Abstract
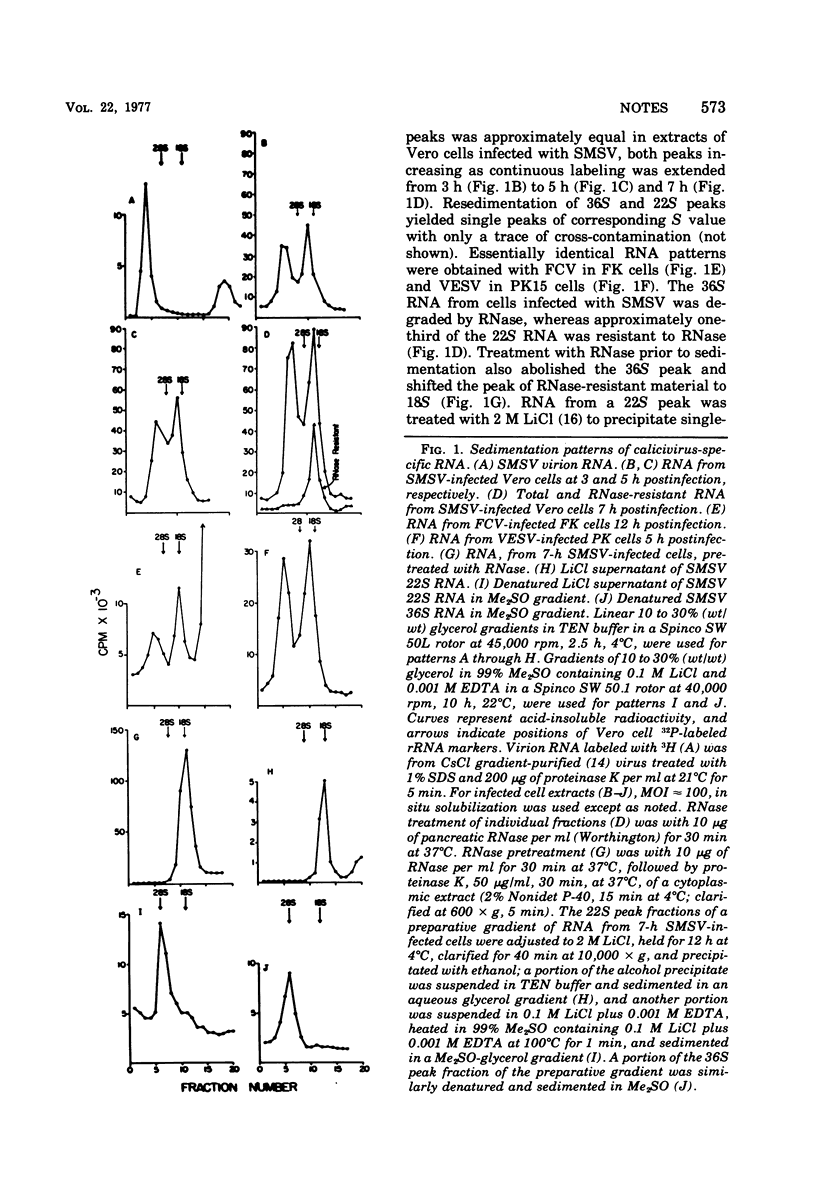
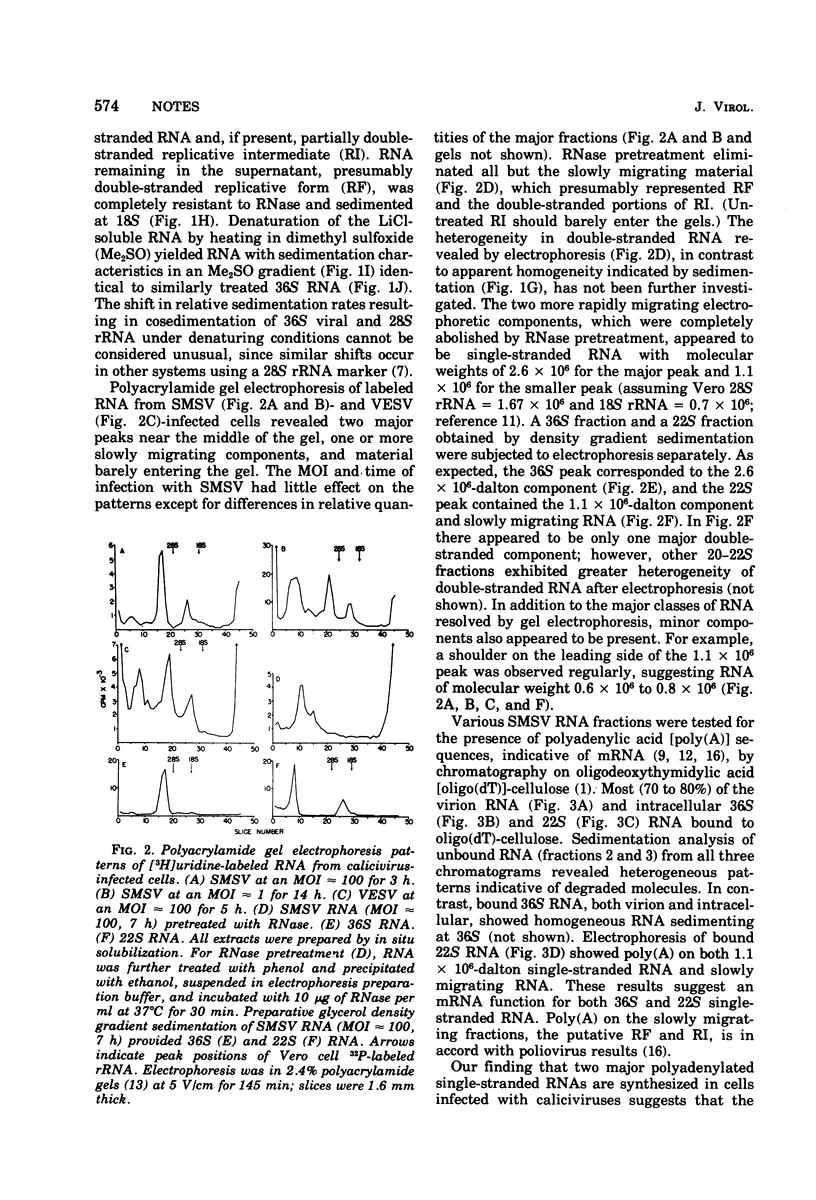
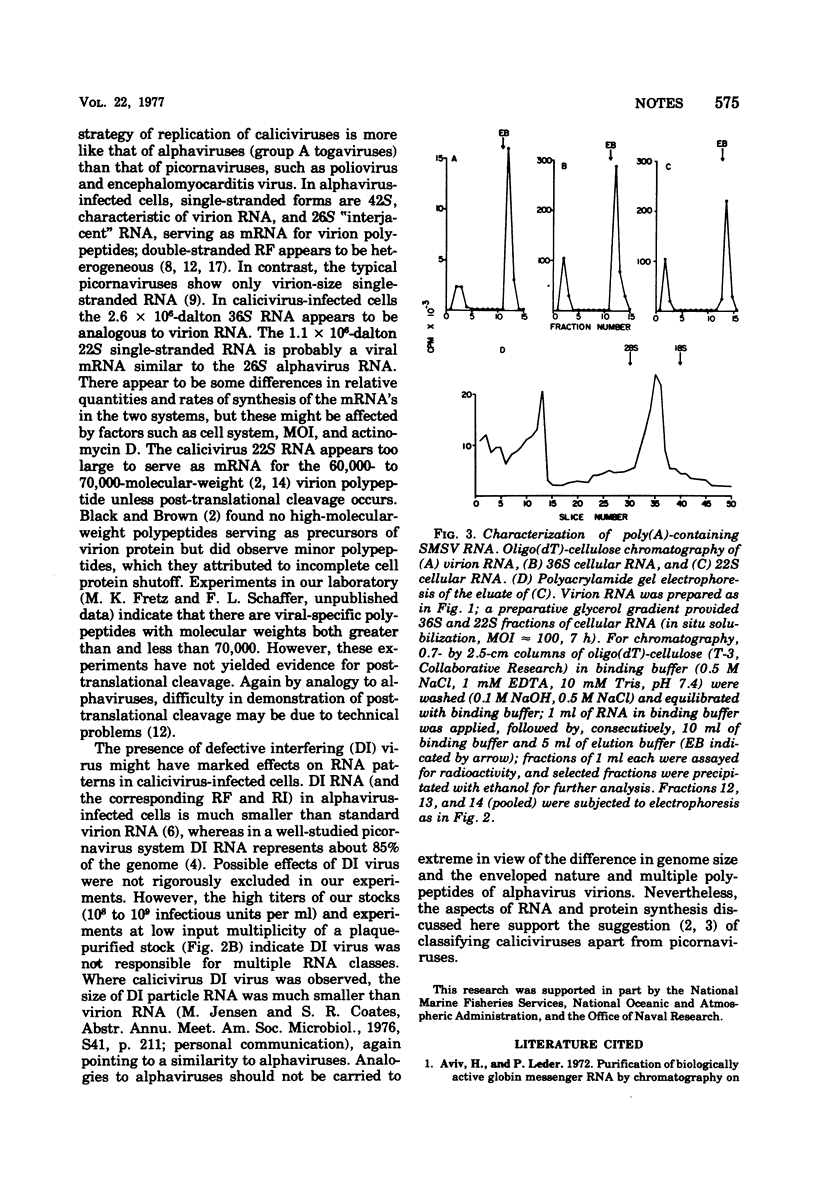
RNA labeled with [3H]uridine from Vero cells infected with San Miguel sea lion virus in the presence of actinomycin D was analyzed by glycerol density gradient sedimentation and polyacrylamide gel electrophoresis. The predominant single-stranded RNA (36S, 2.6 x 10(6) molecular weight) was genome size. There was also a prominent 22S, 1.1 x 10(6)-molecular weight, single-stranded component and one or more double-stranded or partially double-stranded classes. Replicative forms, sedimenting at 18S, contained single-stranded RNA corresponding to the larger-molecular-weight class. All classes of intracellular RNA and virion RNA were polyadenylated. These findings and results with pig kidney cells infected with vesicular exanthema of swine virus and feline cells infected with feline calicivirus indicate that caliciviruses exhibit a strategy of replication different from typical picornaviruses and supports removal of the caliciviruses from the family Picornaviridae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D., Brown F. A major difference in the strategy of the calici- and picornaviruses and its significance in classification. Intervirology. 1975;6(1):57–60. doi: 10.1159/000149454. [DOI] [PubMed] [Google Scholar]
- Burroughs J. N., Brown F. Physico-chemical evidence for the re-classification of the caliciviruses. J Gen Virol. 1974 Feb;22(2):281–286. doi: 10.1099/0022-1317-22-2-281. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. 3. Interference and enrichment. J Mol Biol. 1973 May 25;76(3):345–361. doi: 10.1016/0022-2836(73)90509-3. [DOI] [PubMed] [Google Scholar]
- Fenner F. The classification and nomenclature of viruses. Summary of results of meetings of the International Committee on Taxonomy of Viruses in Madrid, September 1975. J Gen Virol. 1976 Jun;31(3):463–470. doi: 10.1099/0022-1317-31-3-463. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Bruton C. J., Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the defective virion RNA. J Virol. 1976 Sep;19(3):1034–1043. doi: 10.1128/jvi.19.3.1034-1043.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Agol V. I., Bachrach H. L., Brown F., Cooper P. D., Fiers W., Gard S., Gear J. H., Ghendon Y., Kasza L. Picornaviridae. Intervirology. 1974;4(5):303–316. doi: 10.1159/000149863. [DOI] [PubMed] [Google Scholar]
- Oglesby A. S., Schaffer F. L., Madin S. H. Biochemical and biophysical properties of vesicular exanthema of swine virus. Virology. 1971 May;44(2):329–341. doi: 10.1016/0042-6822(71)90264-9. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E. Biochemical and biophysical characterization of calicivirus isolates from pinnipeds. Intervirology. 1973;1(3):210–219. doi: 10.1159/000148848. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E. Single major polypeptide of a calicivirus: characterization by polyacrylamide gel electrophoresis and stabilization of virions by cross-linking with dimethyl suberimidate. J Virol. 1976 Sep;19(3):925–931. doi: 10.1128/jvi.19.3.925-931.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soergel M. E., Smith A. W., Schaffer F. L. Biophysical comparisons of calicivirus serotypes isolated from pinnipeds. Intervirology. 1975;5(3-4):239–244. doi: 10.1159/000149920. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA. II. poly(A) on intracellular RNAs. J Virol. 1975 Jun;15(6):1418–1431. doi: 10.1128/jvi.15.6.1418-1431.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Localization of the 26-S RNA sequence on the viral genome type 42-S RNA isolated from SFV-infected cells. Virology. 1976 Aug;73(1):190–199. doi: 10.1016/0042-6822(76)90073-8. [DOI] [PubMed] [Google Scholar]
- Wiegers U., Hilz H. A new method using 'proteinase K' to prevent mRNA degradation during isolation from HeLa cells. Biochem Biophys Res Commun. 1971 Jul 16;44(2):513–519. doi: 10.1016/0006-291x(71)90632-2. [DOI] [PubMed] [Google Scholar]


