Abstract
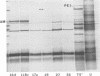
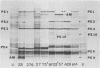
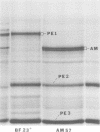
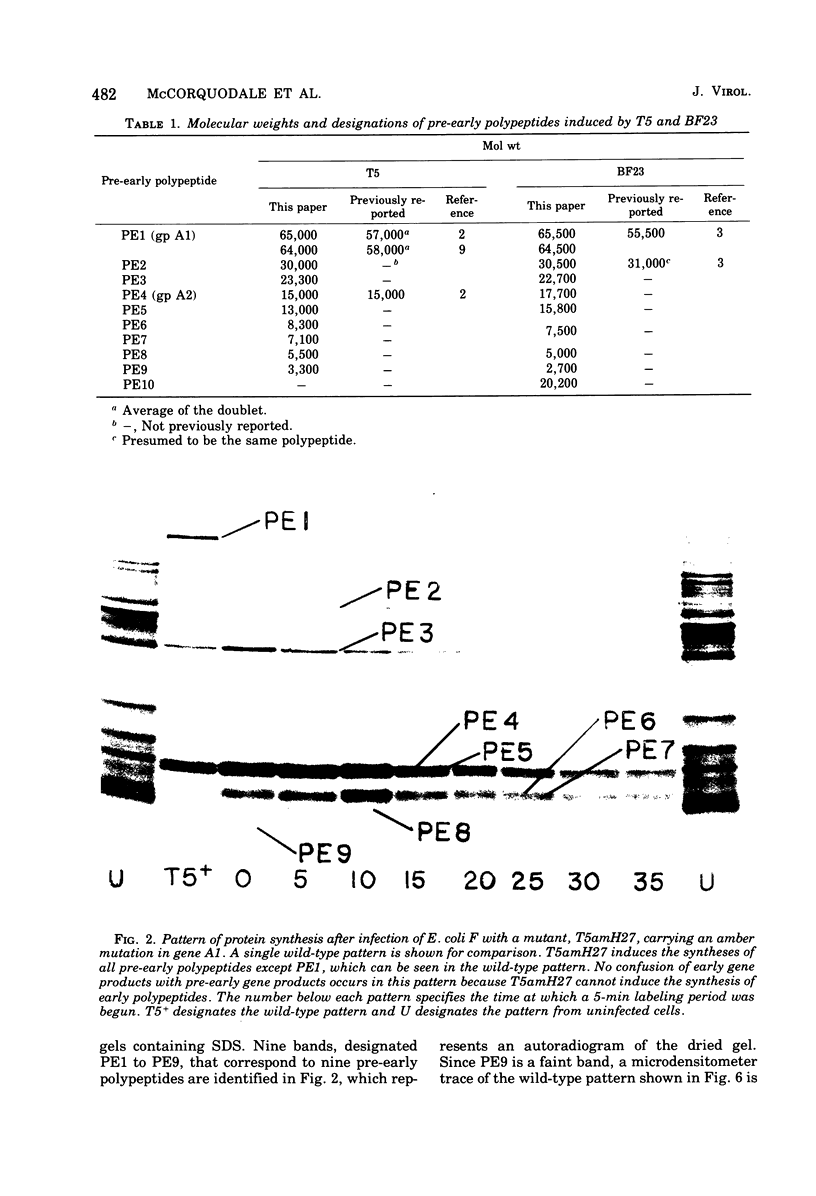
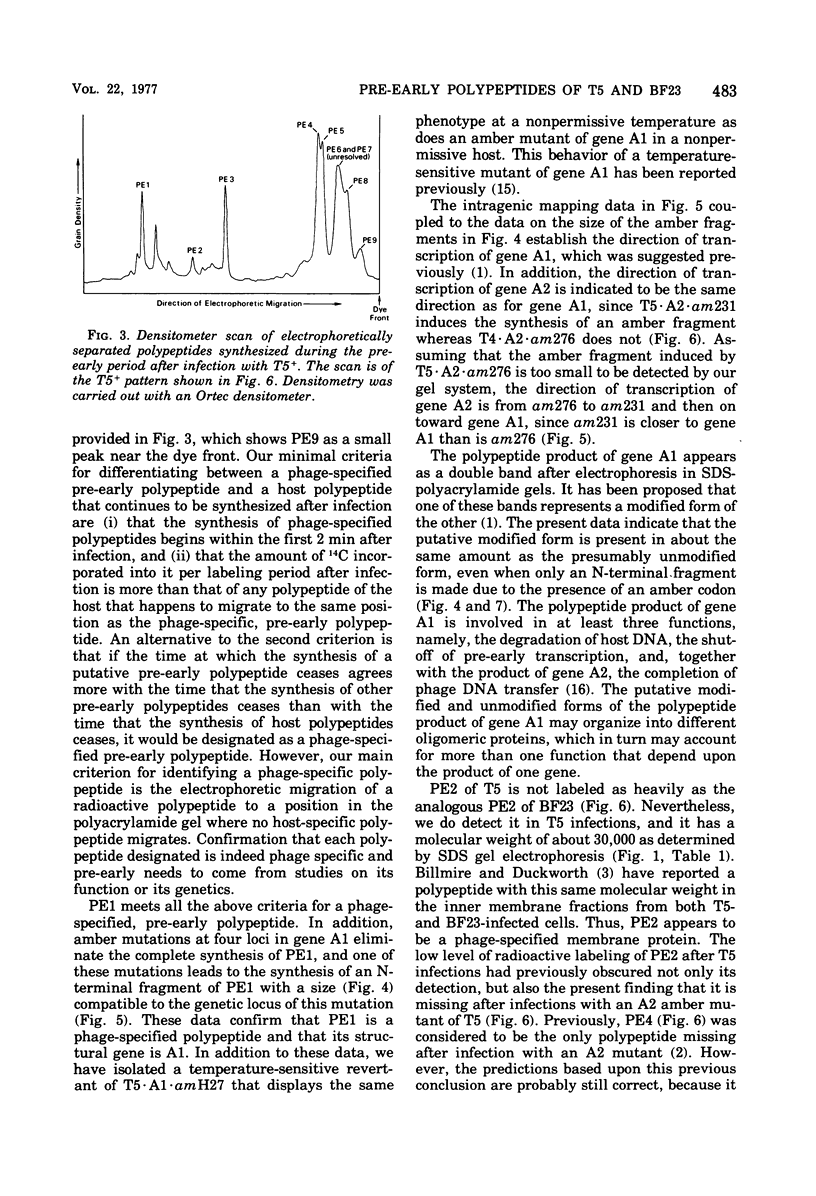
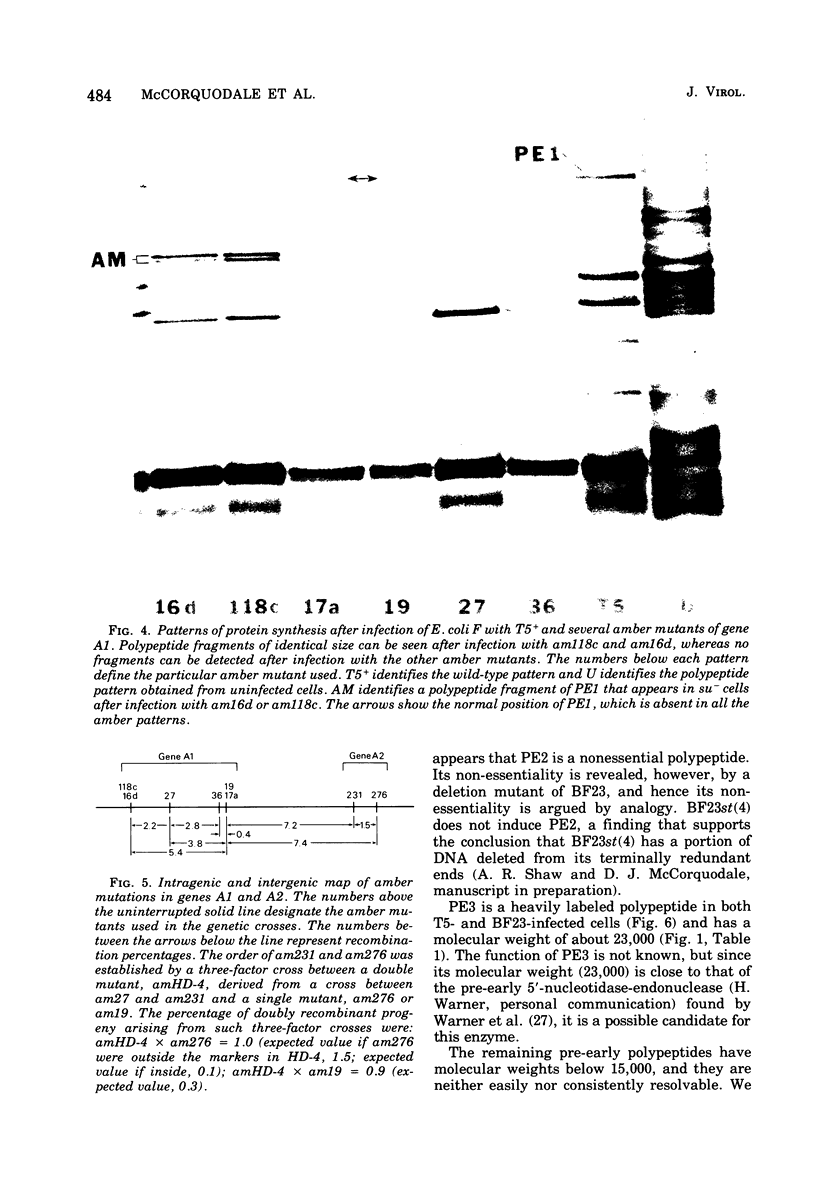
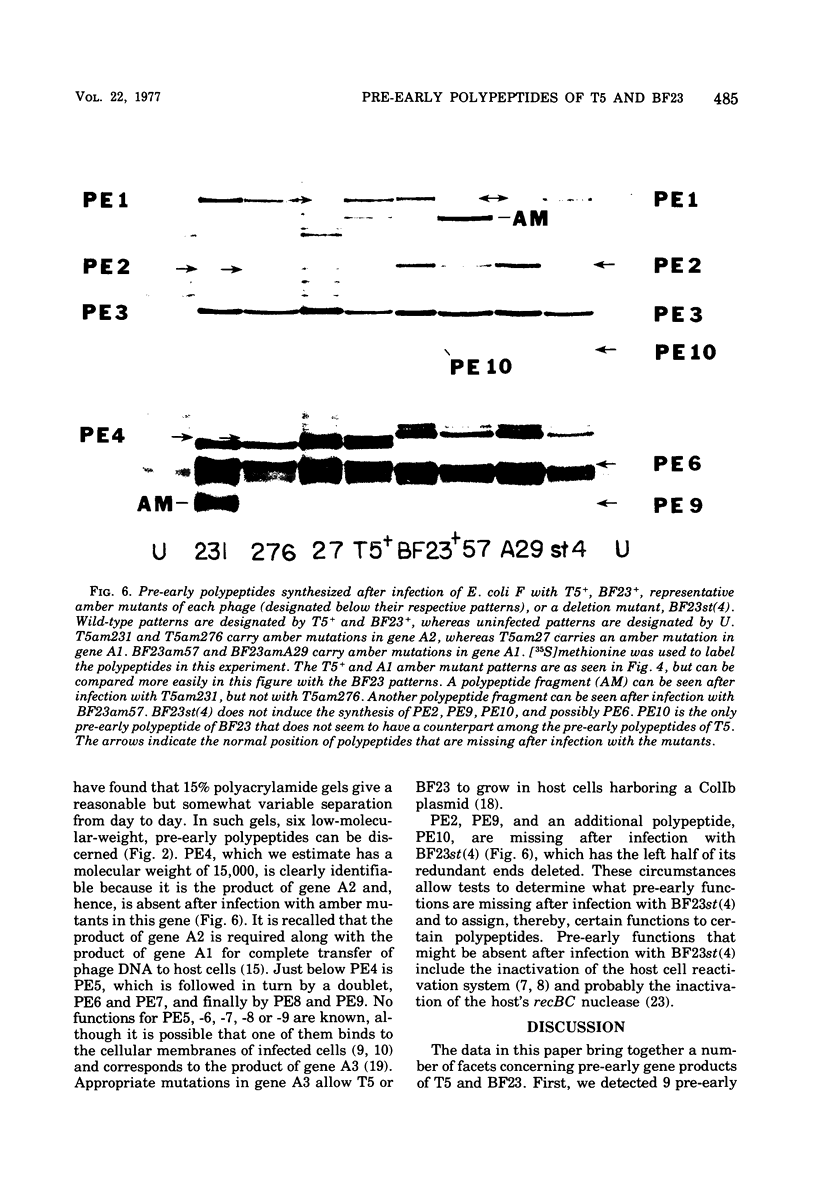
Nine pre-early polypeptides have been detected after infection with bacteriophage T5 and 10 pre-early polypeptides have been detected after infection with bacteriophage BF23. Only about one-half of the coding capacity of the redundant ends of the phage DNA, which code for pre-early proteins, is needed for these 9 to 10 pre-early polypeptides. The direction of transcription of pre-early genes A1 and A2 has been established from the size of N-terminal polypeptide fragments induced by amber mutants and from the known intragenic loci of the amber mutations. Some pre-early functions appear to be nonessential, because a viable deletion mutant of BF23 fails to induce three and possibly four of the detectable pre-early polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman L. D., Anderson G. C., McCorquodale D. J. Arrangement on the chromosome of the known pre-early genes of bacteriophages T5 and BF23. J Virol. 1973 Nov;12(5):1191–1194. doi: 10.1128/jvi.12.5.1191-1194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman L. D., Hoffman M. S., McCorquodale D. J. Pre-early proteins of bacteriophage T5: structure and function. J Mol Biol. 1971 Dec 28;62(3):551–564. doi: 10.1016/0022-2836(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Billmire E. W., Duckworth D. H. Membrane protein biosynthesis in bacteriophage BF23-infected Escherichia coli. J Virol. 1976 Aug;19(2):475–489. doi: 10.1128/jvi.19.2.475-489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Bremer H. Identification of single-stranded DNA fragments of bacteriophage T5. Virology. 1976 Oct 1;74(1):104–115. doi: 10.1016/0042-6822(76)90133-1. [DOI] [PubMed] [Google Scholar]
- Chen C. Transcription map of bacteriophage T5. Virology. 1976 Oct 1;74(1):116–127. doi: 10.1016/0042-6822(76)90134-3. [DOI] [PubMed] [Google Scholar]
- Chiang T., Harm W. On the lack of host-cell reactivation of UV-irradiated phage T5 II. Further characterization of the repair inhibition exerted by T5 infection. Mutat Res. 1976 Aug;36(2):135–146. doi: 10.1016/0027-5107(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Chiang T., Harm W. On the lack of host-cell reactivation of UV-irradiated phage T5. I. Interference of T5 infection with the host-cell reactivation of phage T1. Mutat Res. 1976 Aug;36(2):121–134. doi: 10.1016/0027-5107(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B., McCorquodale D. J. Identification of the gene controlling the synthesis of the major bacteriophage T5 membrane protein. J Virol. 1976 May;18(2):542–549. doi: 10.1128/jvi.18.2.542-549.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B. Membrane protein biosynthesis in T5 bacteriophage-infected Escherichia coli. Arch Biochem Biophys. 1976 Feb;172(2):319–328. doi: 10.1016/0003-9861(76)90083-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. E., Bujard H. Structure and function of the genome of coliphage T5. 2. Regions of transcription of the chromosome. Eur J Biochem. 1973 Mar 15;33(3):529–534. doi: 10.1111/j.1432-1033.1973.tb02712.x. [DOI] [PubMed] [Google Scholar]
- LANG D., Shaw A. R., McCorquodale D. J. Molecular weights of DNA from bacteriophages T5, T5st(O), BF23, and BF23st(4). J Virol. 1975 Jan;17(1):296–297. doi: 10.1128/jvi.17.1.296-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K., Anderson G. C., McCorquodale D. J. Abortive infection by bacteriophage BF23 due to the colicin Ib factor. I. Genetic studies of nonrestricted and amber mutants of bacteriophage BF23. Genetics. 1971 Jul;68(3):323–340. doi: 10.1093/genetics/68.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi K., McCorquodale D. J. Abortive infection by bacteriophage BF23 due to the colicin Ib factor. II. Involvement of pre-early proteins. J Mol Biol. 1974 May 5;85(1):67–74. doi: 10.1016/0022-2836(74)90129-6. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Buchanan J. M. Patterns of RNA synthesis in T5-infected cells. I. As studied by the technique of DNA-RNA hybridization-competition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1249–1256. doi: 10.1073/pnas.64.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbasku D. A., Buchanan J. M. Patterns of ribonucleic acid synthesis in T5-infected Escherichia coli. 3. Separation of low molecular weight ribonucleic acid species by disc electrophoresis on acrylamide gel columns. J Biol Chem. 1970 May 25;245(10):2693–2703. [PubMed] [Google Scholar]
- Sirbasku D. A., Buchanan J. M. Patterns of ribonucleic acid synthesis in T5-infected Escherichia coli. II. Separation of high molecular weight ribonucleic acid species by disc electrophoresis on acrylamide gel columns. J Biol Chem. 1970 May 25;245(10):2679–2692. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Drong R. F., Berget S. M. Early events after infection of Escherichia coli by bacteriophage T5. Induction of a 5'-nucleotidase activity and excretion of free bases. J Virol. 1975 Feb;15(2):273–280. doi: 10.1128/jvi.15.2.273-280.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]