Abstract
Objective
This study tested the effect of early neglect on defensive and appetitive physiology during puberty.
Method
Emotion-modulated reflexes, eye-blink startle (defensive) and postauricular (appetitive), were measured in 12-to-13-year-old internationally adopted youth (from foster care or from institutional settings) and compared to non-adopted US born controls.
Results
Startle Reflex: adopted youth displayed lower overall startle amplitude across all valences and startle potentiation to negative images was negatively related to severity of pre-adoption neglect. Postauricular reflex (PAR): adopted youth showed larger PAR magnitude across all valences. Puberty: adopted youth showed diminished PAR potentiation to positive images and startle potentiation during mid/late puberty versus the opposite pattern in not-adopted.
Conclusions
Early neglect was associated with blunted fast defensive reflexes and heightened fast appetitive reflexes. After puberty, early neglected youth showed physiological hyporeactivity to threatening and appetitive stimuli versus heightened reactivity in not adopted youth. Behavioral correlates in this sample and possible neurodevelopmental mechanisms of psychophysiological differences are discussed.
Keywords: Neglect, Physiology of Emotion, Neural development, Puberty, Startle, Postauricular Reflex, Adolescence, Fast Emotional Processing, Behavior
Introduction
Early childhood adversity of a variety of forms (neglect, abuse, institutional deprivation) has a profound impact on the developing child with persistent effects reported for emotional functioning and neural and physiological development. Here we focus on deprivation and disruptions in care as experienced by children who were adopted as infants from international institutions and foster care. Emotional disturbances are frequently noted in neglected children raised in institutions (Hanson et al., 2013) or those removed from their families and placed in foster care (Giannopoulou, 2012; Perna et al., 2013). Institutional neglect is also associated with alterations in the stress neuraxis (Quevedo et al., 2009) and in neural systems underlying fear (Tottenham et al., 2010). It is unsurprising that lack of adequate parenting and/or disruptions in parenting results in altered physiology and psychopathology; the quality of the early caregiving relationship guides the development of the physiological basis of emotional experiences and their regulation (Heim et al., 2001; Sanchez et al., 2001). For example, a secure parent-child attachment relationship buffers against stressors (Hertsgaard et al., 1995; Zeanah et al., 1997) and supports acquisition of regulatory skills. These two motivational states -defensive and appetitive- evolved to ensure an organism's survival. Fast defensive physiological reactions evolved to respond automatically to threats with escape, hide, or defensive behaviors. Fast appetitive physiological responses are triggered by rewarding stimuli and promote behaviors toward sustenance and procreation (i.e. food and sex cues).
Deprivation or disruption of parental care affects the development of the physiological bases of these motivational systems separately (Gilmer et al., 2003; Joseph, 1999), yet research has not examined its enduring impact on the physiology of both appetitive and defensive reflexes in the same sample. The first goal of this study was to study the effect of early deprivation or disruption of adequate parenting on defensive and appetitive physiological responses measured respectively by the startle and the postauricular reflexes. Current emotional disturbances were screened out to examine fast physiological systems that process positive and negative stimuli, while minimizing confounds due to heterogeneous psychiatric symptoms that often result from early neglect.
The physiology of fast emotional reflexes, however, is also shaped by developmental processes. Puberty signals the restructuring and calibration of motivational systems due to increases in sexual hormones in reciprocal transaction with central nervous system reorganization (Spielberg et al., 2014; Urosevic et al., 2014). Puberty-linked changes are evident in increased emotional reactivity measured by the startle and the postauricular reflexes in typically developing adolescents. There are no studies in the human literature examining whether puberty interacts with early deprivation/disruption in care and neglect to shape the reactivity of appetitive and defensive systems. The second goal of this study was to examine whether early life neglect interacts with pubertal stage to predict startle and postauricular responses to negative and positive stimuli.
Twelve to 13 year old adolescents who were internationally adopted as young children from either institutional or foster care settings were compared to non-adopted U.S. born adolescents from families of similar socioeconomic status. Children adopted internationally have experienced disruption of early care relationships and may have experienced varying degrees of neglect. Children adopted internationally from institutional and foster care settings share factors associated with being an abandoned infant (e.g., poverty, parental psychopathology) and/or being an adopted child (e.g., higher risk for emotional and behavioral problems; (B. C. Miller et al., 2000). They are also more likely to have experienced poor prenatal conditions and lower birth weights (Van Ijzendoorn et al., 2007) relative to their non-adopted peers. In addition, different early care experiences of extended institutional care relative to foster care overseas are associated with different trajectories. Children internationally adopted from institutions experienced varying levels of deprivation and profound neglect including low levels of responsive caregiving due to large child-to caregiver ratios and inflexible routines, as well as impoverished emotional, cognitive and linguistic stimulation. Importantly, the use of multiple, changing caregivers affects formation of attachment relationships. Institutionalized children are also typically adopted at an older age (Hellerstedt et al., 2008). These children lag behind academically, need more intervention services, and exhibit more emotional and behavior problems compared to those adopted from foster care (Loman et al., 2009; Nelson et al., 2007).
Deprivation and the Psychophysiology of Affect
Startle Reflex
This measure of fast processing of threat indexes defensive motivations is heightened following early deprivation in animal models (Finamore et al., 2000; Sanchez et al., 2005). In humans, however, studies of adults and children with a history of trauma, including exposure to war or childhood physical and sexual abuse (Pole, 2007), have yielded inconsistent results. Some report enhanced startle suggestive of a hypersensitive defensive motivational system due to early adversity (Jovanovic et al., 2009; Pole, 2007). Others have found blunting of the startle response and argue that early adversity or/and persistent dysphoria depletes defensive responding (Klorman et al., 2003; McTeague et al., 2010; Reichmann-Decker et al., 2009). These contradictory results appear to be due to heterogeneous psychiatric symptoms and varied modalities of adversity being more prevalent in some studies versus others. Heterogeneity of symptoms influences the startle potentiation and magnitude. Specifically, anxiety disorders characterized by focal fears (e.g. phobias) show reliable heightened startle to aversive contents; but anxiety disorders characterized by enduring apprehension, avoidance and depression comorbidity (PTSD, generalized anxiety disorder: GAD) show paradoxically diminished startle to all aversive contents. Furthermore within PTSD, single trauma is linked to heightened startle but chronic/multiple traumas are linked to blunted startle. Suggesting that “adaptive defensive engagement during imagery may be compromised by long-term dysphoria and stress” (McTeague et al., 2012). In other words pervasive versus circumscribed activation of defensive neural systems are linked to opposite profiles of startle response. Blunted versus augmented startle are linked respectively to multiple versus single trauma, and to type of symptoms (pervasive fears and depression versus focal fears). We do not know whether early traumatic experiences, such as pervasive deprivation and chronic neglect during periods of high neural plasticity (prenatal and post-natal years) result in blunted or enlarged startle. Our unpublished study of younger (age 8-9 years) internationally adopted versus healthy control children yielded blunted startle magnitude among adopted children (Wiik et al., 2009).
Postauricular Reflex (PAR)
With regards to appetitive motivations and behaviors, decreased food intake (i.e. cocaine and sucrose consumption) has been reported in early deprived animals (Leventopoulos et al., 2009; Martini et al., 2012) and early chronic stress has been associated with a reduced sensitivity to reward (Stamford et al., 1991). Further, children adopted from institutions show hyporesponsive basal ganglia activity during anticipation of rewards (Mehta et al., 2010). These results would suggest that the PAR, a measure of appetite motivation (Benning et al., 2004; Benning, 2009; Benning, 2011; Gable et al., 2009; Hess et al., 2007; Parks et al., 2009; Sandt et al., 2009), would be blunted in adolescents who had experienced deprivation and/or disruption in care as infants, but there is no actual research regarding how these putatively traumatic early experiences may impact the PAR magnitude and potentiation. In the current study, PAR was used to test the impact of early deprivation on fast appetitive reflexes. Study hypotheses regarding the impact of early deprivation on the physiology of defensive and appetitive motivations included: 1) internationally adopted adolescents would show dampened defensive physiology in the form of low startle potentiation and magnitude, particularly for those exposed to severe early neglect prior to adoption (i.e. adopted from institutional care), 2) internationally adopted adolescents would show dampened reward physiology in the form of low PAR potentiation and magnitude, especially those adopted following severe neglect (i.e., adopted from institutional care).
Puberty and the Psychophysiology of Affect after Early Deprivation
Puberty is a developmental window characterized by increased reactivity and sensitivity to emotionally laden stimuli in defensive and appetitive related brain functions (Ladouceur, 2012; Spear, 2009). Research is limited with regard to the impact of early chronic neglect or trauma on appetitive and defensive physiology (e.g. startle and PAR potentiation or suppression) during key developmental transitions such as puberty.
Studies with humans and animal models show increases in social interaction, increased sensitivity to reward, and increased risk-taking behaviors (Cameron, 2004; Kuhn et al., 2010). Similarly, stress and anxiety reactivity increases during adolescence resulting in enduring behavioral traits (Eiland et al., 2013; Romeo, 2010; van Duijvenvoorde et al., 2014). These changes involve transformations in neural areas supporting the startle response. Typically developing youth at mid/late puberty, compared to early puberty, show less amygdala and medial prefrontal cortex (mPFC) reactivity to fearful and neutral faces (Forbes et al., 2011; Forbes et al., 2010). The amygdala is part of the circuit that underlies potentiation of the startle response (i.e. larger responses to aversive versus neutral stimuli) and acquisition of conditioned fear startle responses (Antoniadis et al., 2009; Fendt, 2001; Fendt et al., 2010). With regards to appetitive motivations, youth with more advanced pubertal maturation show less striatal and more mPFC reactivity to rewards than same aged youth with less advanced maturation (Forbes et al., 2011; Forbes et al., 2010), possibly indicating higher emotional reactivity to rewards and lower regulatory control supported by the mPFC. Our research shows larger startle overall magnitude (in startle circuitry: arousal and preparedness effect sub-served by the brain stem, not the amygdala), and PAR potentiation in youth at mid/late puberty versus those at early puberty (Quevedo, et al., 2009). Overall, research indicates that the neurobiology of defensive and appetitive motivations increases in overall reactivity with the onset of puberty in typically developing youth and that personality and affective physiology become aligned after puberty.
Early experience affects the process and timing of puberty. For those with adverse early life histories, puberty is marked by emotional problems increase (Colvert et al., 2008). Early hardships and family dysfunction have been shown to delay or accelerate puberty depending on gender, window and type of deprivation (Boynton-Jarrett et al., 2012; Sheppard et al., 2012; Tither et al., 2008). This indicates interactions between quality of parenting experience, early stress systems, and pubertal maturation that may result in underlying physiology of defensive and appetitive motivations (Charmandari et al., 2003). On the other hand, puberty may also open a window of opportunity for reorganization of defensive and appetitive motivational systems within current new environments (Quevedo et al., 2011). We previously found that the HPA axis, measured via the cortisol awakening response, differed between non-adopted and internationally adopted youth at early stages of puberty, but not during mid/late stages of puberty. Our third and final hypothesis with regard to the effect of early neglect on pubertal process and timing was that: 3) Internationally adopted adolescents would show both reduced startle and PAR potentiation with advanced puberty, in comparison to their not-adopted peers, who would show higher physiological reactivity with advanced puberty.
Methods
Participants
Participants were adolescents (12.0 – 13.0 years; M = 12.9, SD = 0.7) divided into three groups based on early experience. Post-Institutionalized (PI, n=55, 29 females) youth were internationally adopted at 8 months or older (M = 25.3 months) after having spent most of their pre-adoptive life in institutional care overseas. Post-Foster Care (PFC, n=44, 25 female) youth were internationally adopted between 2 and 8 months (M = 4.2 months) after spending most of their pre-adoptive lives in foster care overseas and/or no more than 2 months in an institution. Non-Adopted (NA, n=58, 28 female) youth were reared in U.S. birth families of similar education and income as those who adopted internationally. Groups did not differ in parental education level, income, or symptoms in self or parent report measures.
Procedures
Recruitment
Participants were recruited from a registry of families willing to be contacted with information about research participation. Participants with a physical or psychiatric diagnosis were screened out. Participants completed the Behavior Assessment System for Children, Second Edition (BASC-2, Doyle et al., 1997), which confirmed that the groups did not differ on internalizing or externalizing problems, F(1, 108) = 0.08−3.6, p <0.7 −0.08. We purposely recruited psychologically healthy youth to examine the long term impact of early adversity on the physiology of emotion, independent of concurrent symptoms and their treatment which would be a confounding factor when comparing the psychophysiology of adoptees versus US born and reared youth. Parent report of puberty (Petersen et al., 1988) was used to balance sex and adoption groups by puberty and confirmed through youth report at testing. Participants completed a measure of adolescent personality (MPQ, Patrick et al., 2002) scales. See Table 1 for sample composition and demographics.
Table 1.
Participant Demographic Characteristics by Group
| Post-Institutionalized n = 54 M (SD) | Post-Foster Care n = 44 M (SD) | Non-Adopted n = 58 M (SD) | |
|---|---|---|---|
| Age at session (yrs) | 12.9 (0.6) | 12.8 (0.7) | 13.0 (0.8) |
| Age at adoption (mos) | 25.3 (19.3) | 4.2 (1.7) | - |
| Time in institution (mos) | 22.3 (15.9) | 0.7 (1.0) | - |
| Region of origin (n) | |||
| Eastern Europe | 28 | 0 | - |
| Asia | 21 | 30 | - |
| Central & South America | 4 | 14 | - |
| Africa | 1 | 0 | - |
Emotion modulation and sound probe
Participants sat 135 cm from a 21” computer monitor. ERP-W 32 software (New Boundaries Technologies Inc.) was used to collect responses and present stimulus. Seventy-five pictures from the International Affective Picture System (Lang et al., 1999) were presented for 6 sec each in 5 orders per gender, counterbalanced at the level of valence content so that no content was presented successively (14 min total). Bursts of white noise were delivered bilaterally via insert ear-buds with nearly instantaneous rise time (Duration: 40 ms, M intensity = 100.94 dB, SD = 1.11; M inter-probe interval=11019 ms, SD = 2601.8; M inter-trial interval (ITI) =5881 ms, SD = 687). Pictures included 5 images per content: (1) Pleasant Valence Contents: Romantic, Food, Adventure, Nurturing/Attachment, Attractive People, (2) Neutral Valence Contents: Buildings, Landscape, Objects, Humans, and (3) Aversive Valence Contents: Threat, Disgust, Disgusting Animals, Victims, and Dangerous Animals. During picture exposure (6 sec) mean probe delivery was at 3646.2 ms, SD = 1044.8 ms. Six probes were also delivered during ITI's and 9 pictures per valence (3 by content) for all orders were presented without probes. After electrode removal, participants viewed and rated the pictures on emotional valence and arousal (Lang, 1980) on a 1-6 scale using the Self-Assessment Manikin system (Bradley et al., 1994). As expected, aversive pictures were rated as most aversive, pleasant pictures were rated as most pleasant, and neutral pictures were intermediate, F(2, 286) = 449.11, p < .001, η2p = .758. All valence contrasts were significant, F(1, 143) = 760.71, p < .001, η2p = .842. Neutral pictures were rated as less exciting than both aversive and positive pictures, F(2, 288) = 210.91, p < .001, η2p = .594. There was no significant group difference or group by valence interactions, p's > .10.
Psychophysiological data
Eye-blink startle and PAR reflexes were measured by recording electromyographic (EMG) activity with disk electrodes (Grass bioamplifier = 20,000x, Sampling = 1000 Hz, Impedance < 10 kΩ). Startle EMG: Filtering band pass = 30-300 Hz, peak EMG activity window = 20-175 ms after noise. Postauricular EMG: Filtering band pass = 10-1000 Hz, EMG activity window = 5-175 ms after noise. Sensor placement for the startle reflex was done as described by Blumenthal and colleagues (2005) and the PAR as described by Sollers and Hackley (1997). Rectification, integration, and (for PAR) averaging of trials were done employing ERP-W 32 software [initial trial excluded, startle time constant integration=20 ms, PAR time constant integration=1 ms, Baseline (Startle and PAR) = EMG 100 ms before noise]. Startle trials were inspected visually, whereas the PAR reflex was inspected after signal averaging of trials across valenced context due to restrictions on adapting ERP-W to sampling this fast occurring reflex. Average PAR waveforms per content were exported to a spread sheet and maximum EMG peak activity was selected within a window of 8-30 ms after noise onset. Peak EMG startle was coded as: response, non-response, or artifact for noisy background EMG activity (these were excluded from all analyses). The first response for all participants was not included in analysis to allow for adaptation. Responders were participants who showed elevated signal on at least 30% of all combined trials, while those with less (non-responders) were removed from analyses as recommended by Blumenthal et al. (2005). Coders had 90% reliability on 32% of the sample. The final sample of responders with artifact-free startle responses was: PI=39 (23 females), PFC=33 (19 females), NA=52 (27 females). For this final sample, there were no significant difference in the number of not responders in adopted versus not-adopted youth; PI= 16, PFC=11, NA=8; χ2 (2) = 4.5, p =0.12. There were no differences in the frequency of artifact trials or non-responders between main variable groupings including when PI and PFC were grouped together and compared to not-adopted youth.
Measures
Startle amplitude was computed with EMG that included only trials with noticeable reflexive responses.
Postauricular magnitude was based on EMG for PARs that were noise/artifact free and included trials with both noticeable and non-noticeable average reflex responses within valence contents.
Potentiated Startle and Postaricular Reflexes
For startle, potentiation was calculated as EMG amplitude during aversive images minus amplitude during neutral images (i.e. potentiated startle). PAR potentiation was calculated as EMG magnitude during pleasant pictures minus magnitude during neutral images (i.e. potentiated PAR). These measures represent how much more the reflexes respond to emotionally arousing (negative or positive) versus not arousing images.
Pubertal status
Adolescents completed a semi-structured interview to establish Tanner Stage (Morris et al., 1980) and a pubertal development scale (Petersen, et al., 1988). Participants who endorsed Tanner stage 3 across both instruments were classified as mid/late puberty and those below Tanner stage 3 were classified as pre/early puberty. Females who had had menarche were also classified as mid/late puberty.
Pre-adoption neglect
Adoptive parents responded to questions about their child's experiences of care prior to being adopted as previously reported in samples of children adopted internationally (e.g., Loman et al., 2009; Hellerstedt et al., 2008). Specifically, parents provided a rating to a Likert-style question of their overall impression of the quality of their child's pre-adoptive care as an index of early neglect on a scale of low (1) to very high (5) (L. C. Miller et al.). Because there was little reporting at the highest level possible (i.e., no 5s and few 4s), the highest levels were collapsed to yield a 3-point scale of chronic early neglect (l = low, 2 = medium, 3 = high). Pre adoption neglect was unrelated to sex or puberty but PI youth had more frequent medium and high neglect than PFC youth, χ2 (2) = 32.5, p < .001. PI youth received scores across the neglect range (Frequencies: 1= 16, 2=16, 3=17), while PFC youth were mostly rated low neglect (Frequencies: 1= 39, 2=3, 3=1).
Data Analysis
Startle amplitude values were negatively skewed and thus log transformed; PAR values were normally distributed, thus raw values were used. Mixed analysis of variance (RM-ANOVA) on baseline corrected peak responses were used for the main analyses of startle and PAR. Specifically: 3 (Image Valence) x 2 (Pubertal Status) x 2 (Gender) x 3 (Group). While there was an effect of 3 Groups, F(2,286) = 10.9, p <.01, η2p = 0.16, there was no difference between post-institutionalized and post-foster care groups in post hoc Bonferroni comparisons, yet they both significantly differed from the not-adopted group. Thus the adopted groups were combined and examined against non-adopted youth. Chi-square analyses were also conducted to test group differences in the frequency of high versus low overall raw startle reflex and PAR reflex amplitudes. Furthermore, in a previous sample, we noted significant effects of being SE Asian origin on eye-blink startle amplitude, but not on PAR magnitude or potentiation for either reflex (Johnson et al., in review). Similar effects were noted in this sample, yet when Not Asian adopted were compared with NA, the former still showed significantly lower startle magnitude than NA, F(1,84) = 4.9 p < .05. Nonetheless, all analyses of startle amplitude were computed covarying for ethnicity (Asian or Non-Asian as a dummy variable). Follow up analyses examined interaction effects among valence, puberty, and group for startle and PAR reflexes on the principal analyses. Splitting by pre-early or mid-late puberty groups, adopted vs. non-adopted adolescents were compared via a RM-ANOVA: 3 (Image Valence) x 2 (Gender) x 2 (Group). Additional tests examined the effect of pre adoption early chronic neglect (3 levels) on startle potentiation via a one way ANOVA on the combined (PI and PFC) group of internationally adopted adolescents. Chi-square analyses tested differences in startle responders versus non-responders by group. RM-ANOVA: 5(Content) x 2 (Group) were conducted for PAR responses within Positive Valence to clarify results. Statistical significance and direction of all results were replicated for both startle amplitude and magnitude. Figures show raw amplitude for startle and raw magnitude for postauricular reflex to place both signals in a similar scale.
Results
Effectiveness of Emotion Manipulation on Reflex Responses
There were no effects of gender for PAR magnitude. However, females showed higher overall startle amplitude than males F(1,117) = 6.74, p < .05. There was a significant effect of picture's valence on startle size, F(2, 224) = 10.7, p < .01, η2p = 0.07. Repeated contrast showed that amplitude was larger during aversive than neutral and pleasant pictures, F(1,112) = 8.34, p < .01, η2p = .07, and larger during neutral than pleasant pictures, F(1,112) = 3.68, p = .06, η2p = .03. PAR magnitude was larger during positive than negative pictures F(2,286) = 3.37, p <.05, η2p = .02. Neutral slides tended to elicit smaller responses than positive ones.
Startle Reflex
Early experience
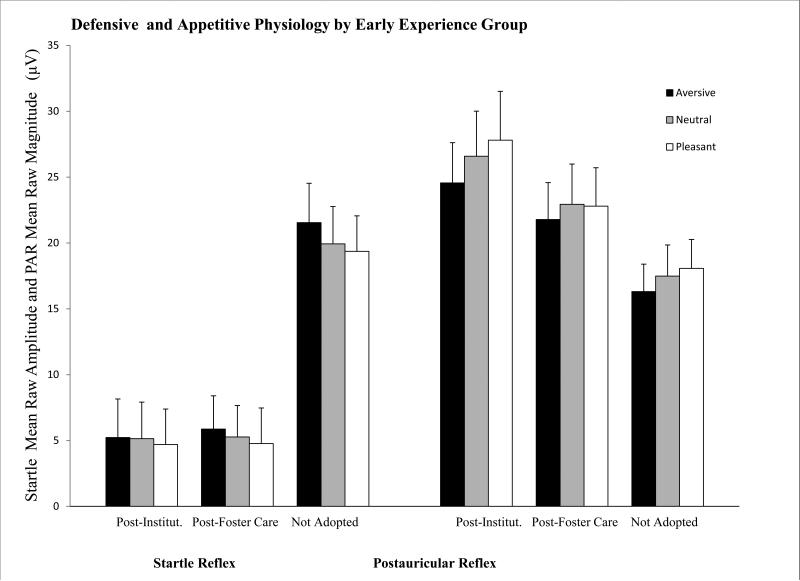
PI and PFC youth showed lower overall startle amplitude than NA youth, F(2,109) = 16.5, p < 0.01, η2p = 0.23 even after using Asian ethnicity as a covariate. However, PI and PFC youth did not differ in overall startle amplitude when examined with both Bonferroni and Tukey post-hoc tests (Raw values: MPI = 8.40, SD = 6.69; MFC= 6.83, SD = 6.30; MNA= 17, SD = 14). There was no group by valence interaction but there was an interaction between slides valence, group and puberty on startle amplitude, F(4, 218) = 2.56, p < 0.05, η2p = 0.50, indicating associations of puberty with startle amplitude potentiation within early experience groups that were explored below. Because PFC and PI adolescents were no different in startle amplitude or number of responders they were subsequently analyzed as a group.
Similar results were obtained with the pooled adopted youth showing lower startle amplitude than NA, F(1,115) = 33.7, p < 0.01, η2p = 0.227 (Figure 1). Additionally, χ2 analyses, using a mean split (M=11.4) of startle overall raw amplitude, showed lower frequency of high startle responses among internationally adopted (Nhigh=13, Nlow =59) versus not-adopted youth (Nhigh=25 Nlow =27), χ2 (1,124) = 12.8 p<0.01.
Figure 1.
Chronic early neglect in adopted adolescents is associated with blunted startle amplitude and enhanced PAR magnitude in comparison with non-adopted peers.
Pre-adoption early chronic neglect.
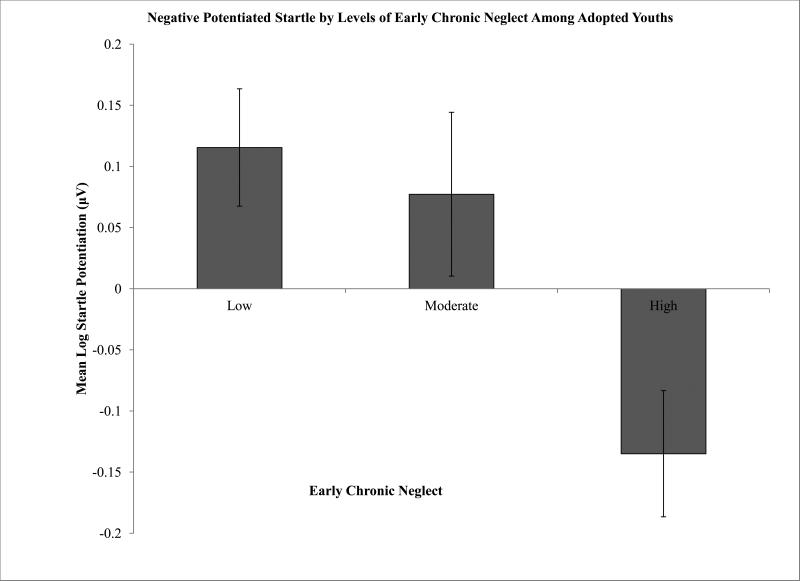
Among adopted youth, higher levels of parent reported pre-adoption neglect were associated with lower startle amplitude potentiation, F(2,67) = 3.62, p < 0.05, η2p = 0.09. Bonferroni and Tukey post-hoc (p<0.05) tests showed that the most severe level of reported neglect (3) was linked to significantly lower startle potentiation relative to low levels (1),with moderate levels falling in between (2) (Figure 2).
Figure 2.
Among adopted youth, higher chronic early neglect is associated with increasingly blunted startle amplitude potentiation.
Puberty, startle potentiation, and early experience
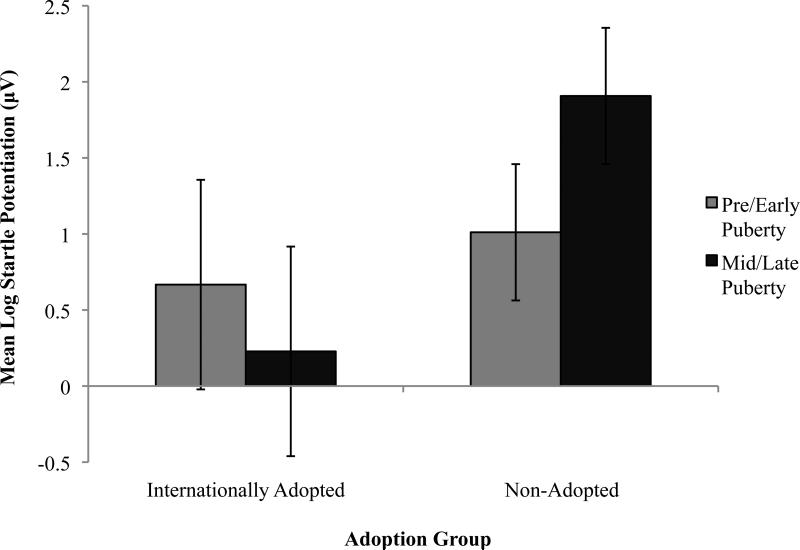
During pre/early puberty there were no significant differences in startle amplitude potentiation (startle to negative vs. to neutral images) between non-adopted and adopted adolescents but there were differences between the groups in potentiation during mid/late puberty: puberty by group by valence interaction: F(1, 119) = 5.53, p < .05, η2p = 0.44. Follow up analyses showed that during mid/late puberty startle potentiation increased for non-adopted F(1, 50) = 7.93, p < 0.05, η2p = 0.14 but decreased for adopted adolescents mostly because there were no significant differences between their startle amplitude during negatives versus neutral stimuli. Figure 3 shows these results grouped by internationally adopted versus non-adopted youth. There was however no interaction between puberty and levels of chronic neglect on startle potentiation within adopted adolescents. The only significant physiology to personality traits results were correlations between startle potentiation and MPQ subscales and their resulting factor scores, (MPQ, Patrick, et al., 2002) of risk-avoidance (r = − 0.32, p<.05) and emotionality (positive: r = − 0.4 p<0.1, negative: r = − 0.33 p<0.5) only for the mid/late puberty adopted sub-group
Figure 3.
Blunted potentiated started in adopted youth, with increase potentiation emerging among mid/late pubertal nondopted youth.
Postauricular Reflex
Early experience
There was a trend for an effect of the three groups on PAR magnitude, F(2,143) = 2.62, p = .07. However, neither reflex magnitude or PAR potentiation differed between PI and PFC youth; thus, as with startle, the two groups were combined and compared with NA youth. The adopted group showed larger overall PAR magnitude compared to the NA group, F(1,145) = 4.62, p < .05, η2p = .03 (Figure 1). Additionally there was a valence by group by puberty interaction on PAR magnitude, F(1,125) = 3.8, p <.05, η2p =.26, suggesting effects of group and puberty on PAR potentiation to positive stimuli that were then further explored below. There were no significant associations between parent-reported pre-adoption neglect and PAR reflex magnitude or potentiation, p > .10. Additionally, χ2 analyses, using a mean split (M=22.0) of PAR overall raw amplitude, showed higher frequency of high PAR responses among internationally adopted (Nhigh=56, Nlow =39) versus not-adopted (Nhigh=14, Nlow =41) youth, χ2 (1,150) = 3.7 p<0.05.
Post-hoc analyses of positive slide contents yielded a significant within positive contents (5) by group (2) interaction. When contents were sorted in ascending PAR magnitude for the not-adopted group, initial significant polynomial contrast resolved into a significant linear contrast, F(1,145) = 8.15, p <0.01, η2p =.05, confirmed by repeated within content contrasts. Table 2 shows that internationally adopted have less PAR magnitude to adventure images versus all other contents, while not-adopted youth have similar PAR magnitude (e.g. food and adventure).
Table 2.
Positive Valence Contents by Group
| Image Content | Internationally Adopted M (SE) | Not Adopted M (SE) |
|---|---|---|
| Nurture/Attachment | 26.4 (2.4) | 17.6 (3.1) |
| Attractive-Other Gender | 26.5 (2.3) | 18.0 (3.0) |
| Romantic Couples | 25.2 (2.2) | 18.6 (2.8) |
| Food | 26.1 (2.3) | 18.9 (3.0) |
| Adventure | 22.5 (2.0) | 18.6 (2.6) |
Puberty, PAR potentiation, and early experience
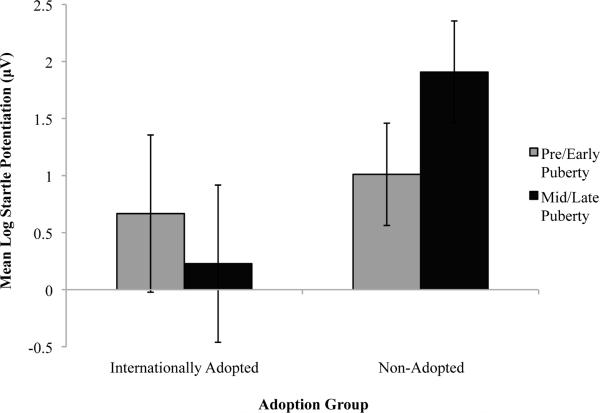
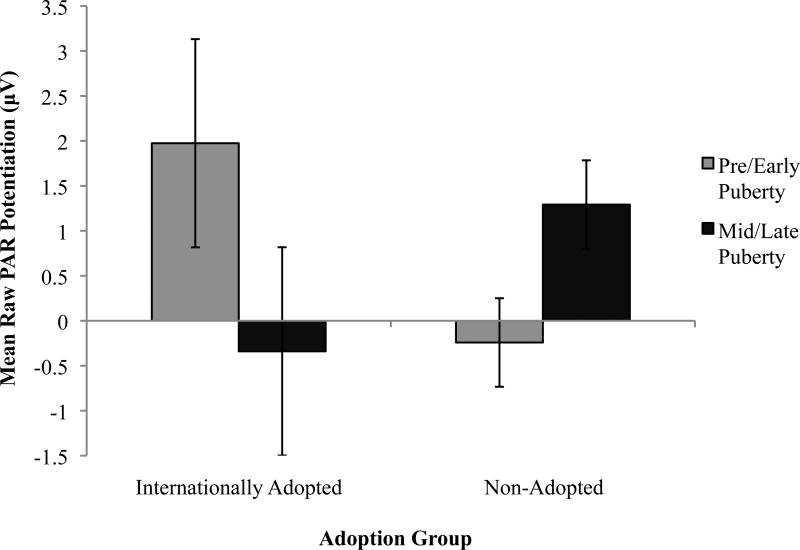
A puberty by group interaction on PAR potentiation, F(1,146) = 4.28, p <0.05, η2p = 0.03 yielded in follow up analyses that adopted adolescents during pre/early puberty showed typical PAR positive emotional potentiation, while their mid/late pubertal peers showed significantly diminished potentiationF(1,93) = 3.99, p <0.05, η2p = 0.41. By contrast, pre/early puberty non-adopted adolescents showed less PAR potentiation and their mid/late pubertal peers showed enhanced PAR potentiation (Figure 4).
Figure 4.
Within pleasant contents, PAR magnitude diminishes from nurture/attachment images to adventure images in adopted versus non-adopted youth, who show similar PAR magnitude to food and adventure and less to nurture/attachment images.
Discussion
Early Adversity and Defensive Physiological Responses
Our first finding was that startle amplitude was smaller among the adopted youth, which held for both youth who experienced long term institutionalization (PI), and those who spent less than 2 months in institutions (PFC). Physiological hyporesponsivity for adopted youth was present across all emotional experimental contexts. This suggests that chronic early neglect may have altered the development of the very early maturing neural bases of the startle response. Possibly brain stem structures that support the startle response and mature during pre- and very early postnatal development (Joseph, 1999). Specifically, the brain stem supports basic arousal and sensorimotor gating functions (the nucleus reticularis pontis caudalis) and it is sufficient for the startle response (Koch, 1999). It is important to underscore that youth adopted internationally may experience multiple and varying degrees of adversity early in life. During gestation they are at risk due to maternal poverty. Many children who end up in foster and institutional care are born small-for-gestational age. During the postnatal period, children in institutional care experience multiple caregivers and little cognitive and social stimulation. Children in foster care overseas may experience more typical family care, but quality likely varies across settings and countries and their experience still involves separation from the biological parent and adaptation to new early environments. Given this complex chain of early insults and disruptions, whether blunted startle magnitude and damage to brain stem structures is caused by maternal stress during gestation, early life malnutrition, dearth of sensory/social stimulation, or the accumulation of these risk in an institution, cannot be determined by this study.
It is notable; however, that the adoptive parent's report of degree of pre-adoption neglect experienced by their children was associated with the degree of blunted startle potentiation. The circuitry of startle potentiation requires amygdala input (Davis, 2006), which animal models show it develops prenatally and may reach full function soon after birth (Ehrlich et al., 2013; Jagalska-Majewska et al., 2003). Rodent models suggest that the electrophysiological properties of neurons in the human amygdala may undergo the greatest maturation before 1 year of age (Ehrlich et al., 2012), and in non-human primates the most robust amygdala maturational changes occur before the age of 8 months and earlier than other areas supporting stress response i.e. the hippocampus (Payne et al., 2010). Dramatic volumetric and functional amygdala changes observed in the first two years of life in animals suggest that this is a sensitive neurodevelopmental period for the startle circuitry. This makes defensive motivations highly susceptible to perturbations that may have permanent effects on brain structure and function. So in addition to possible alterations to brain stem structures, our findings strongly suggest altered amygdala activity in response to threatening stimuli is a long standing effect of early deprivation. Our findings are also consistent with imaging research that shows impact of institutionalization on amygdala development, including decreased glucose metabolism (Chugani et al., 2001; Tottenham et al., 2011). Although, we lacked objective measures of pre-adoption care, and relied on parent report of pre-adoptive care adversity, these findings are consistent with the proposition that blunted startle potentiation results from adverse early life histories, particularly neglect.
With regards to the direction of startle anomalies, our unpublished data (Wiik, et al., 2009) and studies of startle in severely traumatized samples, would have predicted blunted startle responses subsequent to neglect and deprivation. There is in fact a growing body of research suggesting that adversity and trauma may cause blunted affect and discordant emotional experiences in a subset of affected individuals (Bremner et al., 1999; Kraus et al., 2009; Rauch et al., 1996). This cluster of anomalies includes restricted range of affect, dissociation, depression, and persistent avoidance symptoms. Our findings are consistent with blunted startle response found in physically abused children (Klorman, et al., 2003) and severely traumatized adults. For example, adults with PTSD due to multiple traumatic events exhibit blunted startle when compared with adults with only a single traumatic event experience. Furthermore, their blunted startle was associated with greater mood and anxiety comorbidiy (McTeague, et al., 2010). Physiological hyporeactivity and a presentation of discordance between high negative emotions yet blunted defensive physiology also characterizes peri-traumatic dissociation in adults (Pole et al., 2005). However, it is not only parental neglect during early infancy that may be linked to blunted defensive physiology. Hyporeactive defensive physiology in this sample is likely to reflect a continuum of stressful adversity that includes pre- and perinatal experience, structural insults, and caregiving and environmental disruption. Physiological defensive hyporeactivity probably aroused from sustained high stress and negative affectivity that compromised defensive responding and(via experience-dependent programming of the neural basis of emotion processing) resulted in blunted defensive reflexes as an adaptation to chronic environmental disruptions, transitions in caregiving and/or chronic neglect of emotional and physical needs.
Early Adversity and Appetitive Reflexive Responses
The actual neural circuit of the PAR is unknown, but it is likely similar to the auditory eye blink reflex circuit in afferent input (the cochlear nucleus) and efferent output (the facial-motor nucleus). However, the PAR is heightened to anticipation of eating appetizing food (Hackley et al., 2009); and reward anticipation has been consistently linked to the activity of the basal ganglia (Ernst et al., 2004), particularly the ventral striatum, which supports processing of food and sex's visual cues, that reliably elicit the PAR in adults (Sandt, et al., 2009). Startle and PAR are uncorrelated here and in the available literature; suggesting that these are, to some extent, independent reflexes. The basal ganglia might play a role in PAR potentiation and overall magnitude in addition to-, or with stronger efferent output to muscle responses than the amygdala (Kensinger et al., 2006), though these limbic areas are structurally connected.
Previous research has found that adopted youth with early stress experience show deficient modulation of inhibitory control by reward processes (Mueller et al., 2012). Increased overall PAR magnitude, in this sample, means that lower thresholds of auditory and visually stimulation arise this appetitive reflexive response. This is not necessarily an aberrant sign in a psychiatrically healthy sample, as it may reflect compensatory alertness to cues that promote nutrition, social attachments and procreation while also eschewing risks. Deprivation of adequate parenting – among many insults- during a sensitive nerodevelopmental window may have spurred fast reflective processes to detect all potentially rewarding cues to increase chances of later survival, yet further interpretation of these results ought to wait until these results are replicated.
Puberty, Early Adversity and Reflexive Emotional Responses
Our third hypothesis pertained to physiological changes that typically emerge with puberty and their possible interaction with a history of early adversity. Startle and PAR potentiation became more blunted for adopted youth during mid/late puberty relative to non-adopted youth who showed heightened potentiation. It must be underscored that this puberty by early deprivation interactions are not explained by more advanced pubertal development among the more severely neglected youth as there are no significant early neglect by puberty interactions in this sample. This strengthens the notion of puberty as a sensitive window that moderates previous developmental history. It should be noted that in this same sample, the cortisol awakening response was blunted among post-institutionalized youth at pre/early stages of puberty but it was similar to non-adopted youth by mid/late puberty (Quevedo et al., 2011). Thus, puberty does not induce changes in stress or threat-reactivity across all systems for youth with adverse early life histories. Rather, in early adolescence, the impact of early adversity may vary by branches of the defensive system and there is a need to understand how the combination of effects on different systems affects mental and emotional functioning of high risk youth. In this sample, diminished emotional reactivity (measured by startle and PAR potentiation) appeared to onset with puberty for youth with a history of early adversity, contrary to increased emotional reactivity that characterizes typical pubertal development in this and in previous studies. More on the meaning of these findings follows.
Physiology and Behavior
PI and PFC groups showed similar physiology of affect despite reported differences in their behavioral trajectories. The common insults they suffered during pre- and perinatal development (some PFC spent at least 2 months in an institution) may have altered the neurobiology of defensive and appetitive motivations (e.g. amygdala, basal ganglia and brain stem), but in a healthy sample, such alterations are an endophenotype for risks not currently expressed in clinical disorders. It is plausible that early shared insults superimposed to the rapid structural and functional changes of infants’ reflexive neurobiology, particularly for reflexes sub-served by amygdala and brain stem, resulted in modified physiology of emotions for both PI and PFC youth. Furthermore, parents rarely reported severe neglect for the PI youth (i.e. scores of 4 or 5), which made the groups more similar, but this in turn goes back to the decision to study adopted youth with insignificant clinical symptoms.
The literature shows that blunted startle magnitude and potentiation are linked to anxiety comorbid with depression pursuant to complex trauma and/or predominance of anxious avoidance. These behavioral and clinical profiles associated to the underlying phenotype of blunted startle may place PI, and to a lesser extent PFC youth, at risk for depression and GAD. We selected a sample free of clinically significant symptoms, but this would suggest that healthy individuals exposed to early neglect or continuous early disruptions of attachment and parenting may avoid slightly negatively arousing and mildly risky experiences. In this same sample, institutional care was associated with less risk-taking and sensation-seeking: PI youth had a preference for safe choices which was in turn linked to depressive signs (Loman et al., 2014). This is confirmed by lower PAR magnitude to adventure images in adopted versus not-adopted youth. The significant group by linear contrast interaction in positive valence contents also means that PAR magnitude is higher for nurturance/attachment images and drops for cues of adventure for adopted youth. Instead, nurture/attachment images elicit the lowest PAR for the not adopted youth. Given their different early attachment histories, internationally adopted youth might be expected to find nurturance/attachment cues pleasurable and less so undertaking pleasurable risks. US born and reared youth instead seem to respond to food and adventure, i.e. pleasure linked to exploratory behavior that accompanies separation from attachment figures during adolescence.
The significant correlations between startle potentiation and personality traits (MPQ, Patrick, et al., 2002) of risk-avoidance (r = − 0.32, p<.05) and emotionality (positive: r = − 0.4 p<0.1, negative: r = − 0.33 p<0.5) only for the mid/late puberty adopted sub-group suggest a different trajectory for personality development (and its underlying affective physiology) for individuals with a history of early adversity and neglect. Here, among the adopted youth, low potentiated startle is linked to higher emotionality and impulsivity, which is known to increase with puberty. We have found that puberty moderates the association between physiology and personality traits before. Yet the underlying physiology of affect is very different in maturing youth with a history of early neglect, as typically developing pubertal youth show heightened overall startle magnitude linked to their personality traits (Quevedo, et al., 2009). This also suggests that low startle potentiation in this sub-sample may be linked to discordant emotional experiences and/or emotion regulation difficulties in pubertal adopted youth, as blunted startle potentiation is linked to both high negative and positive emotionality.
Finally, overall enhanced reward-related vigilance to all cues could compensate for some of these risks, e.g. anhedonia symptoms. While, no different from each other, PI youth exhibited larger PAR magnitude to positive images than PFC youth and both less than not-adopted, and PAR magnitude potentiation was unrelated to personality traits in this sample. This configuration (high overall PAR paired with low startle amplitude and potentiation) might represent a broad measure of resilience in the population of internationally adopted youth given that this is a healthy sub-sample. Fast disposition to seize natural rewards may be protective against depression and added to low risk taking it may prevent adolescent morbidity linked to poor emotion regulation and impulsivity. These hypotheses though would have to be tested.
Limitations
First, we need to be cautious in interpreting any finding that includes parental ratings of pre-adoption care. We knew the setting of early care prior to adoption (foster home versus institution) but actual international pre-adoption histories are difficult to reconstruct. Second, the representativeness of the sample of both adopted and non-adopted youth needs to be viewed cautiously. For the adopted youth, the family registry represents about 60% of the total available families formed through international adoption in our state. They are well-educated and of high income, they are may not be representative of the general population. We controlled for the socioeconomic status of the post-adoption home; therefore in our study the non-adopted children were drawn from families of similar socioeconomic status as the families for the internationally adopted youth. However, in selecting high functioning youth, we eliminated youth with clinically significant symptoms thus the lack of significant differences between our adopted and non-adopted group. Whether the findings generalize to adopted youth with emotional problems will depend on the clinical outcome of interest. This study is pertinent to eventual development of internalizing disorders such as depression, anxiety and trauma related disorders. Finally, although we statistically controlled SE Asian origin, this may not have completely eliminated the effect of race on startle. Yet our analyses revealed no association of SE Asian origin on startle potentiation or PAR magnitude. It would be useful to conduct similar analyses in samples large enough to allow separate analyses by race/ethnicity, and clinical research must consider racial/ethnic differences in the biology of affect.
Figure 5.
Potentiated PAR is larger among pre/early pubertal adopted versus non-adopted youth, yet among mid/late pubertal youth the opposite pattern is observed.
Acknowledgments
This research was conducted at the Center for Neurobehavioral Development, at the University of Minnesota and supported by NIH via HH01684, K01 MH092601, T32 HD007151 to Karina Quevedo, MH080905 and MH078105 to Megan R. Gunnar, and T32 HD007151 to Anna Johnson and Michelle Loman
Footnotes
No competing interests.
References
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biol Psychiatry. 2009;65(3):241–248. doi: 10.1016/j.biopsych.2008.07.007. doi: 10.1016/j.biopsych.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning S, Patrick C, Lang AR. Emotional modulation of the post-auricular reflex. Psychophysiology. 2004;41(3):426–432. doi: 10.1111/j.1469-8986.00160.x. [DOI] [PubMed] [Google Scholar]
- Benning SD. Reduced appetitive postauricular reflex potentiation in depressed undergraduates. Psychophysiology. 2009;46:S103. [Google Scholar]
- Benning SD. Postauricular and superior auricular reflex modulation during emotional pictures and sounds. [Research Support, N.I.H., Extramural]. Psychophysiology. 2011;48(3):410–414. doi: 10.1111/j.1469-8986.2010.01071.x. doi: 10.1111/j.1469-8986.2010.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Harville EW. A prospective study of childhood social hardships and age at menarche. Ann Epidemiol. 2012;22(10):731–737. doi: 10.1016/j.annepidem.2012.08.005. doi: 10.1016/j.annepidem.2012.08.005 S1047-2797(12)00339-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Ann N Y Acad Sci. 2004;1021:110–123. doi: 10.1196/annals.1308.012. doi: 10.1196/annals.1308.012 1021/1/110 [pii] [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59(4):161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, et al. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Dev Psychopathol. 2008;20(2):547–567. doi: 10.1017/S0954579408000278. doi: S0954579408000278 [pii]10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. The American Psychologist. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Doyle A, Ostrander R, Skare S, Crosby RD, August GJ. Convergent and criterion-related validity of the Behavior Assessment System for Children-Parent Rating Scale. J Clin Child Psychol. 1997;26(3):276–284. doi: 10.1207/s15374424jccp2603_6. [DOI] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol. 2013;110(4):926–941. doi: 10.1152/jn.01105.2012. doi: 10.1152/jn.01105.2012 jn.01105.2012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Rainnie DG. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol. 2012;590(Pt 19):4819–4838. doi: 10.1113/jphysiol.2012.237453. doi: 10.1113/jphysiol.2012.237453 jphysiol.2012.237453 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. doi: 10.1016/j.neuroscience.2012.10.048 S0306-4522(12)01072-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: An fMRI study. [Original]. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Fendt M. Injections of the NDMA receptor antagonist aminophosphonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. Journal of Neuroscience. 2001;21(11):4111–4115. doi: 10.1523/JNEUROSCI.21-11-04111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Imobersteg S, Burki H, McAllister KH, Sailer AW. Intra-amygdala injections of neuropeptide S block fear-potentiated startle. Neurosci Lett. 2010;474(3):154–157. doi: 10.1016/j.neulet.2010.03.028. doi: 10.1016/j.neulet.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Finamore TL, Port RL. Developmental stress disrupts habituation but spares prepulse inhibition in young rats. Physiology & Behavior. 2000;69(4-5):527–530. doi: 10.1016/s0031-9384(00)00205-5. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2011;36(4):429–452. doi: 10.1080/87565641.2010.550178. doi: 10.1080/87565641.2010.550178 936794297 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, et al. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49(2):162–172. e161–165. doi: 10.1097/00004583-201002000-00010. doi: 00004583-201002000-00010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Postauricular reflex responses to pictures varying in valence and arousal. [Research Support, U.S. Gov't, Non-P.H.S.]. Psychophysiology. 2009;46(3):487–490. doi: 10.1111/j.1469-8986.2009.00794.x. doi: 10.1111/j.1469-8986.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Giannopoulou I. [Neurobiological inscriptions of psychological trauma during early childhood]. Psychiatrike. 2012;23(Suppl 1):27–38. [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75(2):97–113. doi: 10.1016/s0165-0327(03)00046-6. doi: S0165032703000466 [pii] [DOI] [PubMed] [Google Scholar]
- Hackley SA, Munoz MA, Hebert K, Valle-Inclan F, Vila J. Reciprocal modulation of eye-blink and pinna-flexion components of startle during reward anticipation. Psychophysiology. 2009;46(6):1154–1159. doi: 10.1111/j.1469-8986.2009.00867.x. doi: 10.1111/j.1469-8986.2009.00867.x. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev. 2013;84(5):1566–1578. doi: 10.1111/cdev.12069. doi: 10.1111/cdev.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: population-based surveillance of Minnesota parents who adopted children internationally. Maternal and Child Health Journal. 2008;12(2):162–171. doi: 10.1007/s10995-007-0237-9. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical responses to the Strange Situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66(4):1100–1106. [PubMed] [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44(3):431–435. doi: 10.1111/j.1469-8986.2007.00516.x. doi: doi:10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Jagalska-Majewska H, Wojcik S, Dziewiatkowski J, Luczynska A, Kurlapska R, Morys J. Postnatal development of the basolateral complex of rabbit amygdala: a stereological and histochemical study. J Anat. 2003;203(5):513–521. doi: 10.1046/j.1469-7580.2003.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Wiik K, Quevedo K, Gunnar M. Race and startle reactivity in internationally adopted children. in review. [Google Scholar]
- Joseph R. Environmental influences on neural plasticity, the limbic system, emotional development and attachment: a review. Child Psychiatry Hum Dev. 1999;29(3):189–208. doi: 10.1023/a:1022660923605. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, Ressler KJ. Childhood abuse is associated with increased startle reactivity in adulthood. Depress Anxiety. 2009;26(11):1018–1026. doi: 10.1002/da.20599. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Klorman R, Cicchetti D, Thatcher JE, Ison JR. Acoustic startle in maltreated children. J Abnorm Child Psychol. 2003;31(4):359–370. doi: 10.1023/a:1023835417070. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59(2):107–128. doi: 10.1016/s0301-0082(98)00098-7. doi: S0301008298000987 [pii] [DOI] [PubMed] [Google Scholar]
- Kraus A, Esposito F, Seifritz E, Di Salle F, Ruf M, Valerius G, et al. Amygdala deactivation as a neural correlate of pain processing in patients with borderline personality disorder and co-occurrent posttraumatic stress disorder. Biol Psychiatry. 2009;65(9):819–822. doi: 10.1016/j.biopsych.2008.10.028. doi: S0006-3223(08)01317-6 [pii]10.1016/j.biopsych.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, et al. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58(1):122–137. doi: 10.1016/j.yhbeh.2009.10.015. doi: 10.1016/j.yhbeh.2009.10.015 S0018-506X(09)00246-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front Integr Neurosci. 2012;6:65. doi: 10.3389/fnint.2012.00065. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: Computer applications. In: Sidowski JB, J. J. H., W. T. A., editors. Technology in mental health care delivery systems. Ablex: Ablex; NJ: 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM. International affective picture system (IAPS): Instruction manual and affective ratings (Tech. Rep. No. A-4) University of Florida, The Center for Research in Psychophysiology; Gainesville, FL: 1999. [Google Scholar]
- Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology. 2009;56(3):692–701. doi: 10.1016/j.neuropharm.2008.12.005. doi: 10.1016/j.neuropharm.2008.12.005 S0028-3908(08)00526-1 [pii] [DOI] [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Quevedo K, Lafavor TL, Gunnar MR. Risk-taking and sensation-seeking propensity in postinstitutionalized early adolescents. J Child Psychol Psychiatry. 2014;55(10):1145–1152. doi: 10.1111/jcpp.12208. doi: 10.1111/jcpp.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Wiik KL, Frenn KA, Pollak SD, Gunnar MR. Postinstitutionalized children's development: growth, cognitive, and language outcomes. J Dev Behav Pediatr. 2009;30(5):426–434. doi: 10.1097/DBP.0b013e3181b1fd08. doi: 10.1097/DBP.0b013e3181b1fd08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Valverde O. A single episode of maternal deprivation impairs the motivation for cocaine in adolescent mice. Psychopharmacology (Berl) 2012;219(1):149–158. doi: 10.1007/s00213-011-2385-2. doi: 10.1007/s00213-011-2385-2. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety. 2012;29(4):264–281. doi: 10.1002/da.21891. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 2010;67(4):346–356. doi: 10.1016/j.biopsych.2009.08.023. doi: S0006-3223(09)01025-7 [pii]10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22(10):2316–2325. doi: 10.1162/jocn.2009.21394. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Miller BC, Fan X, Grotevant HD, Christensen M, Coyl D, van Dulmen M. Adopted adolescents' overrepresentation in mental health counseling: Adoptees' problems or parents' lower threshold for referral? J Am Acad Child Adolesc Psychiatry. 2000;39 doi: 10.1097/00004583-200012000-00011. [DOI] [PubMed] [Google Scholar]
- Miller LC, Hendrie NW. Health of children adopted from China. Pediatrics. 2000;105(6):E76. doi: 10.1542/peds.105.6.e76. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9(3):271–280. doi: 10.1007/BF02088471. doi: 10.1007/bf02088471. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Korelitz K, Daniele T, Bemis J, Dozier M, et al. Incentive effect on inhibitory control in adolescents with early-life stress: an antisaccade study. Child Abuse Negl. 2012;36(3):217–225. doi: 10.1016/j.chiabu.2011.10.010. doi: 10.1016/j.chiabu.2011.10.010 S0145-2134(12)00031-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. doi: 318/5858/1937 [pii] 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Parks NA, Hilimire MR, Corballis PM. Visual perceptual load modulates an auditory microreflex. Psychophysiology. 2009;46(3):498–501. doi: 10.1111/j.1469-8986.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20(8):922–935. doi: 10.1002/hipo.20688. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna RB, Kiefner M. Long-term cognitive sequelae: abused children without PTSD. Appl Neuropsychol Child. 2013;2(1):1–5. doi: 10.1080/09084282.2011.595460. doi: 10.1080/09084282.2011.595460. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133(5):725–746. doi: 10.1037/0033-2909.133.5.725. doi: 2007-12463-001 [pii]10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Pole N, Cumberbatch E, Taylor WM, Metzler TJ, Marmar CR, Neylan TC. Comparisons between high and low peritraumatic dissociators in cardiovascular and emotional activity while remembering trauma. J Trauma Dissociation. 2005;6(4):51–67. doi: 10.1300/j229v06n04_04. [DOI] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, Gunnar M. The confluence of adverse early experience and puberty on the cortisol awakening response. International Journal of Behavioral Development. 2011 doi: 10.1177/0165025411406860. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Dev Psychopathol. 2009;21(1):27–45. doi: 10.1017/S0954579409000030. doi: 10.1017/S0954579409000030 S0954579409000030 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53(5):380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Reichmann-Decker A, DePrince AP, McIntosh DN. Affective responsiveness, betrayal, and childhood abuse. J Trauma Dissociation. 2009;10(3):276–296. doi: 10.1080/15299730902956788. doi: 912960867 [pii]10.1080/15299730902956788. [DOI] [PubMed] [Google Scholar]
- Romeo R. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Frontiers in Neuroendocrinology. 2010;31(2):232–240. doi: 10.1016/j.yfrne.2010.02.004. doi: S0091-3022(10)00008-7 [pii]10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry. 2005;57(4):373–381. doi: 10.1016/j.biopsych.2004.11.032. doi: S0006-3223(04)01235-1 [pii] 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sandt AR, Sloan DM, Johnson KJ. Measuring appetitive responding with the postauricular reflex. Psychophysiology. 2009;46(3):491–497. doi: 10.1111/j.1469-8986.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Sheppard P, Sear R. Father absence predicts age at sexual maturity and reproductive timing in British men. Biol Lett. 2012;8(2):237–240. doi: 10.1098/rsbl.2011.0747. doi: 10.1098/rsbl.2011.0747rsbl.2011.0747 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollers JJ, Hackley SA. Effects of foreperiod duration on reflexive and voluntary responses to intense noise bursts. Psychophysiology. 1997;34:518–526. doi: 10.1111/j.1469-8986.1997.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21(1):87–97. doi: 10.1017/S0954579409000066. doi: 10.1017/S0954579409000066 S0954579409000066 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: does pubertal development alter threat processing? Dev Cogn Neurosci. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. doi: 10.1016/j.dcn.2014.01.004S1878-9293(14)00006-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Muscat R, O'Connor JJ, Patel J, Trout SJ, Wieczorek WJ, et al. Voltammetric evidence that subsensitivity to reward following chronic mild stress is associated with increased release of mesolimbic dopamine. Psychopharmacology (Berl) 1991;105(2):275–282. doi: 10.1007/BF02244322. [DOI] [PubMed] [Google Scholar]
- Tither JM, Ellis BJ. Impact of fathers on daughters' age at menarche: a genetically and environmentally controlled sibling study. Dev Psychol. 2008;44(5):1409–1420. doi: 10.1037/a0013065. doi: 10.1037/a00130652008-12114-019 [pii] [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urosevic S, Collins P, Muetzel R, Lim KO, Luciana M. Pubertal status associations with reward and threat sensitivities and subcortical brain volumes during adolescence. Brain Cogn. 2014 doi: 10.1016/j.bandc.2014.01.007. doi: S0278-2626(14)00009-8 [pii] 10.1016/j.bandc.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde AC, Op de Macks ZA, Overgaauw S, Gunther Moor B, Dahl RE, Crone EA. A cross-sectional and longitudinal analysis of reward-related brain activation: Effects of age, pubertal stage, and reward sensitivity. Brain Cogn. 2014 doi: 10.1016/j.bandc.2013.10.005. doi: S0278-2626(13)00152-8 [pii] 10.1016/j.bandc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Bakermans-Kranenburg MJ, Juffer F. Plasticity of growth in height, weight, and head circumference: meta-analytic evidence of massive catch-up after international adoption. J Dev Behav Pediatr. 2007;28(4):334–343. doi: 10.1097/DBP.0b013e31811320aa. doi: 10.1097/DBP.0b013e31811320aa00004703-200708000-00012 [pii] [DOI] [PubMed] [Google Scholar]
- Wiik K, Quevedo K, Frenn K, Schlaak M, Pollak S, Gunnar M. Fear-Potentiated Startle in Post-Institutionalized Children; Paper presented at the Society for Research in Child Development; Denver, Colorado. 2009.2009. [Google Scholar]
- Zeanah CH, Boris NW, Scheeringa MS. Psychopathology in infancy. J Child Psychol Psychiatry. 1997;38(1):81–99. doi: 10.1111/j.1469-7610.1997.tb01506.x. [DOI] [PubMed] [Google Scholar]