Abstract
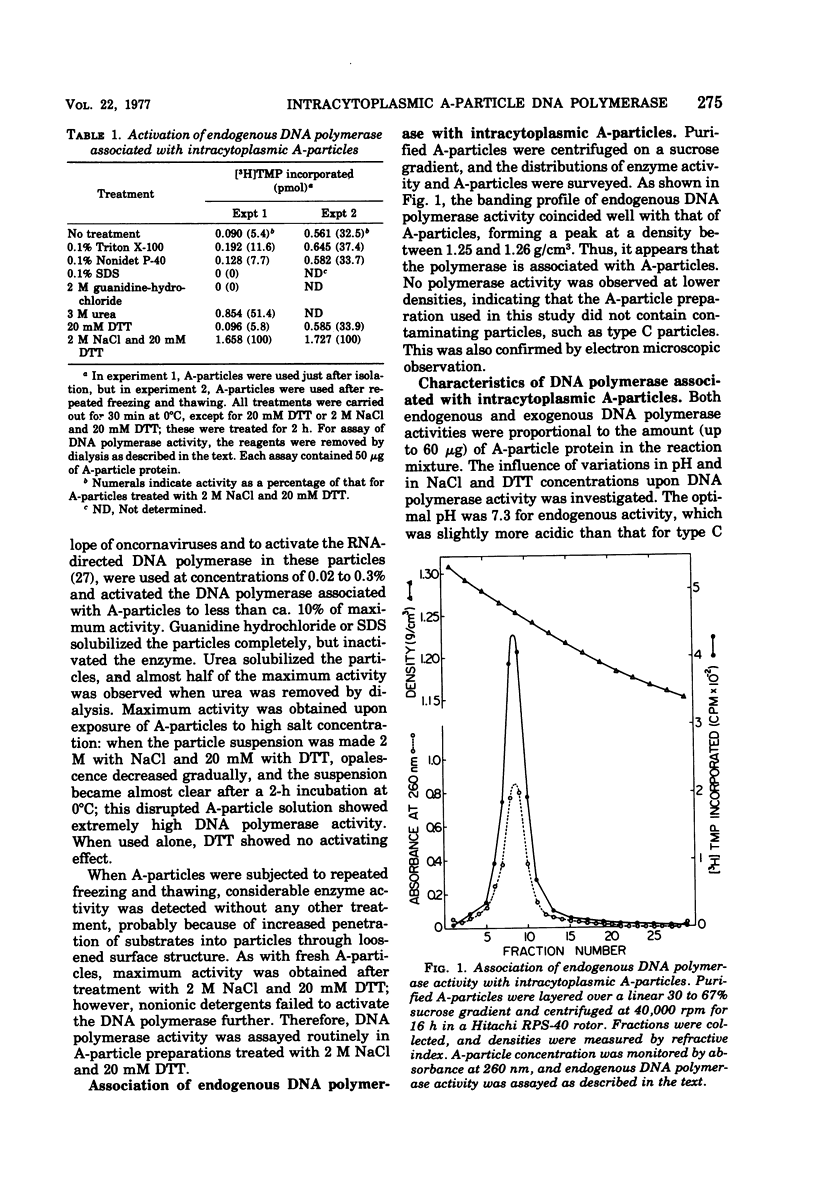
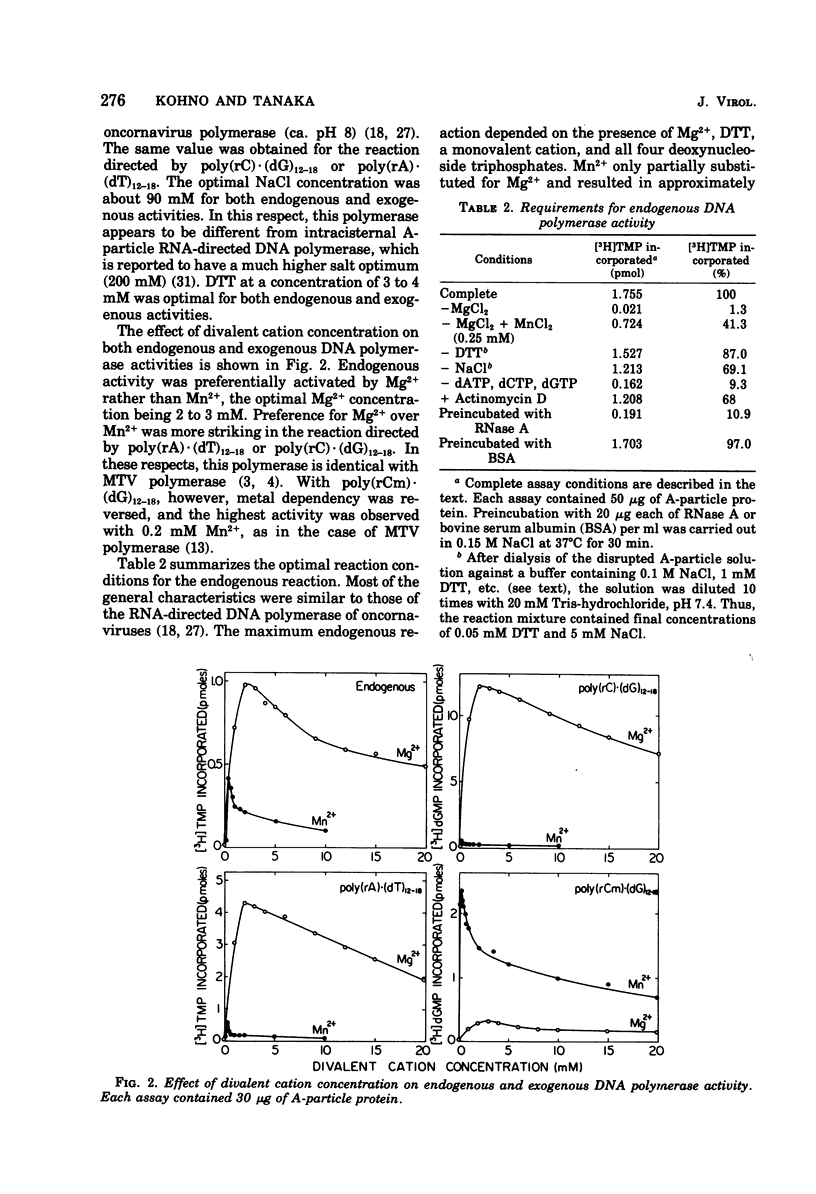
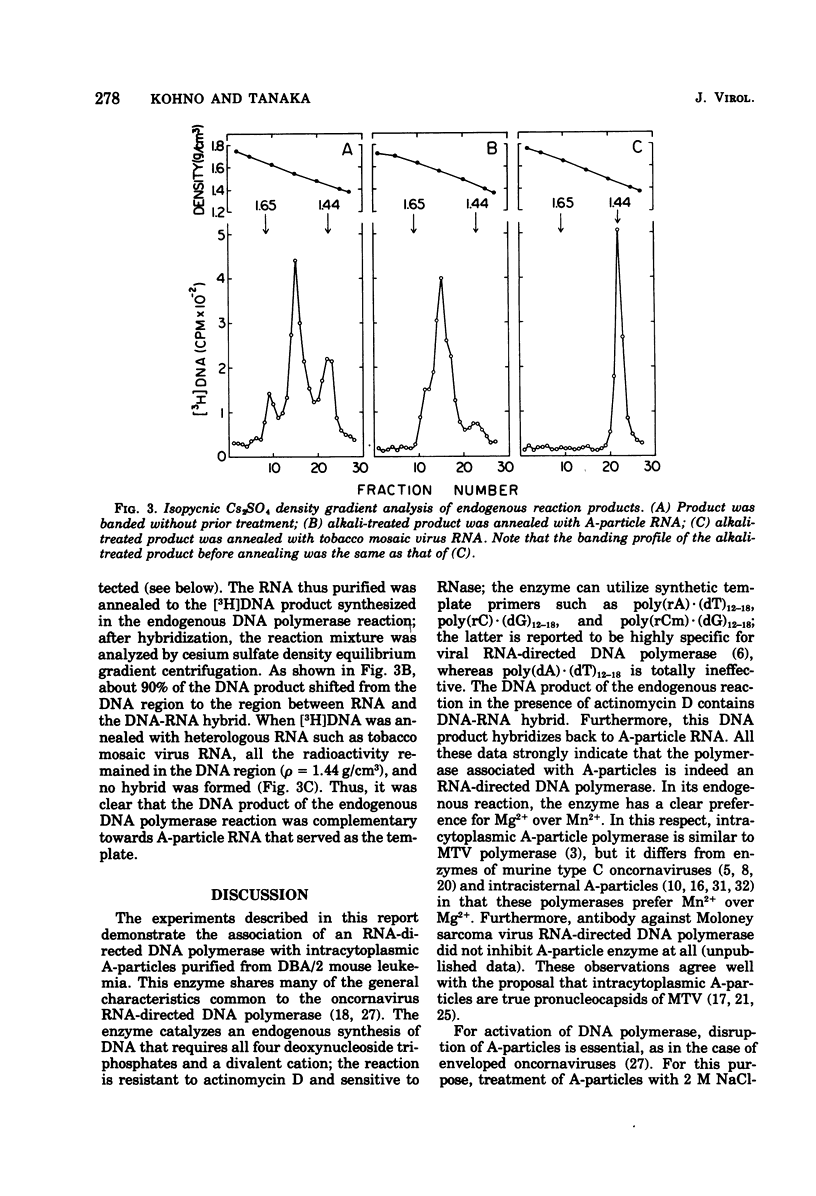
An RNA-directed DNA polymerase was found to be associated with intracytoplasmic A-particles from DBA/2 mouse leukemia cells. The enzyme activity was detected after disrupting the purified particles with 2 M NaCl-20 mM dithiothreitol. The presence of a divalent cation and all four deoxyribonucleoside triphosphates was essential for this enzyme activity. The enzyme had a clear preference for Mg2+ over Mn2+. Cesium sulfate isopycnic gradient centrifugation of the DNA product synthesized in the actinomycin D-containing reaction revealed the presence of DNA-RNA hybrid. Furthermore, the purified DNA product was found to hybridize with RNA isolated from A-particles. These observations strongly indicate that the endogenous A-particle RNA serves as the template for the DNA polymerase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD W. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 1958 Jun;18(5):491–509. [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Vaidya A. B., Fout G. S. Cation preferences for poly(rC)-oligo(dG)-directed DNA synthesis by RNA tumor viruses and human milk particulates. Cancer Res. 1974 Dec;34(12):3509–3515. [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Gerard G. F. Poly (2'O-methylcytidylate).oligodeoxyguanylate, a template-primer specific for reverse transcriptase, is not utilized by HeLa cell gamma DNA polymerases. Biochem Biophys Res Commun. 1975 Apr 7;63(3):706–711. doi: 10.1016/s0006-291x(75)80441-4. [DOI] [PubMed] [Google Scholar]
- Green M., Rokutanda M., Fujinaga K., Ray R. K., Rokutanda H., Gurgo C. Mechanism of carcinogenesis by RNA tumor viruses. I. An RNA-dependent DNA polymerase in murine sarcoma viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):385–393. doi: 10.1073/pnas.67.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Krueger R. G. Intracisternal A particles from FLOPC-1 BALB/c myeloma: presence of high-molecular-weight RNA and RNA-dependent DNA polymerase. J Virol. 1976 May;18(2):745–756. doi: 10.1128/jvi.18.2.745-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Longfellow D. G., Smith G. H. Further characterization of intracytoplasmic A particle-associated DNA. J Virol. 1976 Mar;17(3):816–823. doi: 10.1128/jvi.17.3.816-823.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. L., Sarkar N. H., Modak M. J. Purification and properties of murine mammary tumor virus DNA polymerase. Virology. 1976 May;71(1):242–254. doi: 10.1016/0042-6822(76)90109-4. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Abrell J. W., Trainor C. D., Gallo R. C. Precipitation of nucleic acids with cetyltrimethylammonium bromide: a method for preparing viral and cellular DNA polymerase products for cesium sulfate density gradient analysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):30–38. doi: 10.1016/0006-291x(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Baenziger N. L., Dobbertin D. C., Thach R. E. Characterization of DNA polymerase and RNA associated with A-type particles from murine myeloma cells. J Virol. 1975 Feb;15(2):407–415. doi: 10.1128/jvi.15.2.407-415.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Dion A. S. Polypeptides of the mouse mammary tumor virus. I. Characterization of two group-specific antigens. Virology. 1975 Apr;64(2):471–491. doi: 10.1016/0042-6822(75)90125-7. [DOI] [PubMed] [Google Scholar]
- Schlom J., Harter D. H., Burny A., Spiegelman S. DNA polymerase activities in varions of visna virus, a causative agent of a "slow" neurological disease. Proc Natl Acad Sci U S A. 1971 Jan;68(1):182–186. doi: 10.1073/pnas.68.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. H., Lee B. K. Mouse mammary tumor virus polypeptide precursors in intracytoplasmic A particles. J Natl Cancer Inst. 1975 Aug;55(2):493–496. [PubMed] [Google Scholar]
- Smith G. H., Litwack M., Longfellow D. G. High-molecular-weight DNA associated with intracytoplasmic A-particle inclusions. Cancer Res. 1974 Oct;34(10):2654–2662. [PubMed] [Google Scholar]
- Smith G. H., Wivel N. A. Intracytoplasmic A particles: mouse mammary tumor virus nucleoprotein cores? J Virol. 1973 Apr;11(4):575–584. doi: 10.1128/jvi.11.4.575-584.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H. Precursor-product relationship between nonglycosylated polypeptides of A and B particles of mouse mammary tumor virus. Virology. 1977 Feb;76(2):835–850. doi: 10.1016/0042-6822(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Tamura A., Tsujimura D. Properties of the intracytoplasmic A particles purified from mouse tumors. Virology. 1972 Jul;49(1):61–78. doi: 10.1016/s0042-6822(72)80007-2. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Tsujimura D., Nakamura K. Localization of intracytoplasmic A particles in mouse tumors by light microscopy. Cancer Res. 1974 Jun;34(6):1465–1474. [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Tsujimura D., Tanaka H. Quantitative studies on intracytoplasmic A particles formed in DBA-2 mouse leukemias. Cancer Res. 1974 Jun;34(6):1475–1486. [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Trainor C. D., Gallo R. C. Murine intracisternal type A particles: a biochemical characterization. J Virol. 1975 Oct;16(4):887–896. doi: 10.1128/jvi.16.4.887-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Characterization of an endogenous RNA-dependent DNA polymerase associated with murine intracisternal A particles. J Virol. 1974 Mar;13(3):712–720. doi: 10.1128/jvi.13.3.712-720.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


