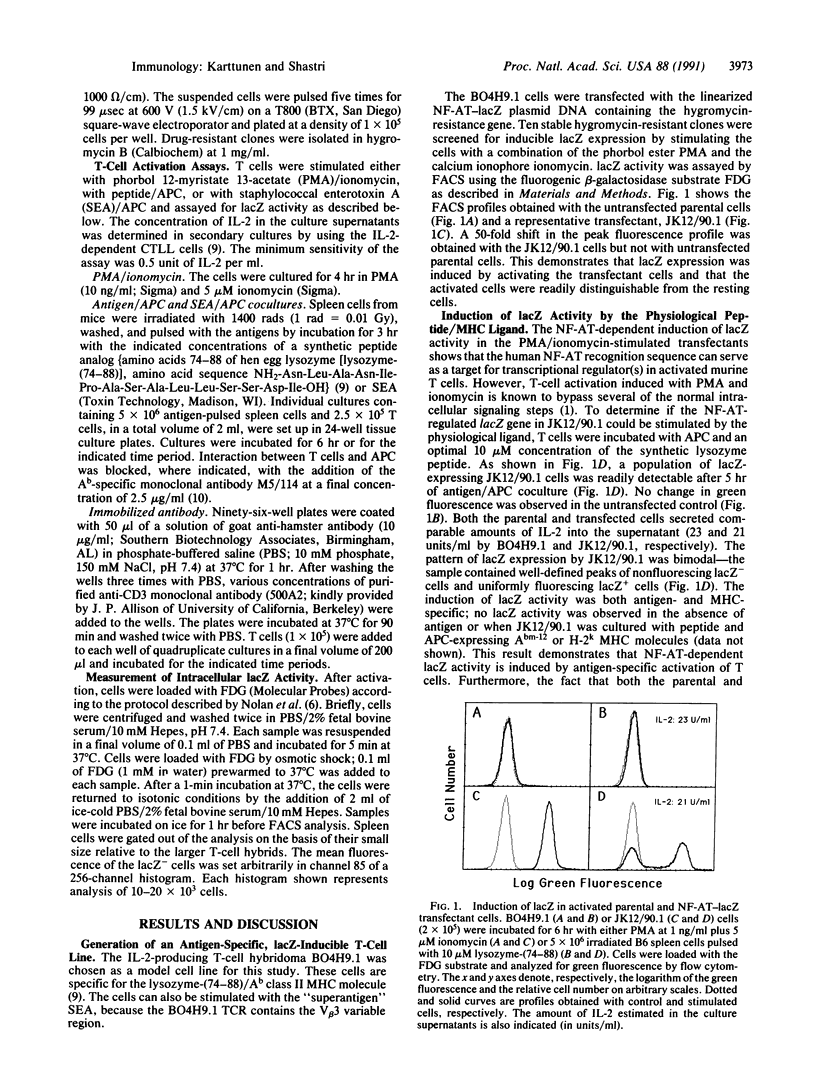
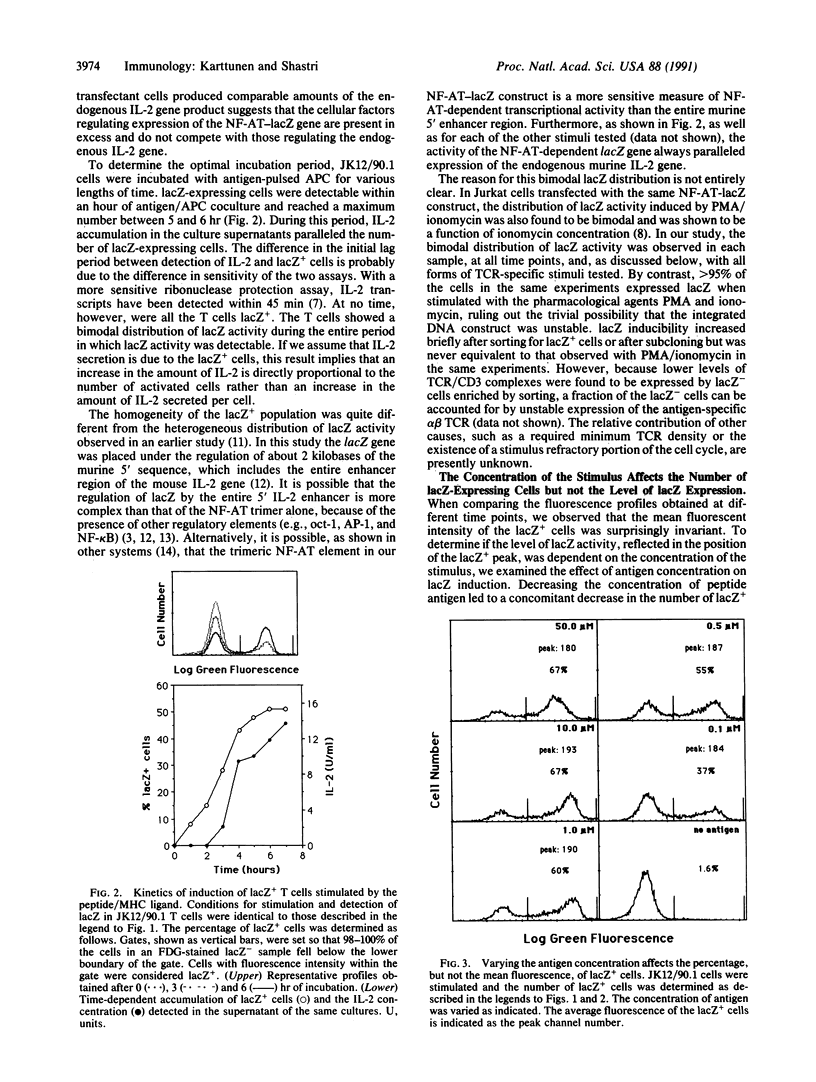
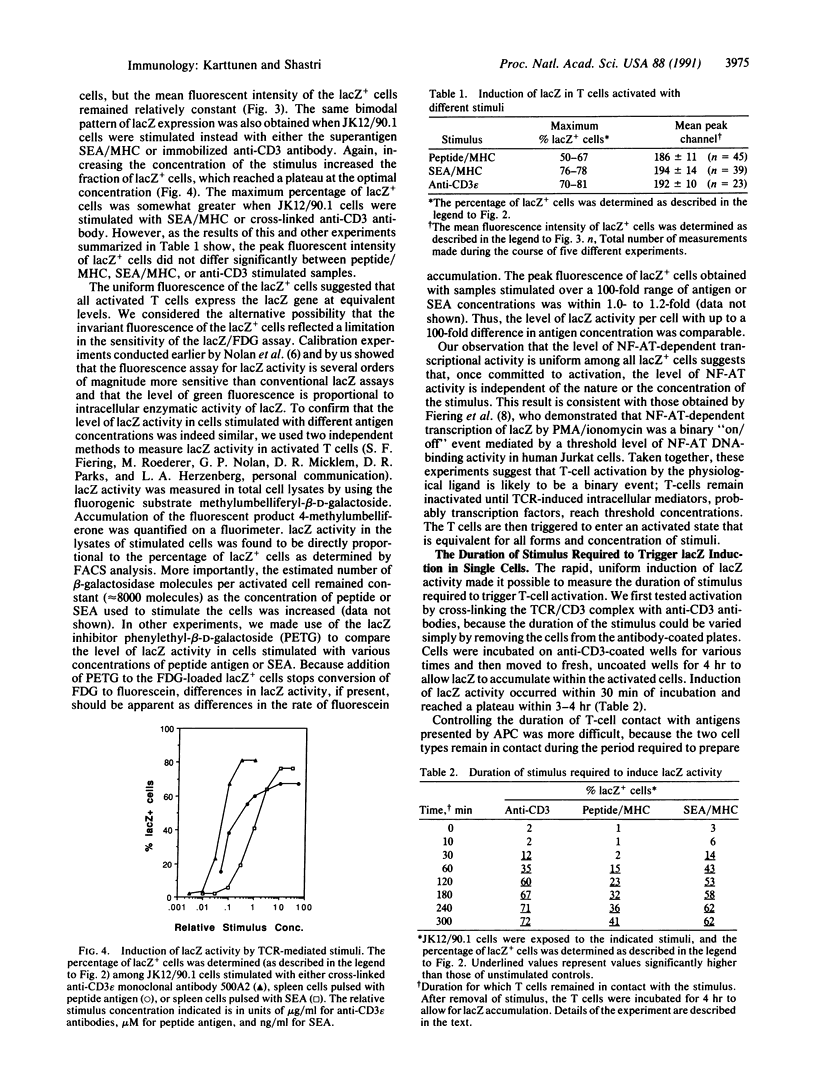
Abstract
We have used the bacterial beta-galactosidase gene (lacZ) as a reporter gene for the rapid measurement of T-cell antigen receptor (TCR)-mediated activation of individual T cells. The reporter construct contained the lacZ gene under the control of the nuclear factor of activated T cells (NF-AT) element of the human interleukin 2 enhancer [Fiering, S., Northrop, J. P., Nolan, G. P., Matilla, P., Crabtree, G. R. & Herzenberg, L. A. (1990) Genes Dev. 4, 1823-1834]. The activity of the intracellular lacZ enzyme was analyzed by flow cytometric measurement of fluorescein accumulation in cells loaded with the fluorogenic beta-galactosidase substrate fluorescein di-beta-D-galactopyranoside. As a model system, the T-cell hybridoma BO4H9.1, which is specific for the lysozyme peptide (amino acids 74-88)/Ab complex, was transfected with the NF-AT-lacZ construct. lacZ activity was induced in 50-100% of the transfectant cells following exposure to pharmacological agents, to the physiological peptide/major histocompatibility complex ligand, or to other TCR-specific stimuli. Interestingly, increasing concentrations of the stimulus increased the fraction of lacZ+ cells, but not the level of lacZ activity per cell. Even under widely varying levels of stimulus, the level of lacZ activity in individual lacZ+ cells remained within a remarkably narrow range. These results demonstrate that TCR-mediated activation can be readily measured in single T cells and strongly suggest that, once committed to activation, the level of NF-AT transcriptional activity in individual T cells is independent of the form or concentration of stimulus. This assay is likely to prove useful for the study of early activation events in individual T cells and of TCR ligands.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Hodes R. J. T-cell recognition of minor lymphocyte stimulating (Mls) gene products. Annu Rev Immunol. 1989;7:683–708. doi: 10.1146/annurev.iy.07.040189.003343. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Emilie D., Peuchmaur M., Barad M., Jouin H., Maillot M. C., Couez D., Nicolas J. F., Malissen B. Visualizing interleukin 2 gene expression at the single cell level. Eur J Immunol. 1989 Sep;19(9):1619–1624. doi: 10.1002/eji.1830190915. [DOI] [PubMed] [Google Scholar]
- Engel I., Hedrick S. M. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988 Aug 12;54(4):473–484. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- Fiering S., Northrop J. P., Nolan G. P., Mattila P. S., Crabtree G. R., Herzenberg L. A. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990 Oct;4(10):1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Tougne C., MacDonald H. R., Smith K. A., Nabholz M. Antigenic stimulation regulates the expression of IL 2 receptors in a cytolytic T lymphocyte clone. J Immunol. 1985 Feb;134(2):931–939. [PubMed] [Google Scholar]
- Milner S. M. Activation of mouse spleen cells by a single short pulse of mitogen. Nature. 1977 Aug 4;268(5619):441–442. doi: 10.1038/268441a0. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke A. M., Mescher M. F., Webb S. R. Activation of polyphosphoinositide hydrolysis in T cells by H-2 alloantigen but not MLS determinants. Science. 1990 Jul 13;249(4965):171–174. doi: 10.1126/science.2164711. [DOI] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Russell J. K., Pontzer C. H., Johnson H. M. The I-A beta b region (65-85) is a binding site for the superantigen, staphylococcal enterotoxin A. Biochem Biophys Res Commun. 1990 Apr 30;168(2):696–701. doi: 10.1016/0006-291x(90)92377-c. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri N., Gammon G., Miller A., Sercarz E. E. Ia molecule-associated selectivity in T cell recognition of a 23-amino-acid peptide of lysozyme. J Exp Med. 1986 Sep 1;164(3):882–896. doi: 10.1084/jem.164.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Sinha A. A., Lopez M. T., McDevitt H. O. Autoimmune diseases: the failure of self tolerance. Science. 1990 Jun 15;248(4961):1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Woodland D., Happ M. P., Bill J., Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990 Feb 23;247(4945):964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]



