation of Macrophage Function during Nanoparticle-Induced Oxidative Stress. ACS nano. 2016;10:524–538. doi: 10.1021/acsnano.5b05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Y, Wang F, Liu Z, Qin H, et al. Five-plex isotope dimethyl labeling for quantitative proteomics. Chemical communications. 2014;50:1708–1710. doi: 10.1039/c3cc47998f. [DOI] [PubMed] [Google Scholar]
- 97.Evans AR, Robinson RA. Global combined precursor isotopic labeling and isobaric tagging (cPILOT) approach with selective MS(3) acquisition. Proteomics. 2013;13:3267–3272. doi: 10.1002/pmic.201300198. [DOI] [PubMed] [Google Scholar]
- 98.Spickett CM. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox biology. 2013;1:145–152. doi: 10.1016/j.redox.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wakita C, Honda K, Shibata T, Akagawa M, Uchida K. A method for detection of 4-hydroxy-2-nonenal adducts in proteins. Free radical biology & medicine. 2011;51:1–4. doi: 10.1016/j.freeradbiomed.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 100.Han B, Hare M, Wickramasekara S, Fang Y, Maier CS. A comparative ‘bottom up’ proteomics strategy for the site-specific identification and quantification of protein modifications by electrophilic lipids. Journal of proteomics. 2012;75:5724–5733. doi: 10.1016/j.jprot.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 102.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. Journal of the American Society for Mass Spectrometry. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 103.Borges CR, Sherma ND. Techniques for the analysis of cysteine sulfhydryls and oxidative protein folding. Antioxidants & redox signaling. 2014;21:511–531. doi: 10.1089/ars.2013.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu H, Zhang L, Freitas MA. Identification and characterization of disulfide bonds in proteins and peptides from tandem MS data by use of the MassMatrix MS/MS search engine. J Proteome Res. 2008;7:138–144. doi: 10.1021/pr070363z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi S, Jeong J, Na S, Lee HS, et al. New algorithm for the identification of intact disulfide linkages based on fragmentation characteristics in tandem mass spectra. J Proteome Res. 2010;9:626–635. doi: 10.1021/pr900771r. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt A, Forne I, Imhof A. Bioinformatic analysis of proteomics data. BMC systems biology. 2014;8(Suppl 2):S3. doi: 10.1186/1752-0509-8-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991;19(Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ceroni A, Passerini A, Vullo A, Frasconi P. DISULFIND: a disulfide bonding state and cysteine connectivity prediction server. Nucleic acids research. 2006;34:W177–181. doi: 10.1093/nar/gkl266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salsbury FR, Jr, Knutson ST, Poole LB, Fetrow JS. Functional site profiling and electrostatic analysis of cysteines modifiable to cysteine sulfenic acid. Protein science : a publication of the Protein Society. 2008;17:299–312. doi: 10.1110/ps.073096508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han S. Force field parameters for S-nitrosocysteine and molecular dynamics simulations of S-nitrosated thioredoxin. Biochem Biophys Res Commun. 2008;377:612–616. doi: 10.1016/j.bbrc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 111.Zhao X, Ning Q, Ai M, Chai H, Yin M. PGluS: prediction of protein S-glutathionylation sites with multiple features and analysis. Molecular bioSystems. 2015;11:923–929. doi: 10.1039/c4mb00680a. [DOI] [PubMed] [Google Scholar]
- 112.Chen YJ, Lu CT, Lee TY, Chen YJ. dbGSH: a database of S-glutathionylation. Bioinformatics. 2014;30:2386–2388. doi: 10.1093/bioinformatics/btu301. [DOI] [PubMed] [Google Scholar]
- 113.Zhou F, Xue Y, Yao X, Xu Y. CSS-Palm: palmitoylation site prediction with a clustering and scoring strategy (CSS) Bioinformatics. 2006;22:894–896. doi: 10.1093/bioinformatics/btl013. [DOI] [PubMed] [Google Scholar]
- 114.Ren J, Wen L, Gao X, Jin C, et al. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein engineering, design & selection : PEDS. 2008;21:639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun MA, Wang Y, Cheng H, Zhang Q, et al. RedoxDB--a curated database for experimentally verified protein oxidative modification. Bioinformatics. 2012;28:2551–2552. doi: 10.1093/bioinformatics/bts468. [DOI] [PubMed] [Google Scholar]
- 116.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiology of aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 117.Baez NOD, Reisz JA, Furdui CM. Mass spectrometry in studies of protein thiol chemistry and signaling: Opportunities and caveats. Free Radical Bio Med. 2015;80:191–211. doi: 10.1016/j.freeradbiomed.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zareba-Koziol M, Szwajda A, Dadlez M, Wyslouch-Cieszynska A, Lalowski M. Global analysis of S-nitrosylation sites in the wild type and APP transgenic mouse brain-clues for synaptic pathology. Molecular & cellular proteomics : MCP. 2014;13:2288–2305. doi: 10.1074/mcp.M113.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakamura T, Lipton SA. Protein S-Nitrosylation as a Therapeutic Target for Neurodegenerative Diseases. Trends in pharmacological sciences. 2016;37:73–84. doi: 10.1016/j.tips.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perez VI, Pierce A, de Waal EM, Ward WF, et al. Detection and quantification of protein disulfides in biological tissues a fluorescence-based proteomic approach. Methods in enzymology. 2010;473:161–177. doi: 10.1016/S0076-6879(10)73008-1. [DOI] [PubMed] [Google Scholar]
- 121.Dogru-Abbasoglu S, Tamer-Toptani S, Ugurnal B, Kocak-Toker N, et al. Lipid peroxidation and antioxidant enzymes in livers and brains of aged rats. Mechanisms of ageing and development. 1997;98:177–180. doi: 10.1016/s0047-6374(97)00082-1. [DOI] [PubMed] [Google Scholar]
- 122.Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiology of aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-e. [DOI] [PubMed] [Google Scholar]
- 123.McDonagh B, Sakellariou GK, Smith NT, Brownridge P, Jackson MJ. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J Proteome Res. 2014;13:5008–5021. doi: 10.1021/pr5006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Menger KE, James AM, Cocheme HM, Harbour ME, et al. Fasting but Not Aging Dramatically Alters the Redox Status of Cysteine Residues on Proteins in Drosophila melanogaster. Cell reports. 2015;11:1856–1865. doi: 10.1016/j.celrep.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brandes N, Tienson H, Lindemann A, Vitvitsky V, et al. Time line of redox events in aging postmitotic cells. eLife. 2013;2:e00306. doi: 10.7554/eLife.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Experimental eye research. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 127.Hains PG, Truscott RJ. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochimica et biophysica acta. 2008;1784:1959–1964. doi: 10.1016/j.bbapap.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 128.Fan X, Zhou S, Wang B, Hom G, et al. Evidence of highly conserved beta-crystallin disulfidome that can be mimicked by in vitro oxidation in age-related human cataract and glutathione depleted LEGSKO mouse lens. Molecular & cellular proteomics : MCP. 2015 doi: 10.1074/mcp.M115.050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 130.Abdul HM, Sultana R, Clair DKS, Markesbery WR, Butterfield DA. Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radical Bio Med. 2008;45:1420–1425. doi: 10.1016/j.freeradbiomed.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sinclair AJ, Bayer AJ, Johnston J, Warner C, Maxwell SRJ. Altered plasma antioxidant status in subjects with Alzheimer’s disease and vascular dementia. Int J Geriatr Psych. 1998;13:840–845. doi: 10.1002/(sici)1099-1166(1998120)13:12<840::aid-gps877>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 132.Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochimica et biophysica acta. 2000;1502:139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 133.Robinson RA, Cao Z, Williams C. Oxidative stress in CD90+ T-cells of AbetaPP/PS-1 transgenic mice. Journal of Alzheimer’s disease : JAD. 2013;37:661–666. doi: 10.3233/JAD-130665. [DOI] [PubMed] [Google Scholar]
- 134.Correani V, Di Francesco L, Cera I, Mignogna G, et al. Reversible redox modifications in the microglial proteome challenged by beta amyloid. Molecular bioSystems. 2015;11:1584–1593. doi: 10.1039/c4mb00703d. [DOI] [PubMed] [Google Scholar]
- 135.Nakamura T, Tu S, Akhtar MW, Sunico CR, et al. Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron. 2013;78:596–614. doi: 10.1016/j.neuron.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 137.Choi YB, Tenneti L, Le DA, Ortiz J, et al. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nature neuroscience. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 138.Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao QF, Yu JT, Tan L. S-Nitrosylation in Alzheimer’s disease. Molecular neurobiology. 2015;51:268–280. doi: 10.1007/s12035-014-8672-2. [DOI] [PubMed] [Google Scholar]
- 140.Nakamura T, Lipton SA. Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell death and differentiation. 2011;18:1478–1486. doi: 10.1038/cdd.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. Journal of chemical neuroanatomy. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 142.Heales SJ, Bolanos JP, Stewart VC, Brookes PS, et al. Nitric oxide, mitochondria and neurological disease. Biochimica et biophysica acta. 1999;1410:215–228. doi: 10.1016/s0005-2728(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 143.Riederer IM, Schiffrin M, Kovari E, Bouras C, Riederer BM. Ubiquitination and cysteine nitrosylation during aging and Alzheimer’s disease. Brain research bulletin. 2009;80:233–241. doi: 10.1016/j.brainresbull.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 144.Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sultana R, Poon HF, Cai J, Pierce WM, et al. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiology of disease. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 146.Perluigi M, Sultana R, Cenini G, Di Domenico F, et al. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: Role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteomics. Clinical applications. 2009;3:682–693. doi: 10.1002/prca.200800161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Okamoto S, Lipton SA. S-Nitrosylation in neurogenesis and neuronal development. Biochimica et biophysica acta. 2015;1850:1588–1593. doi: 10.1016/j.bbagen.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kragten E, Lalande I, Zimmermann K, Roggo S, et al. Glyceraldehyde-3-phosphate dehydrogenase, the putative target of the antiapoptotic compounds CGP 3466 and R-(−)-deprenyl. The Journal of biological chemistry. 1998;273:5821–5828. doi: 10.1074/jbc.273.10.5821. [DOI] [PubMed] [Google Scholar]
- 149.Gu L, Robinson RAS. High-throughput endogenous measurement of S-nitrosylation in Alzheimer’s disease using oxidized cysteine-selective cPILOT. 2016 doi: 10.1039/c6an00417b. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boyd-Kimball D, Castegna A, Sultana R, Poon HF, et al. Proteomic identification of proteins oxidized by Abeta(1–42) in synaptosomes: implications for Alzheimer’s disease. Brain research. 2005;1044:206–215. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 151.Sultana R, Reed T, Perluigi M, Coccia R, et al. Proteomic identification of nitrated brain proteins in amnestic mild cognitive impairment: a regional study. Journal of cellular and molecular medicine. 2007;11:839–851. doi: 10.1111/j.1582-4934.2007.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Korolainen MA, Goldsteins G, Nyman TA, Alafuzoff I, et al. Oxidative modification of proteins in the frontal cortex of Alzheimer’s disease brain. Neurobiology of aging. 2006;27:42–53. doi: 10.1016/j.neurobiolaging.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 153.Hoyer S, Nitsch R, Oesterreich K. Ammonia Is Endogenously Generated in the Brain in the Presence of Presumed and Verified Dementia of Alzheimer Type. Neurosci Lett. 1990;117:358–362. doi: 10.1016/0304-3940(90)90691-2. [DOI] [PubMed] [Google Scholar]
- 154.Johnson WM, Wilson-Delfosse AL, Mieyal JJ. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients. 2012;4:1399–1440. doi: 10.3390/nu4101399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Newman SF, Sultana R, Perluigi M, Coccia R, et al. An increase in S-glutathionylated proteins in the Alzheimer’s disease inferior parietal lobule, a proteomics approach. Journal of neuroscience research. 2007;85:1506–1514. doi: 10.1002/jnr.21275. [DOI] [PubMed] [Google Scholar]
- 156.Sultana R, Boyd-Kimball D, Poon HF, Cai J, et al. Oxidative modification and down-regulation of Pin1 in Alzheimer’s disease hippocampus: A redox proteomics analysis. Neurobiology of aging. 2006;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 157.Chen CH, Li W, Sultana R, You MH, et al. Pin1 cysteine-113 oxidation inhibits its catalytic activity and cellular function in Alzheimer’s disease. Neurobiology of disease. 2015;76:13–23. doi: 10.1016/j.nbd.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Innes BT, Sowole MA, Gyenis L, Dubinsky M, et al. Peroxide-mediated oxidation and inhibition of the peptidyl-prolyl isomerase Pin1. Biochimica et biophysica acta. 2015;1852:905–912. doi: 10.1016/j.bbadis.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 159.Furdui CM, Poole LB. Chemical approaches to detect and analyze protein sulfenic acids. Mass spectrometry reviews. 2014;33:126–146. doi: 10.1002/mas.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 161.Choi J, Levey AI, Weintraub ST, Rees HD, et al. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. The Journal of biological chemistry. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 162.Danielson SR, Held JM, Oo M, Riley R, et al. Quantitative mapping of reversible mitochondrial Complex I cysteine oxidation in a Parkinson disease mouse model. The Journal of biological chemistry. 2011;286:7601–7608. doi: 10.1074/jbc.M110.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haltia M. Human prion diseases. Annals of medicine. 2000;32:493–500. doi: 10.3109/07853890009002025. [DOI] [PubMed] [Google Scholar]
- 164.Chen LN, Shi Q, Zhang BY, Zhang XM, et al. Proteomic Analyses for the Global S-Nitrosylated Proteins in the Brain Tissues of Different Human Prion Diseases. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9440-7. [DOI] [PubMed] [Google Scholar]
- 165.Pieragostino D, Del Boccio P, Di Ioia M, Pieroni L, et al. Oxidative modifications of cerebral transthyretin are associated with multiple sclerosis. Proteomics. 2013;13:1002–1009. doi: 10.1002/pmic.201200395. [DOI] [PubMed] [Google Scholar]
- 166.Wang S, Circu ML, Zhou H, Figeys D, et al. Highly sensitive detection of S-nitrosylated proteins by capillary gel electrophoresis with laser induced fluorescence. Journal of chromatography. A. 2011;1218:6756–6762. doi: 10.1016/j.chroma.2011.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang SY, Njoroge SK, Battle K, Zhang C, et al. Two-dimensional nitrosylated protein fingerprinting by using poly (methyl methacrylate) microchips. Lab on a chip. 2012;12:3362–3369. doi: 10.1039/c2lc40132k. [DOI] [PubMed] [Google Scholar]
- 168.Zhang C, Kuo CC, Chiu AWL, Feng J. Prediction of S-glutathionylated Proteins Progression in Alzheimer’s Transgenic Mouse Model Using Principle Component Analysis. Journal of Alzheimers Disease. 2012;30:919–934. doi: 10.3233/JAD-2012-120028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yao C, Behring JB, Shao D, Sverdlov AL, et al. Overexpression of Catalase Diminishes Oxidative Cysteine Modifications of Cardiac Proteins. PloS one. 2015;10:e0144025. doi: 10.1371/journal.pone.0144025. [DOI] [PMC free article] [PubMed] [Google Scholar]