Abstract
Background
Digital tonometry is designed to noninvasively screen for endothelial dysfunction by the detection of impaired flow-induced reactive hyperemia in the fingertip. We determined whether digital reactive hyperemia correlated with risk factors for atherosclerosis in 2 groups of children at increased risk for endothelial dysfunction.
Methods
Fifteen obese children and 23 non-obese, dyslipidemic children, 8-21 years of age, were enrolled and medical histories, anthropometric measurements, carotid wall thickness by ultrasound and fasting blood samples for cardiovascular risk factors were obtained. The standard endoPAT index of digital reactive hyperemia was modified to reflect the true peak response or the integrated response of the entire post-occlusion period. In each group, age, sex, pubertal status, carotid wall thickness and multiple cardiovascular risk factors were tested as predictors of endothelial dysfunction.
Results
In the nonobese, dyslipidemic group, but not the obese group, both indices strongly correlated with height (r=0.55, P=0.007 by peak response) followed by weight, waist circumference and age. In both groups, neither index of reactive hyperemia significantly correlated with any other cardiovascular risk factor.
Conclusions
Contrary to the known age-related increase in atherosclerosis, digital reactive hyperemia increased with age and its correlates in nonobese, dyslipidemic children and was not related to other cardiovascular risk factors in either group. The reason for the lack of this relationship with age in obese children is unknown. The age-dependent physiology of digital microvascular reactivity and the endothelium-independent factors controlling the peak hyperemic response need further study in children with a wide age range.
Keywords: endothelial dysfunction, peripheral arterial tonometry, hyperlipidemia
Introduction
New guidelines emphasize the likely long-term benefit of early screening and intervention in childhood to prevent prevalent adult chronic diseases such as diabetes and cardiovascular disease 1. In teenagers and adults, digital tonometry has been shown to be a simple and reproducible screening technique to assess the health of arteries 2,3,4. With this method, reactive hyperemia is induced in the fingertip after the temporary occlusion of blood flow with a blood pressure cuff, and proprietary software produces a reactive hyperemia index from the change in the pulse wave amplitude. When studied in adults, a low hyperemic response was shown to reflect endothelial dysfunction, defined as an imbalance between vasodilating and vasoconstricting factors produced by vascular endothelial cells. Major contributors to this imbalance are impaired nitric oxide-dependent mechanisms controlling vascular dilation 5. In adults, a low level of reactive hyperemia was associated with cardiovascular risk factors 6, coronary disease measured by angiography 7 and vascular events 8,9. In adolescents, reactive hyperemia was blunted in Type 1 diabetes 10, 11, obesity 12, 13,14 and insulin resistance 15 and correlated in the expected direction with several cardiovascular risk factors14, 15.
Although digital tonometry may be useful for the assessment of the arterial health of older teens and adults, several recent studies that included younger, pre-pubertal normal children revealed a strong, positive relationship between digital reactive hyperemia and age, height or pubertal status 12, 16-18. This is surprising given the strongly positive relationship between age and atherosclerosis from childhood through adulthood 19. However, in 2 of these studies, obese children did not show a relationship between reactive hyperemia index and age or its correlates 12, 18. Recently, it has been recommended that the standard automated reactive hyperemia index produced by the widely used endoPAT digital tonometer be modified in children to reflect large age-specific differences in the time course of reactive hyperemia20, 21. Using this modified index, a positive correlation with age was still found in young, mostly lean children21. The relationship between the modified index of reactive hyperemia and cardiovascular risk factors has not been reported in young obese children and other children at high risk for endothelial dysfunction and premature cardiovascular disease.
We evaluated the reactive hyperemia of the fingertip microcirculation of obese children and non-obese, dyslipidemic children and young adults 8-21 years of age. In each group, the standard index of reactive hyperemia was modified for the pediatric age range and correlated with age, sex, pubertal status, carotid wall thickness, and multiple other risk factors for premature cardiovascular disease.
Materials and Methods
Children and young adults, 8-21 years of age, were recruited from general pediatric, cardiology, gastroenterology and lipid clinics and studied at the Weill Cornell Medical College Clinical Translational Science Center after telephone screening. Two groups were evaluated: 1) Obese (body mass index >95th percentile) and 2) Non-obese, dyslipidemic (body mass index >5th and <95th percentiles). “Dyslipidemic” was defined as a fasting low- density lipoprotein cholesterol >130 mg/dL, triglycerides >150 mg/dL, high density lipoprotein cholesterol <40 mg/dL, and/or lipoprotein (a)> 2 fold the upper limit of normal. “Dyslipidemia” was not an inclusion criteria for the obese group, but 12 of 15 subjects had a lipid abnormality: all 12 had low HDL cholesterol (HDL-C), and 6 of these had other lipid abnormalities. The majority of the non-obese, dyslipidemic group (20/23) had a lean body mass index (<85th percentile). Exclusion criteria included systemic disorders such as diabetes (defined as fasting blood sugar >126 mg/dL twice) or autoimmune disease, medication and acute illnesses that might affect cardiovascular risk factors or the arteries. Current cigarette smokers or abusers of illicit drugs or alcohol were also excluded. The protocol was approved by the Weill Cornell Medical College Institutional Review Board.
In the morning after an overnight fast, consent and assent were obtained and then medical histories were reviewed. Family history of premature heart disease and stroke, diabetes, hypertension, hyperlipidemia, obesity and fatty liver in first-degree relatives and grandparents were recorded. Weight and height were measured with digital devices to the nearest 0.1 kg and 0.1 cm, respectively. Blood pressure and pulse were measured with an automated digital device and the appropriate cuff size after sitting at least 5 minutes. Body mass index was calculated as weight divided by height squared (kg*m-2). Body mass index z scores were derived from age- and sex-specific norms. Waist circumference was measured at the umbilicus; hip circumference at the widest point over the buttocks to the nearest 0.1 cm. Pubertal stage was classified as pre-pubertal (no pubertal development), pubertal (some development) or post-pubertal (fully developed and, in females, having regular menses) after questioning the parent and/or participant.
Digital tonometry was then performed following a strictly standardized procedure as specified by the manufacturer (EndoPAT2000, Itamar Medical, Caesarea, Israel). Participants were advised to clip long fingernails and fast except water for at least 12 hours before testing. Over-the-counter supplements were not taken at least 2 days before the visit. The designated study room was dimly lit, quiet and temperature-controlled. For the digital tonometry procedure, the participant lay comfortably supine with finger-cuffs placed on each index finger and attached by cables to the computer. After 5 minutes of tracing the baseline oscillations of blood flow, a blood pressure cuff was inflated to occlude blood flow into the non-dominant arm for 5 minutes. Upon release, tracings were obtained for another 5 minutes. Computerized software with a proprietary algorithm automatically calculated the reactive hyperemia index from the fold increase in the pulse wave amplitude relative to baseline, corrected for fold changes relative to baseline in the un-occluded arm, during the 90-150 second interval after the release of the blood pressure cuff. However, as reported by others 20, 21, this interval missed the true peak response in 67% of children in the obese group and 52% in the nonobese, dsylipidemic group and, in the latter, the time to peak was inversely related to age (r= -0.64, P<0.001). For this reason, the true peak response ratio corrected for the ratio in the control arm during the same time interval was calculated for each subject. In addition, the area under the curve was calculated from the peak response ratios in the occluded arm corrected for changes in the control arm over the entire 5 minutes post-occlusion using the trapezoid rule20.
After the completion of measurements of digital reactive hyperemia, blood was sampled and assayed for traditional and nontraditional cardiovascular risk factors. The cholesterol, triglycerides, and glucose were assayed by enzymatic methods. A direct polymer polyanion method to measure high density lipoprotein cholesterol was performed on a Beckman Coulter UniCel DXC 800 (Beckman Coulter, Incorporated, Brea, California). Lipoprotein (a) was performed by a quantitative immunoturbidity assay. Results were not obtained for 2 samples. Insulin was assayed with a quantitative immunoradiometric assay kit from Millipore (St. Charles, Missouri). The intra-assay and inter-assay coefficients of variation are less than 4.4% and 6.0%, respectively, and the range is 3.125 – 200.0 μU/ml. Fasting insulin resistance was calculated from the homeostasis model assessment from glucose and insulin values 22. The % hemoglobin A1C was determined with a quantitative monoclonal antibody agglutination reaction kit from Siemens Healthcare Diagnostics, Incorporated. (Tarrytown, New York). Homocysteine and high sensitivity C reactive protein were measured with quantitative sandwich enzyme immunoassay kits from ALPCO Diagnostics (Salem, New Hampshire) and R&D systems (Minneapolis, Minnesota), respectively. The intra-assay and inter-assay coefficients of variation are less than 8.3% and 10%, respectively, and the measurement ranges are 2 – 50 mol/L and 0.78 – 50 ng/ml, respectively. The serum concentrations of interleukin-6 and tumor necrosis factor alpha were determined using quantitative electrochemiluminescent assay kits from Meso Scale Discovery (Gaithersburg, Maryland). The intra-assay and inter-assay coefficients of variation are less than 4.4% and 2.8%, respectively, and the measurement ranges are from 0.2 pg/ml to 2,500 pg/ml.
On the same morning, the carotid intima-media thickness was measured by a specialized ultrasound technologist using an ultrasound scanner (Acuson Sequoia 512; Siemens Medical Solutions, Malvern, Pennsylvania) and a high-frequency 15L8-MHz linear-array transducer by following a predetermined standardized scanning protocol. The participants were studied in the supine position with the head turned slightly away from the side that was examined. Images of the arterial wall were obtained from the posterior walls of both common carotid arteries 1 cm below the carotid bulb (bifurcation) during three complete and independent cardiac cycles and digitally stored. An automated computerized edge detection software package (version 1.0, 2002; Siemens Medical Solutions, Malvern, Pennsylvania) was used to determine the carotid wall thickness in the frames of each cycle that depicted the narrowest and widest vessel diameters. The mean and maximum wall thicknesses were calculated for both carotid arteries. All examinations were digitally stored and analyzed by the same researcher (A.K.). The coefficient of variation for wall thickness measurements of the carotid artery using the same device and scanning protocol was previously calculated to be 1.3%23.
Data Analysis
All results are expressed as mean ± standard deviation except for the peak response, which is presented as the median and interquartile range. The group means were compared with student's unpaired t-test or Wilcoxon rank sum/Kruskal-Wallis tests for continuous variables or Fisher's exact test for categorical variables. Univariate analysis evaluated the correlations between either the peak response or area under the curve and cardiovascular risk variables for each group. The distribution of the peak response was skewed, and values were logarithmically transformed before correlation analysis. Variables that were significantly correlated with the outcomes were then included in multiple regression analysis. An association that yielded a P value <0.05 was considered significant. Values less than the detection limit were entered as the detection limit/2. Statistical analysis was carried out with JMP Pro 11 (version 11.0.0, SAS Institute Incorporated, Cary North Carolina).
Results
A total of 41 subjects were enrolled, but 2 were excluded because of the use of metformin and one with a missing reactive hyperemia index value due to noisy signal from excessive movement. Table 1 shows the subject characteristics of the 38 obese and non-obese, dyslipidemic subjects included in this report. There were no significant differences between groups for age, gender and racial/ethnic backgrounds (37% Caucasian, 34% Hispanic, 13% African American, 11% Asian, 5% other). As expected, the obese group had significantly higher levels of triglycerides, lower high density lipoprotein cholesterol, and lower low density lipoprotein cholesterol than the non-obese group. The majority of the obese group (12/15) had low levels of high density lipoprotein cholesterol; the majority of the nonobese, dyslipidemic subjects had high levels of low density lipoprotein cholesterol (12/23, 10 with the genetic lipid disorder, familial hypercholesterolemia, and low density lipoprotein cholesterol ≥ 190 mg/dL). The study groups also differed by pubertal status, with a greater percentage of the obese group either pubertal or post-pubertal (87% vs. 69%). Markers of insulin resistance (insulin levels and homeostasis model assessment score) and inflammation (high sensitivity C reactive protein and interleukin-6) were elevated in the obese group compared to nonobese, dyslipidemic group. The mean values for carotid intima-media thickness did not differ between groups and were one standard deviation higher than published values (0.38 ± 0.04 mm) in a large number of lean and obese children, 5-20 years of age, evaluated with the same ultrasound device and scanning protocol24. Age was not a correlate in this series.
Table 1. Subject characteristics in obese and nonobese, dyslipidemic children.
| Group 1 | Group 2 | P value | |
|---|---|---|---|
| Number | 15 | 23 | |
| Male Gender (%) | 6 (40) | 14 (61) | 0.320 |
| Age (yr) | 13 ± 3 | 14 ± 4 | 0.295 |
| Height (cm) | 160 ± 13 | 159 ± 17 | 0.759 |
| BMI (%) | 98 ± 1 | 59 ± 26 | <0.001 |
| BMI z score | 2.1 ± 0.3 | 0.3 ± 0.8 | <0.001 |
| Waist circ (cm) | 94 ± 14 | 71 ± 10 | <0.001 |
| Systolic BP | 112 ± 9 | 109 ± 8 | 0.411 |
| Pre-pubertal (%) | 2 (13) | 7 (30) | |
| Pubertal (%) | 9 (60) | 4 (17) | 0.026 |
| Post-pubertal (%) | 4 (27) | 12 (52) | |
| Family Hx CVD (%) | 6 (40) | 8 (35) | 1.000 |
| Family Hx DM (%) | 7 (47) | 13 (57) | 0.741 |
| Total Chol (mmol/L) | 4.03 ± 0.96 | 5.77 ± 2.15 | 0.005 |
| LDL-C (mmol/L) | 2.46 ± 0.62 | 4.19 ± 2.15 | 0.005 |
| HDL-C (mmol/L) | 0.96 ± 0.18 | 1.22 ± 0.28 | 0.004 |
| TG (mmol/L) | 1.31 ± 0.82 | 0.80 ± 0.41 | 0.016 |
| Lp(a) (mg/dL) | 33 ± 40 | 41 ± 29 | 0.475 |
| Glucose (mmol/L) | 4.39 ± 0.33 | 4.61 ± 0.44 | 0.302 |
| Insulin (μU/mL) | 202 ± 104 | 104 ± 42 | 0.001 |
| HbA1C (%) | 5.9 ± 1.3 | 5.4 ± 0.3 | 0.096 |
| HOMA-IR | 5.4 ± 2.5 | 3.2 ± 1.6 | 0.002 |
| Homocysteine (μmol/L) | 7.6 ± 2.0 | 7.9 ± 1.3 | 0.525 |
| 25-OH vitamin D (nmol/L) | 52 ± 10 | 69 ± 41 | 0.119 |
| hsCRP (mg/L) | 2.44 ± 1.63 | 1.08 ± 2.41 | 0.064 |
| IL-6 (pg/mL) | 1.59 ± 0.90 | 0.63 ± 0.47 | <0.001 |
| TNF (pg/mL) | 4.85 ± 4.36 | 5.02 ± 2.06 | 0.867 |
| Carotid IMT (mm) | 0.42 ± 0.04 | 0.42 ± 0.05 | 0.799 |
| Peak response | 1.52 [1.22-1.97] | 2.04 [1.61-2.46] | 0.038 |
| Area under the curve (AUC) | 19 ± 4 | 24 ± 6 | 0.010 |
Digital reactive hyperemia expressed as the peak response or area under the curve was significantly lower in the obese group compared to the nonobese, dyslipidemic group (Table 1). Age-specific normal values based on the true peak response have not been established for children; however, our median values for peak response correspond closely to values previously reported in children with narrower age range (12-18 years): 2.15 for lean children with normal lipid levels and 1.50 for severely obese children 14. Thirty-three% of the obese group and 9% of the nonobese, dyslipidemic group had peak responses lower than the abnormal cut-point of 1.35 proposed for adults2. In the nonobese, dyslipidemic group, the subset with LDL cholesterol levels >190 mg/dL and at extremely high risk of premature coronary artery disease did not significantly differ in the 2 indices of microvascular function from the other nonobese, dyslipidemic subjects (1.95 ± 0.53 vs 2.11 ± 0.62, P=0.51 for peak response). This was true despite this subset having a significantly younger age distribution that would lower the reactive hyperemia index.
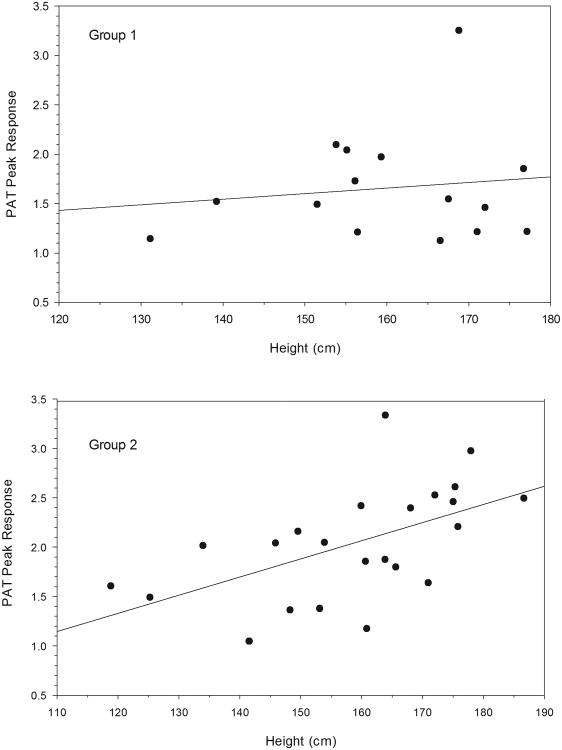
Correlation analysis failed to reveal significant relations in either group between peak response or area under the curve and sex, body mass index z scores, waist/hip circumference ratio, pubertal stage, blood pressure, family history of diabetes or premature cardiovascular disease, or the biochemical cardiovascular risk factors listed in Table 1. However, Table 2 and Figure 1 illustrate the strong, significant, positive relationship between peak response and height in the nonobese, dyslipidemic group (bottom panel) but not the obese group (top panel). A similar significant positive correlation was found in the nonobese, dyslipidemic group for the area under the curve. Less strong positive relationships existed between the 2 indices and age, weight, and waist circumference that are correlates of height. In stepwise regression analysis with height, age, weight and waist circumference as the predictive variables, height was the only significant determinant of both the peak response and area under the curve. There were no significant correlations between carotid wall thickness and indices of reactive hyperemia, height and its correlates, or any of the other tested variables.
Table 2. Significant correlations between digital reactive hyperemia and clinical variables in obese and lean, dyslipidemic children.
| Group 1 | Group 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Peak response1 | Area Under Curve | Peak response1 | Area Under Curve | |||||
| r | P | r | P | r | P | r | P | |
| Age | 0.02 | 0.947 | 0.01 | 0.962 | 0.38 | 0.073 | 0.41 | 0.052 |
| Weight | -0.08 | 0.789 | -0.11 | 0.707 | 0.54 | 0.008 | 0.50 | 0.016 |
| Height | 0.12 | 0.675 | 0.03 | 0.918 | 0.55 | 0.007 | 0.52 | 0.011 |
| Waist circ | 0.07 | 0.808 | 0.06 | 0.819 | 0.48 | 0.021 | 0.45 | 0.029 |
Logarithmically transformed before correlation analysis.
Figure 1.
Relationships between height and reactive hyperemia, expressed as the log-transformed reactive hyperemia index derived from true peak response.
Top panel= 15 obese (r=0.12, P=0.675); Bottom panel= 23 nonobese, dyslipidemic (r=0.55, P=0.007)
Discussion
Endothelial dysfunction occurs early in atherosclerosis and develops in both the large conduit arteries and the microvasculature. Digital tonometry is an attractive noninvasive screening method with the potential to detect endothelial dysfunction early in life and target those who need intensive therapy. However, in our study of obese and non-obese, dyslipidemic children with a wide age span, two indices of digital reactive hyperemia adapted for the pediatric age group did not correlate with any risk marker of endothelial dysfunction and vascular disease. Instead, in the group of non-obese, dyslipidemic children, indices of reactive hyperemia most strongly correlated with height in a positive direction that is unexpected given the expected progression in atherosclerosis with growth in children with hyperlipidemia18. The positive relationships between digital reactive hyperemia and the correlates of growth confirm similar findings by others in normal children16-18 and extend it to lean, dyslipidemic children. The lack of this relationship in the obese group was also reported by others who studied obese children with a wide age distribution using the standard reactive hyperemia index12, 18.
The increase in digital reactive hyperemia with growth has been attributed to an increase in nitric oxide production during puberty, possibly due to increased estrogen and dehydroepiandrosterone sulfate levels17. Another possibility is that endothelium-independent factors that contribute to the reactive hyperemia index vary with childhood development. In adults, an impaired reactive hyperemic response of the digital circulation is associated with impaired dilation of the coronary arteries after the infusion of acetylcholine2 to stimulate production of nitric oxide. Such studies of the coronaries have not been performed in young children, but, flow-induced vasodilation of the brachial artery is blunted in young children with cardiovascular risk factors and not confounded by age 25. However, in adults, the agreement between digital reactive hyperemia and the flow-induced vasodilation of the brachial artery has been inconsistent 7,26. This may be because only 50% of digital reactive hyperemia could be attributed to nitric oxide when assessed in adults after the infusion of a nitrous oxide synthase inhibitor 5. The relative balance between vasodilators that increase levels of nitric oxide and vasoconstrictors such as the renin-aldosterone system27, prostaglandins 28 or sympathetic tone 29 may change with normal childhood development. The complex anatomy of the fingertip circulation that includes arterio-venous anastomoses 30 may also differ in young children. For these reasons, digital reactive hyperemia in children may reflect many growth-dependent factors other than those affecting endothelial function. Our results suggest that these factors and their association with growth may further differ in obese children. Because of these differences, further modifications may be needed in the endoPAT procedure and proprietary algorithms when used in the younger age group.
The strengths of our study include the extensive cardiovascular risk profiles on participants, the carefully standardized measurements of digital reactive hyperemia and carotid intima-media thickness, and the inclusion of children with severe lipid abnormalities and diverse racial/ethnic backgrounds. However, a larger number of participants of all pubertal stages that includes lean children without lipid abnormalities would clarify the true relationships between digital reactive hyperemia and correlates of growth. A professional examination of pubertal stage instead of self-assessment would also improve the accuracy of the analysis.
In conclusion, digital tonometry using methodology developed in adults appears to be useful to stratify at-risk older post-pubertal adolescents and adults, but the reactive hyperemic response by this technique is strongly confounded by correlates of growth in younger non-obese children. Whether the same holds true for young obese children requires further study. The physiological changes underlying this “juvenile micro-vascular response” 16, if it exists, may be relevant to vascular homeostasis and blood pressure control in general. Additional validation and a better understanding of the endothelium-independent factors controlling the digital microcirculation are needed before the endoPAT technique can be successfully used in childhood.
Acknowledgments
The skilled assistance of nurses, research coordinators, laboratory technicians, statisticians, and the REDCAP database manager of the Clinical Translational Science Center of Weill Cornell College of Medicine is greatly appreciated.
Financial Support: ational Institutes of Health Clinical Translational Science Center grant #UL1 TR000457-07, The Starr Foundation (L.H.), and The Sikorski Family (R.C.).
Footnotes
Conflicts of Interest: None.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the Unites States 45 CFR part 46 guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Weill Cornell Medical College Institutional Review Board.
References
- 1.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 2.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 3.Tierney ES, Newburger JW, Gauvreau K, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–5. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 4.McCrea CE, Skulas-Ray AC, Chow M, West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17:29–36. doi: 10.1177/1358863X11433188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. Journal of applied physiology. 2006;101:545–8. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 6.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–6. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 8.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European heart journal. 2010;31:1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 9.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–9. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 10.Haller MJ, Stein J, Shuster J, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes. 2007;8:193–8. doi: 10.1111/j.1399-5448.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahmud FH, Earing MG, Lee RA, Lteif AN, Driscoll DJ, Lerman A. Altered endothelial function in asymptomatic male adolescents with type 1 diabetes. Congenital heart disease. 2006;1:98–103. doi: 10.1111/j.1747-0803.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 12.Mahmud FH, Hill DJ, Cuerden MS, Clarson CL. Impaired vascular function in obese adolescents with insulin resistance. J Pediatr. 2009;155:678–82. doi: 10.1016/j.jpeds.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal C, Cohen HW, Muzumdar RH, Heptulla RA, Renukuntla VS, Crandall J. Obesity, hyperglycemia and endothelial function in inner city Bronx adolescents: a cross-sectional study. International journal of pediatric endocrinology. 2013;2013:18. doi: 10.1186/1687-9856-2013-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruyndonckx L, Hoymans VY, Frederix G, et al. Endothelial progenitor cells and endothelial microparticles are independent predictors of endothelial function. J Pediatr. 2014;165:300–5. doi: 10.1016/j.jpeds.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Osika W, Dangardt F, Gan LM, Strandvik B, Friberg P. High levels of soluble intercellular adhesion molecule-1, insulin resistance and saturated fatty acids are associated with endothelial dysfunction in healthy adolescents. Atherosclerosis. 2010;211:638–42. doi: 10.1016/j.atherosclerosis.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Radtke T, Khattab K, Eser P, Kriemler S, Saner H, Wilhelm M. Puberty and microvascular function in healthy children and adolescents. J Pediatr. 2012;161:887–91. doi: 10.1016/j.jpeds.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Bhangoo A, Sinha S, Rosenbaum M, Shelov S, Ten S. Endothelial function as measured by peripheral arterial tonometry increases during pubertal advancement. Hormone research in paediatrics. 2011;76:226–33. doi: 10.1159/000328455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity. 2012;20:165–71. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGill HC, Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 2008;117:1216–27. doi: 10.1161/CIRCULATIONAHA.107.717033. [DOI] [PubMed] [Google Scholar]
- 20.Bruyndonckx L, Radtke T, Eser P, et al. Methodological considerations and practical recommendations for the application of peripheral arterial tonometry in children and adolescents. International journal of cardiology. 2013;168:3183–90. doi: 10.1016/j.ijcard.2013.07.236. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz E, Friberg P. Age- and sex-related differences in vascular function and vascular response to mental stress. Longitudinal and cross-sectional studies in a cohort of healthy children and adolescents. Atherosclerosis. 2012;220:269–74. doi: 10.1016/j.atherosclerosis.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez J, Wood JC, Dorey FJ, Wren TA, Gilsanz V. Reproducibility of carotid intima-media thickness measurements in young adults. Radiology. 2008;247:465–71. doi: 10.1148/radiol.2472070691. [DOI] [PubMed] [Google Scholar]
- 24.Mittelman SD, Gilsanz P, Mo AO, Wood J, Dorey F, Gilsanz V. Adiposity predicts carotid intima-media thickness in healthy children and adolescents. J Pediatr. 2010;156:592–7 e2. doi: 10.1016/j.jpeds.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–50. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 26.Lee CR, Bass A, Ellis K, et al. Relation between digital peripheral arterial tonometry and brachial artery ultrasound measures of vascular function in patients with coronary artery disease and in healthy volunteers. The American journal of cardiology. 2012;109:651–7. doi: 10.1016/j.amjcard.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer JH. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs & aging. 1993;3:238–45. doi: 10.2165/00002512-199303030-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sun D, Huang A, Smith CJ, et al. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circulation research. 1999;85:288–93. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 29.Thijssen DH, de Groot P, Kooijman M, Smits P, Hopman MT. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. American journal of physiology Heart and circulatory physiology. 2006;291:H3122–9. doi: 10.1152/ajpheart.00240.2006. [DOI] [PubMed] [Google Scholar]
- 30.Braverman IM. The cutaneous microcirculation: ultrastructure and microanatomical organization. Microcirculation. 1997;4:329–40. doi: 10.3109/10739689709146797. [DOI] [PubMed] [Google Scholar]