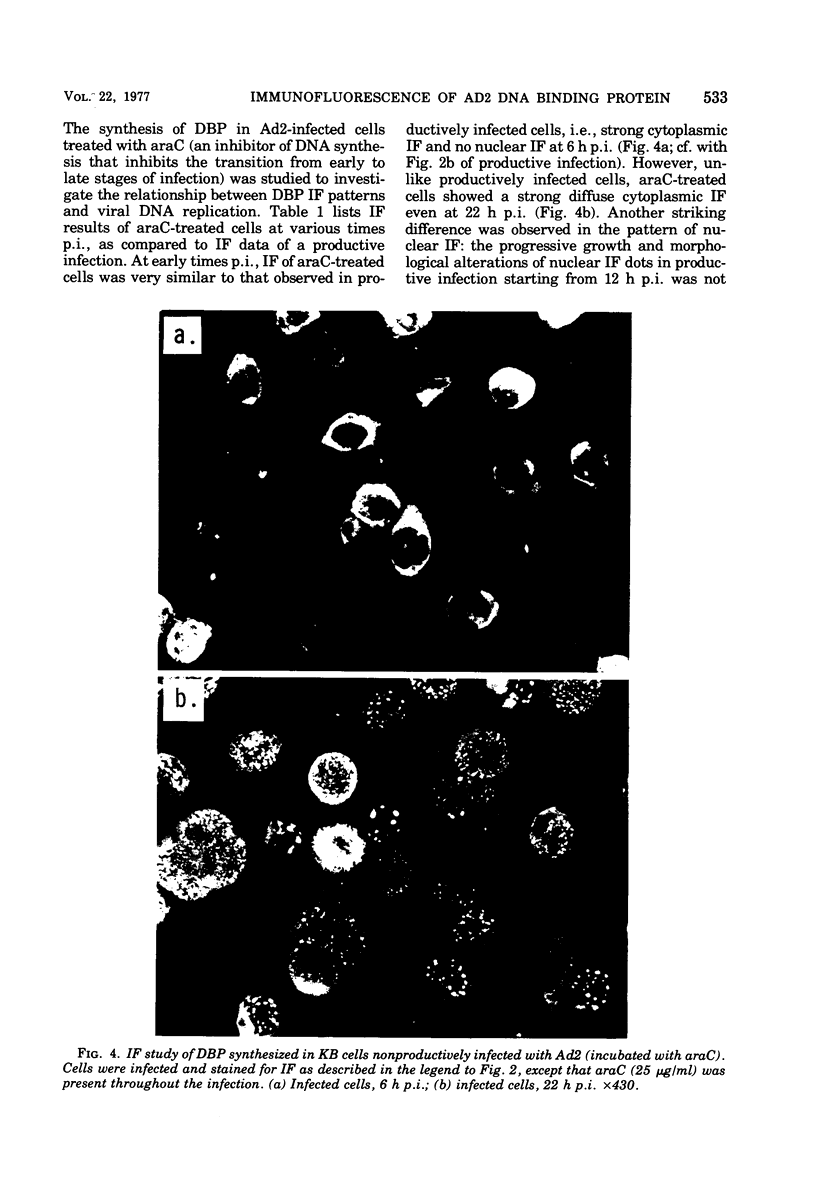
Abstract
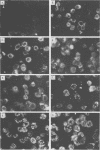
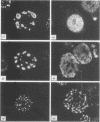
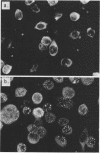
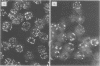
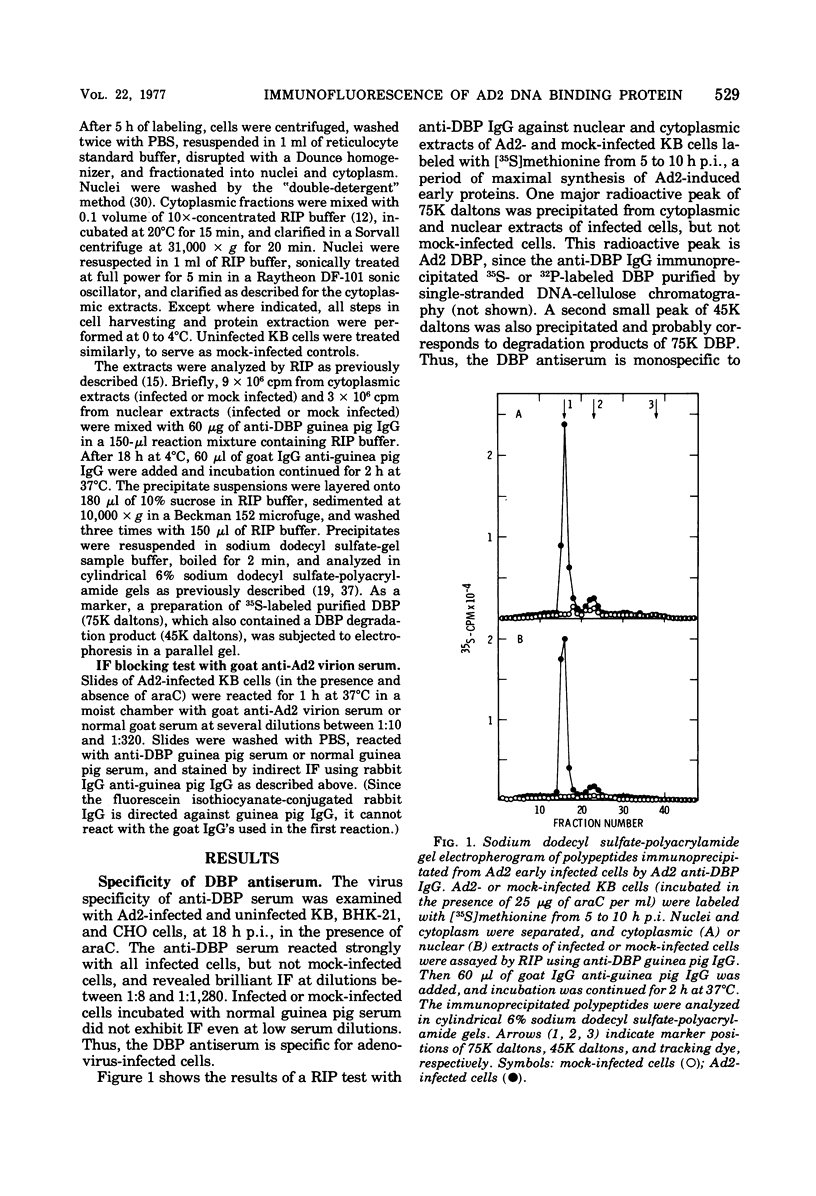
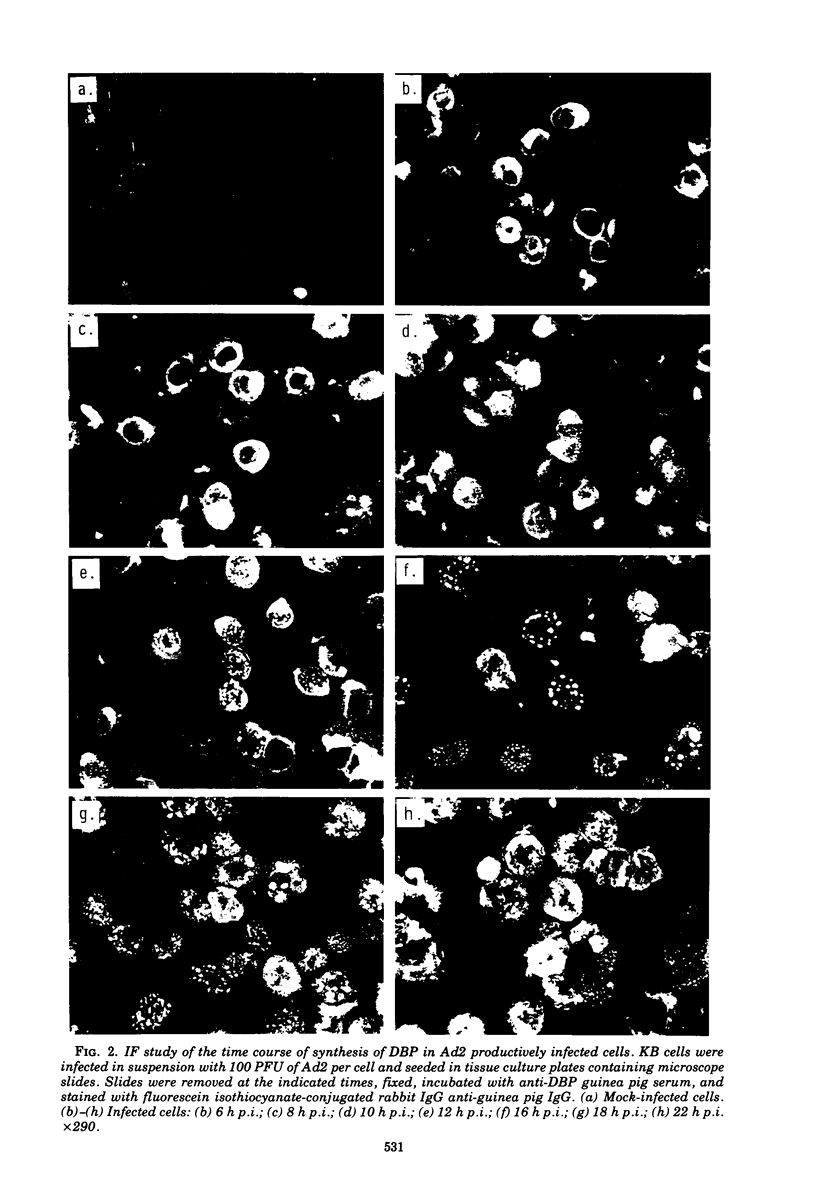
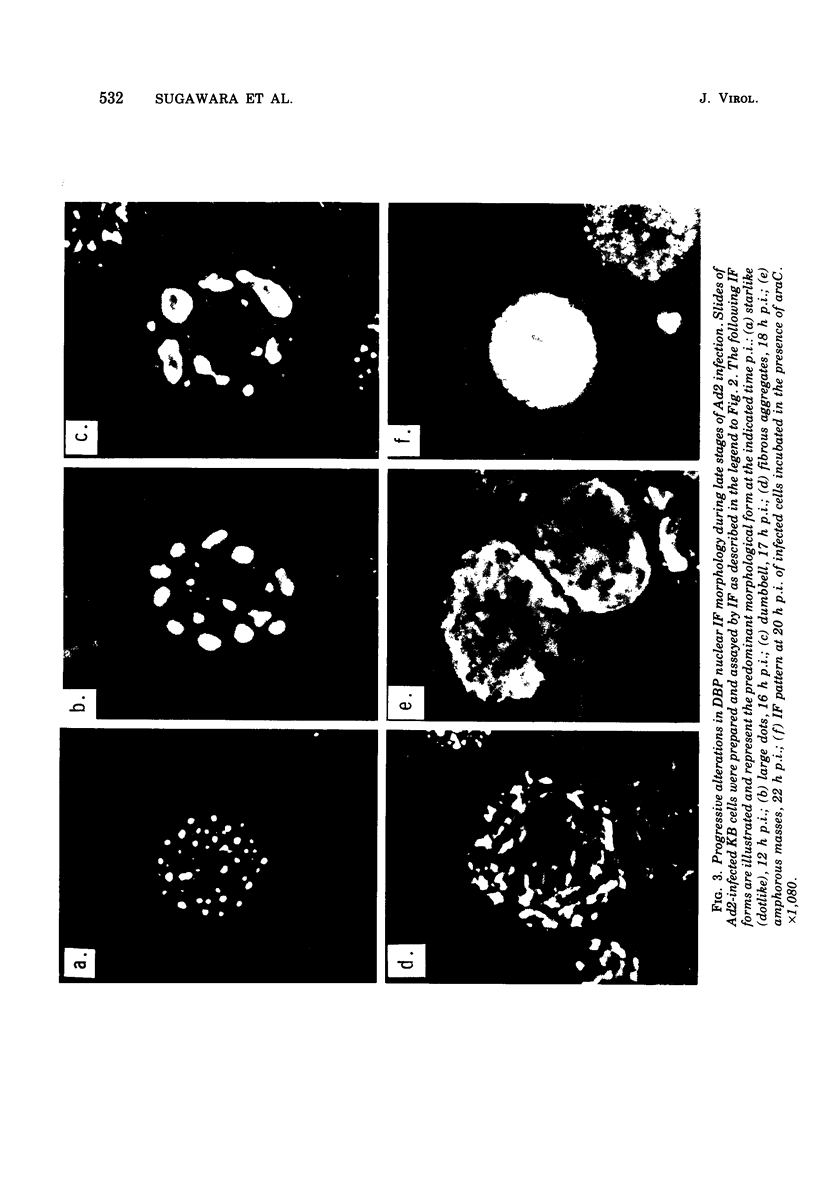
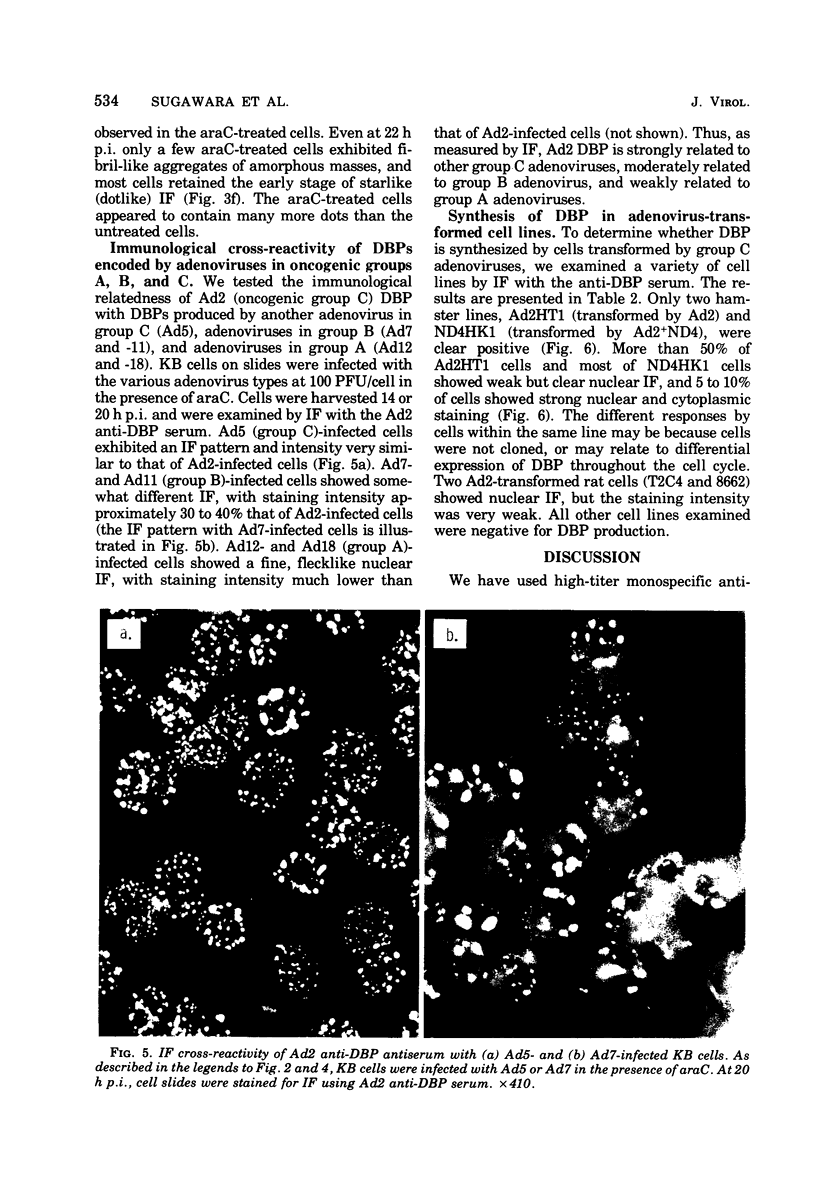
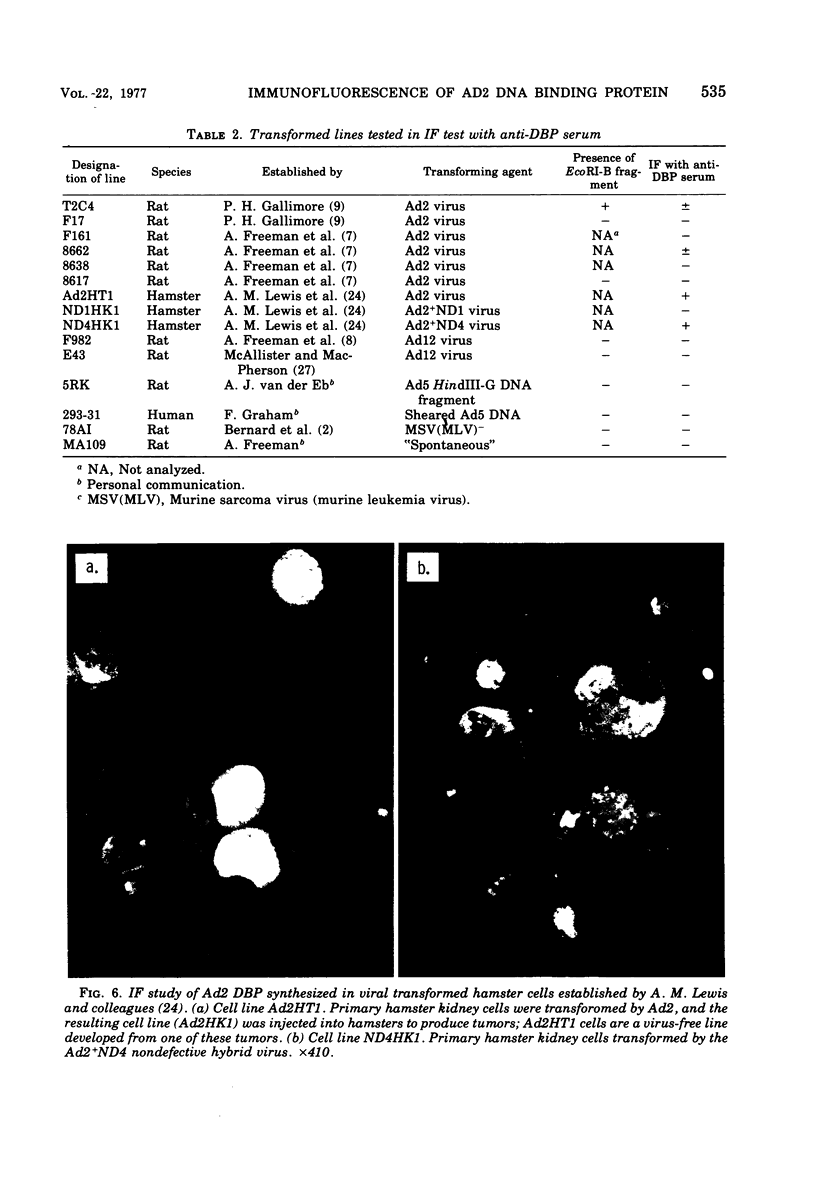
High-titer monospecific antiserum against highly purified adenovirus 2 (Ad2) single-stranded DNA binding protein (DBP) was used to study, by indirect immunofluorescence (IF), the synthesis of DBP in Ad2-infected human cells and adenovirus-transformed rat, hamster, and human cell lines. In infected cells the synthesis of DBP was first detected in the cytoplasm at 2 to 4 h postinfection and reached a maximum intensity at 6 h postinfection. At this time DBP began to accumulate in the nucleus, where it reached maximum intensity at about 14 h postinfection. The cytoplasmic IF was diffuse, whereas nuclear IF appeared as dots that coalesced into large globules as infection progressed. In cells treated with 1-β-d-arabinofuranosylcytosine to inhibit viral DNA synthesis, strong nuclear IF was observed in the form of dots, but the large fluorescent globules were not observed. The Ad2 (oncogenic group C) anti-DBP serum reacted very strongly by IF with Ad5 (group C)-infected, to a lesser extent with Ad7 and Ad11 (group B)-infected, and weakly with Ad12 and Ad18 (group A)-infected KB cells (treated with 1-β-d-arabinofuranosylcytosine). These results may indicate that Ad2 DBP is closely related immunologically to DBPs induced early after infection by adenovirus serotypes in oncogenic group C, moderately related to DBPs of serotypes in oncogenic group B, and perhaps distantly related to DBPs of serotypes in oncogenic group A. The following adenovirus-transformed cell lines were examined for DBP synthesis by IF with the Ad2 anti-DBP serum: six rat cell lines (T2C4, F17, 8662, 8638, 8617, and F161) transformed by Ad2 virus, three hamster cell lines transformed by Ad2 virus (Ad2HT1) and Ad2-simian virus 40 hybrid virus (ND1HK1 and ND4HK4), and one rat (5RK) and one human (293-31) cell line transformed by transfection with Ad5 DNA. T2C4 and 8662 appeared weakly positive, whereas Ad2HT1 and ND4HK1 were strongly positive. The other transformed cell lines did not produce DBP detectable by IF. Thus, some but not all transformed cell lines produce DBP, which indicates that DBP is not required for maintenance of cell transformation and that transformed cells can express “nontransforming” viral genes as protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C., Boiron M., Lasneret J. Transformation et infection chronique de cellules embryonnaires de rat par le virus du sarcome de Moloney. C R Acad Sci Hebd Seances Acad Sci D. 1967 Apr 24;264(17):2170–2173. [PubMed] [Google Scholar]
- Black P. H., Lewis A. M., Jr, Blacklow N. R., Austin J. B., Rowe W. P. The presence of adenovirus-specific antigens in hamster cells rendered neoplastic by adenovirus 1-SV40 and adenovirus 2-SV40 hybrid viruses. Proc Natl Acad Sci U S A. 1967 May;57(5):1324–1330. doi: 10.1073/pnas.57.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Ginsberg H. S. Viral transcription in KB cells infected by temperature-sensitive "early" mutants of adenovirus type 5. J Virol. 1976 Apr;18(1):156–166. doi: 10.1128/jvi.18.1.156-166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Gallimore P. H., Sharp P. A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975 Jul 25;96(1):47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Vanderpool E. A., Henry P. H., Austin J. B., Huebner R. J. Transformation of primary rat embryo cells by adenovirus type 2. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1205–1212. doi: 10.1073/pnas.58.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Wolford R., Huebner R. J. Adenovirus type 12-rat embryo transformation system. J Virol. 1967 Apr;1(2):362–367. doi: 10.1128/jvi.1.2.362-367.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Interactions of adenovirus type 2 with rat embryo cells. Permissiveness, transformation and in vitro characteristics of adenovirus transformed rat embryo cells. J Gen Virol. 1974 Nov;25(2):263–273. doi: 10.1099/0022-1317-25-2-263. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Hierholzer J. C., Rose J. A. Mapping of base sequence heterologies between genomes from different adenovirus serotypes. Virology. 1973 Aug;54(2):414–426. doi: 10.1016/0042-6822(73)90153-0. [DOI] [PubMed] [Google Scholar]
- Gielkens A. L., Van Zaane D., Bloemers H. P., Bloemendal H. Synthesis of Rauscher murine leukemia virus-specific polypeptides in vitro. Proc Natl Acad Sci U S A. 1976 Feb;73(2):356–360. doi: 10.1073/pnas.73.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Kern J., Freeman A. E., Martin C. E., McAllister R. C., Turner H. C., Huebner R. J. T and tumour antigens of adenovirus group C-infected and transformed cells. Nature. 1968 Aug 3;219(5153):517–518. doi: 10.1038/219517a0. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Arens M. Q., Bhaduri S., Shanmugam G., Green M. Tumour antigen specificity of a DNA-binding protein from cells infected with adenovirus 2. Nature. 1975 Apr 10;254(5500):533–536. doi: 10.1038/254533a0. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Sugawara K., Shanmugam G., Green M. Synthesis of the adenovirus-coded DNA binding protein in infected cells. J Virol. 1976 May;18(2):454–460. doi: 10.1128/jvi.18.2.454-460.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Shanmugam G., Wold W. S., Green M. Detection of adenovirus type 2-induced early polypeptides using cycloheximide pretreatment to enhance viral protein synthesis. J Virol. 1976 Jul;19(1):232–242. doi: 10.1128/jvi.19.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng Y. H., Wold W. S., Sugawara K., Gilead Z., Green M. Adenovirus type 2 coded single-stranded DNA binding protein: in vivo phosphorylation and modification. J Virol. 1977 May;22(2):402–411. doi: 10.1128/jvi.22.2.402-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A., Levine A. J., Anderson S., Osborn M., Rosenwirth B., Weber K. The relationship between group C adenovirus tumor antigen and the adenovirus single-strand DNA-binding protein. Cell. 1976 Apr;7(4):575–584. doi: 10.1016/0092-8674(76)90208-7. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Baum S. G., Prigge K. O., Rowe W. P. Occurrence of adenovirus-SV40 hybrids among monkey kidney cell adapted strains of adenovirus. Proc Soc Exp Biol Med. 1966 May;122(1):214–218. doi: 10.3181/00379727-122-31093. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rabson A. S., Levine A. S. Studies of nondefective adenovirus 2-simian virus 40 hybrid viruses. Transformation of hamster kidney cells by adenovirus 2 and the nondefective hybrid viruses. J Virol. 1974 Jun;13(6):1291–1301. doi: 10.1128/jvi.13.6.1291-1301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Macpherson I. Transformation of a hamster cell line by adenovirus type 12. J Gen Virol. 1968 Jan;2(1):99–106. doi: 10.1099/0022-1317-2-1-99. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M. O., Lewis A. M., Jr, Macpherson I., Huebner R. J. Transformation of rat embryo cells by adenovirus type 1. J Gen Virol. 1969 Jan;4(1):29–36. doi: 10.1099/0022-1317-4-1-29. [DOI] [PubMed] [Google Scholar]
- McDongall J. K., Dunn A. R., Gallimore P. H. Recent studies on the characteristics of adenovirus-infected and -transformed cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):591–600. doi: 10.1101/sqb.1974.039.01.073. [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U. Molecular biology of adenoviruses. Virol Monogr. 1975;14:1–115. doi: 10.1007/978-3-7091-8391-5_1. [DOI] [PubMed] [Google Scholar]
- Rosenwirth B., Anderson C., Levine A. J. Tryptic fingerprint analysis of adenovirus types 2, 5 and 12 DNA-Binding proteins. Virology. 1976 Feb;69(2):617–625. doi: 10.1016/0042-6822(76)90490-6. [DOI] [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Arens M., Green M. DNA binding proteins in the cytoplasm and in a nuclear membrane complex isolated from uninfected and adenovirus 2 infected cells. Biochemistry. 1975 Jan 28;14(2):332–337. doi: 10.1021/bi00673a020. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Analysis of adenovirus 12 temperature-sensitive mutants defective in viral DNA replication. Virology. 1974 Oct;61(2):474–485. doi: 10.1016/0042-6822(74)90283-9. [DOI] [PubMed] [Google Scholar]
- Simmons T., Heywood P., Hodge L. D. Intranuclear site of replication of adenovirus DNA. J Mol Biol. 1974 Nov 5;89(3):423–433. doi: 10.1016/0022-2836(74)90473-2. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Gilead Z., Green M. Purification and molecular characterization of adenovirus type 2 DNA-binding protein. J Virol. 1977 Jan;21(1):338–346. doi: 10.1128/jvi.21.1.338-346.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlak J. M., Rozijn R. H., Spies F. Localization of adenovirus DNA replication in KB cells. Virology. 1975 Jun;65(2):535–545. doi: 10.1016/0042-6822(75)90058-6. [DOI] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M., Brackmann K. H., Cartas M. A., Devine C. Genome expression and mRNA maturation at late stages of productive adenovirus type 2 infection. J Virol. 1976 Nov;20(2):465–477. doi: 10.1128/jvi.20.2.465-477.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. II. Synthesis of viral deoxyribonucleic acid in vitro by a nuclear membrane fraction from infected KB cells. J Biol Chem. 1975 May 10;250(9):3273–3279. [PubMed] [Google Scholar]
- Yamashita T., Green M. Adenovirus DNA replication. I. Requirement for protein synthesis and isolation of nuclear membrane fractions containing newly synthesized viral DNA and proteins. J Virol. 1974 Sep;14(3):412–420. doi: 10.1128/jvi.14.3.412-420.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]