Abstract
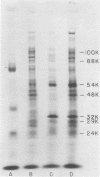
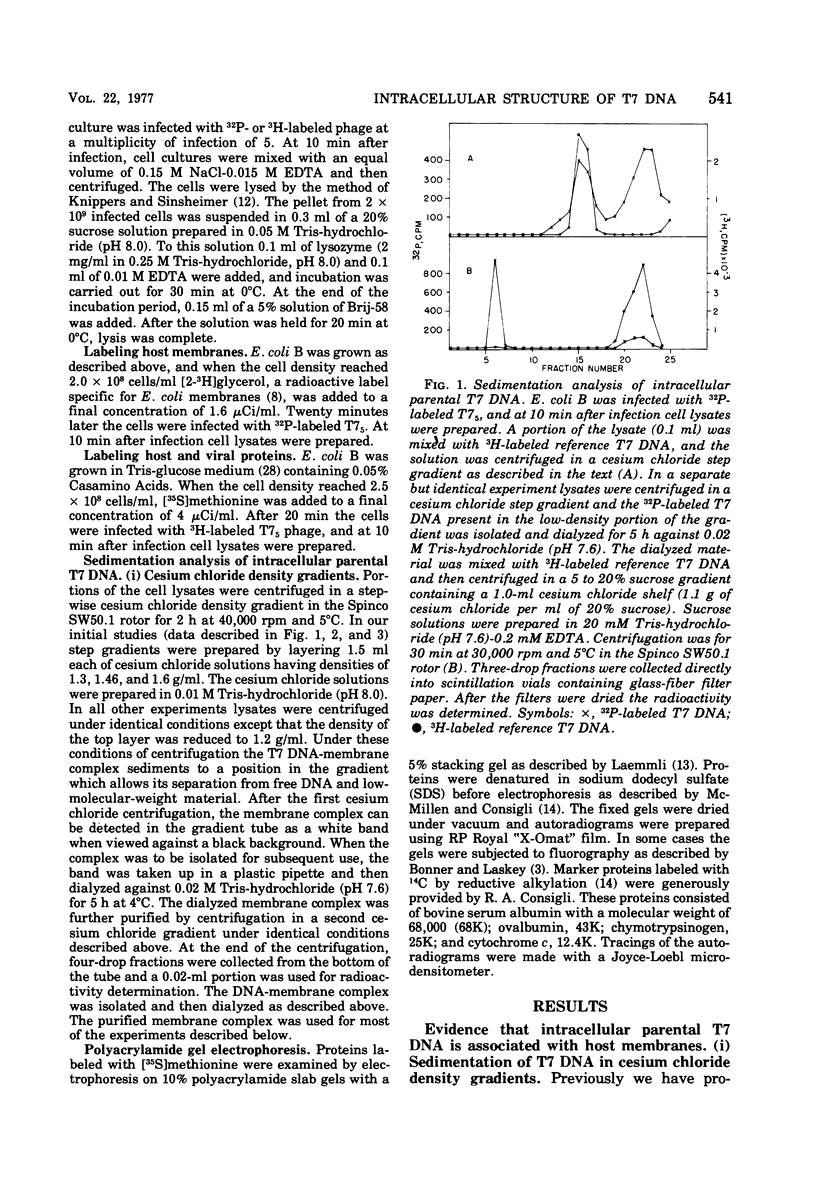
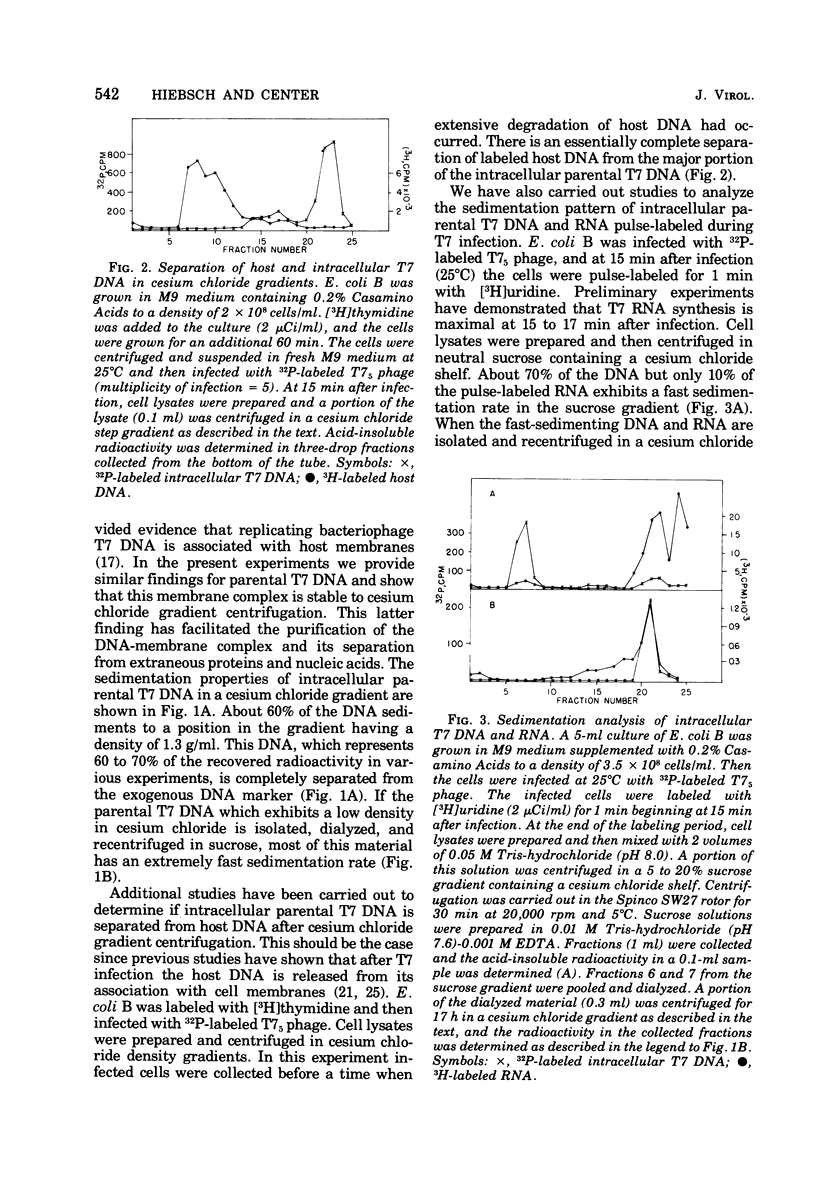
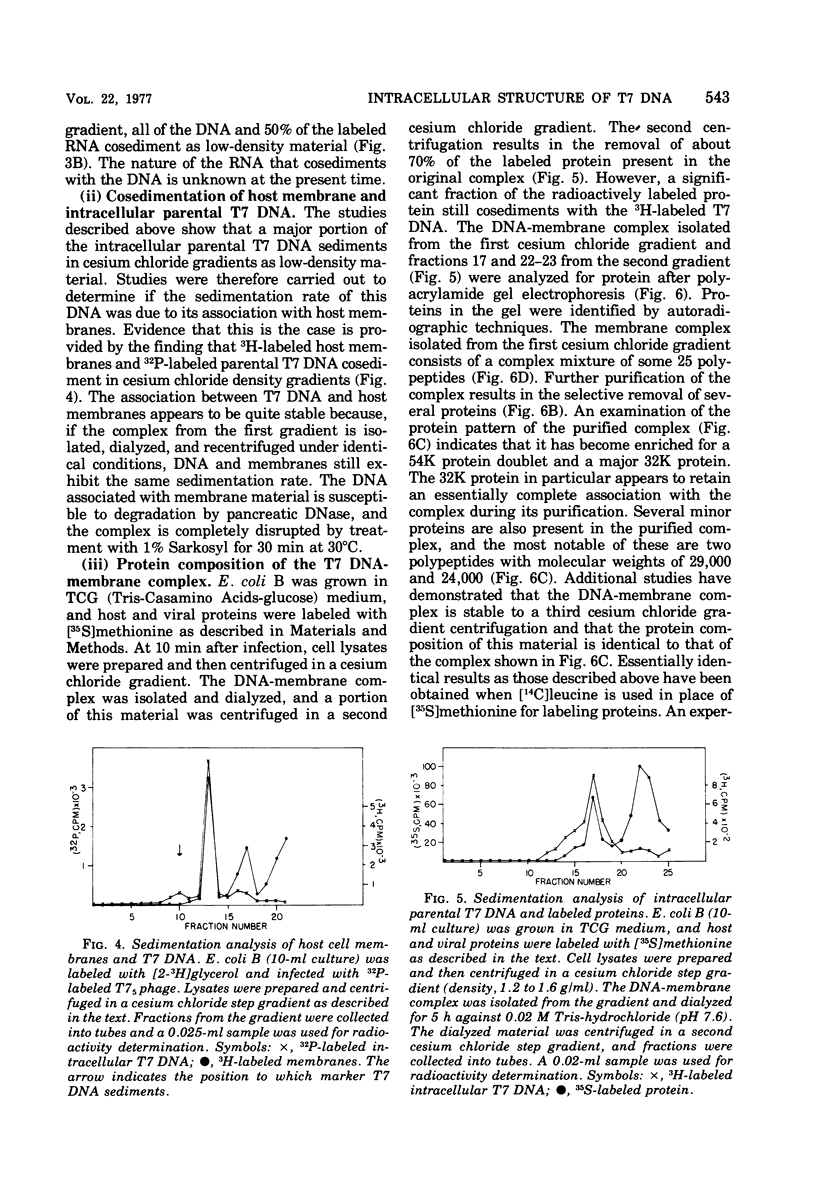
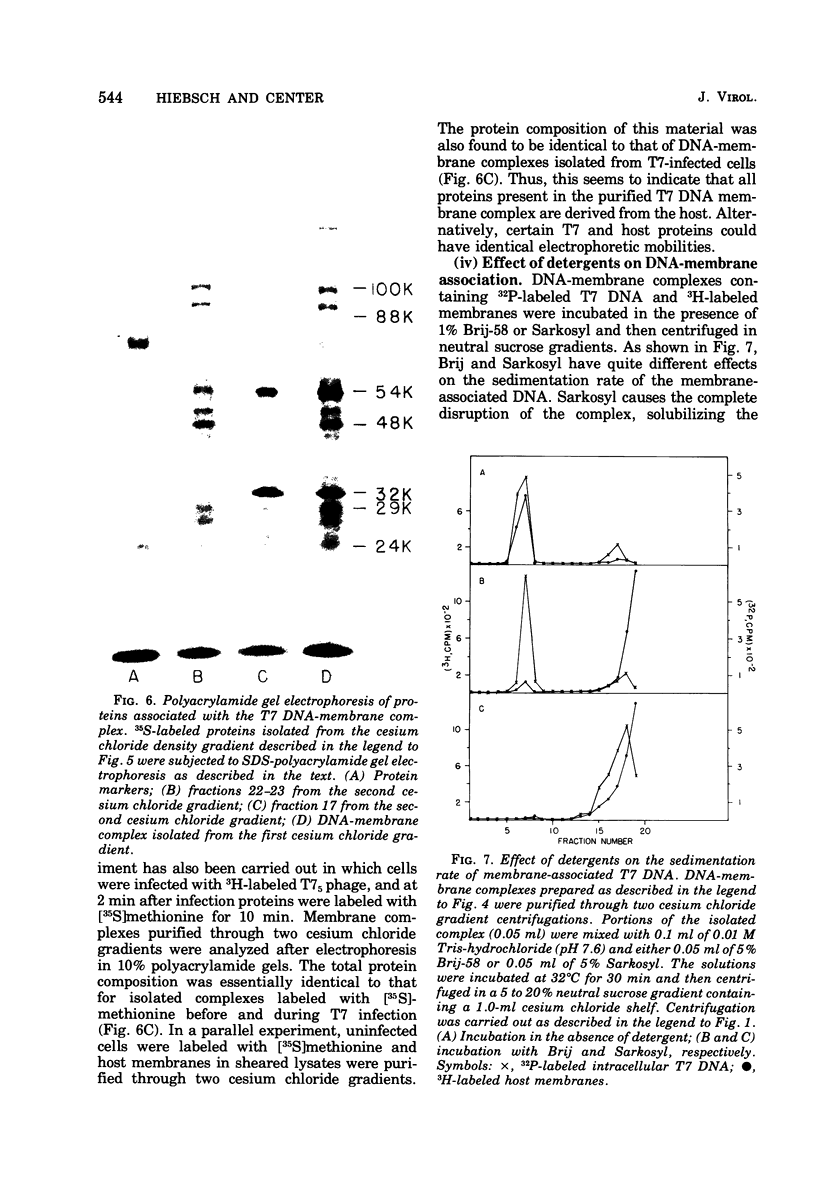
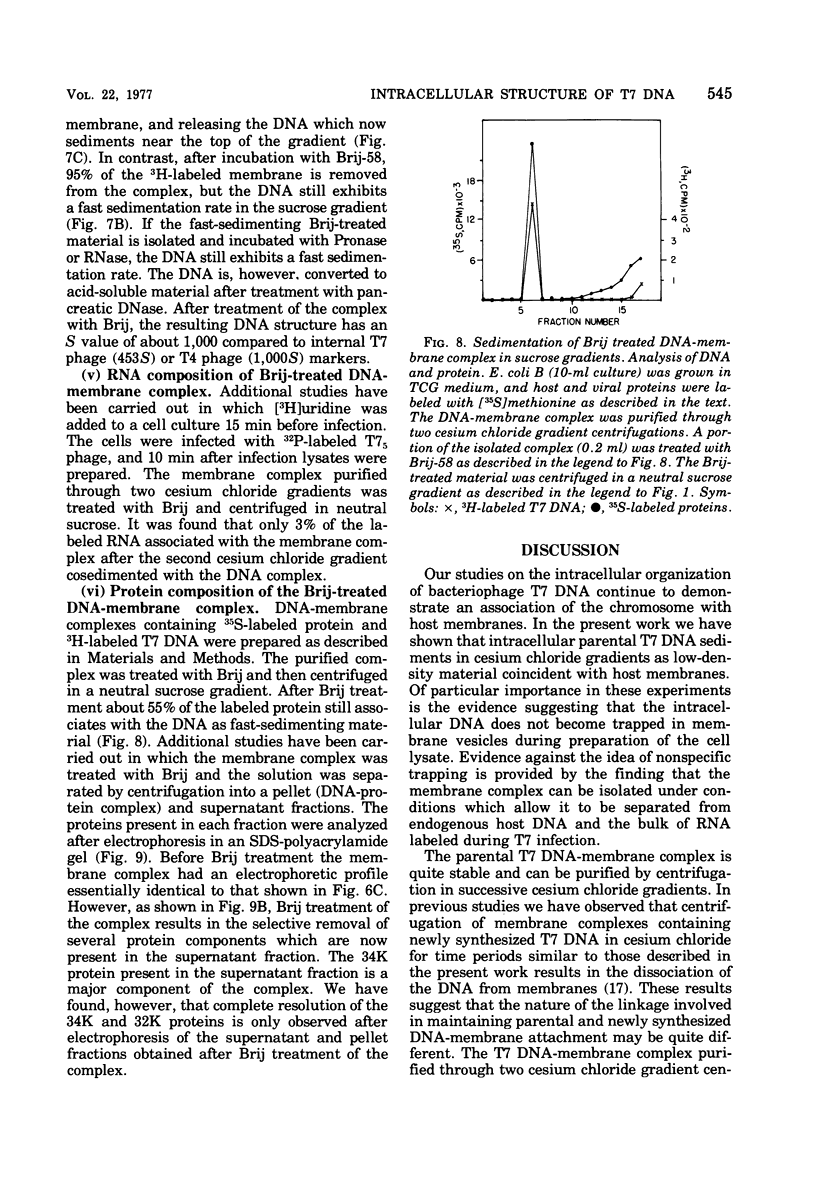
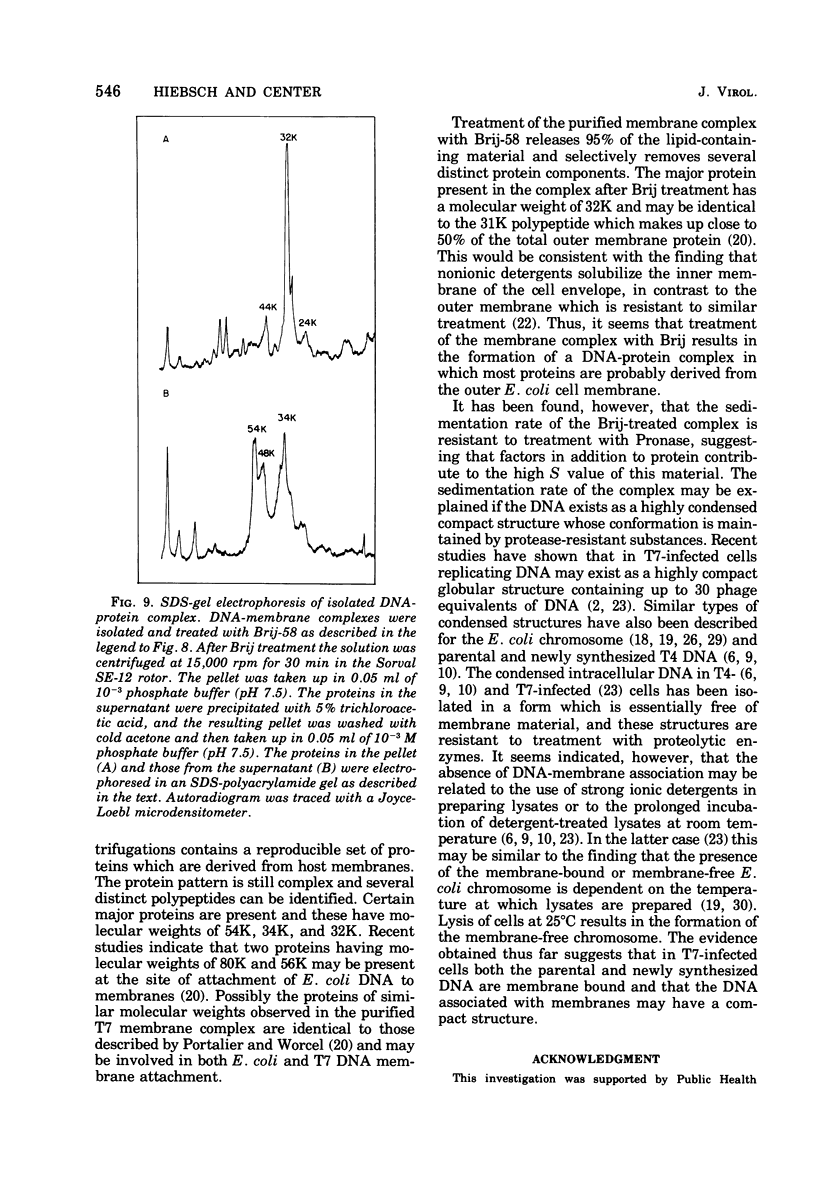
After infection of Escherichia coli with bacteriophage T7, the parenteral DNA forms a stable association with host cell membranes. The DNA-membrane complex isolated in cesium chloride gradients is free of host DNA and the bulk of T7 RNA. The complex purified through two cesium chloride gradients contains a reproducible set of proteins which are enriched in polypeptides having molecular weights of 54,000, 34,000, and 32,000. All proteins present in the complex are derived from host membranes. Treatment of the complex with Bruij-58 removes 95% of the membrane lipid and selectively releases certain protein components. The Brij-treated complex has an S value of about 1,000 and the sedimentation rate of this material is not altered by treatment with Pronase or RNase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C., Bernstein H. Coiled rings of DNA released from cells infected with bacteriophages T7 or T4 or from uninfected Escherichia coli. J Virol. 1974 Jun;13(6):1346–1355. doi: 10.1128/jvi.13.6.1346-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Center M. S. Replicative intermediates of bacteriophage T7 deoxyribonucleic acid. J Virol. 1972 Jul;10(1):115–123. doi: 10.1128/jvi.10.1.115-123.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Richardson C. C. An endonuclease induced after infection of Escherichia coli with bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1970 Dec 10;245(23):6285–6291. [PubMed] [Google Scholar]
- Curtis M. J., Alberts B. Studies on the structure of intracellular bacteriophage T4 DNA. J Mol Biol. 1976 Apr 25;102(4):793–816. doi: 10.1016/0022-2836(76)90292-8. [DOI] [PubMed] [Google Scholar]
- Drlica K., Worcel A. Conformational transitions in the Escherichia coli chromosome: analysis by viscometry and sedimentation. J Mol Biol. 1975 Oct 25;98(2):393–411. doi: 10.1016/s0022-2836(75)80126-4. [DOI] [PubMed] [Google Scholar]
- Green E. W., Schaechter M. The mode of segregation of the bacterial cell membrane. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2312–2316. doi: 10.1073/pnas.69.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S., Pettijohn D. E. Properties of condensed bacteriophage T4 DNA isolated from Escherichia coli infected with bacteriophage T4. J Virol. 1976 Sep;19(3):1012–1027. doi: 10.1128/jvi.19.3.1012-1027.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Characterization of polyoma DNA-protein complexes. I. Electrophoretic identification of the proteins in a nucleoprotein complex isolated from polyoma-infected cells. J Virol. 1974 Dec;14(6):1326–1336. doi: 10.1128/jvi.14.6.1326-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. In vitro radioisotopic labeling of proteins associated with purified polyoma virions. J Virol. 1974 Dec;14(6):1627–1629. doi: 10.1128/jvi.14.6.1627-1629.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication invitro. Bacteriophage T7 DNA polymerase: an an emzyme composed of phage- and host-specific subunits. J Biol Chem. 1975 Jul 25;250(14):5515–5522. [PubMed] [Google Scholar]
- Pacumbaba R. P., Center M. S. Association of replicating bacteriophage T7 DNA with bacterial membranes. J Virol. 1973 Oct;12(4):855–861. doi: 10.1128/jvi.12.4.855-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Portalier R., Worcel A. Association of the folded chromosome with the cell envelope of E. coli: characterization of the proteins at the DNA-membrane attachment site. Cell. 1976 Jun;8(2):245–255. doi: 10.1016/0092-8674(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Kerr C. Degradation of Escherichia coli B deoxyribonucleic acid after infection with deoxyribonucleic acid-defective amber mutants of bacteriophage T7. J Virol. 1970 Aug;6(2):149–155. doi: 10.1128/jvi.6.2.149-155.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P. Fast sedimenting bacteriophage T7 DNA from T7-infected Escherichia coli. Virology. 1974 May;59(1):70–88. doi: 10.1016/0042-6822(74)90207-4. [DOI] [PubMed] [Google Scholar]
- Siegel P. J., Schaechter M. The role of the host cell membrane in the replication and morphogenesis of bacteriophages. Annu Rev Microbiol. 1973;27:261–282. doi: 10.1146/annurev.mi.27.100173.001401. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Inouye M. Studies on the role of bacteriophage T7 lysozyme during phage infection. J Mol Biol. 1975 Jul 25;96(1):1–11. doi: 10.1016/0022-2836(75)90178-3. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]