Abstract
Mengovirus infection of Ehrlich ascites tumor cells caused a change of the intracellular ATP concentration. It increased by 35% within the first 3 h postinfection and then declined to zero within the next 5 h. The decrease in the ATP concentration was due, at least in part, to leakage of ATP into the medium, where it could be demonstrated by the luciferin-luciferase assay. Gross leakage of ATP was observed at 4.5 h postinfection, concomitant with the production of the first intracellular, infectious virus particles. A similar concentration decrease was detected for Mg2+, the polyamines, and K+, whereas an increase in the Na+ concentration was observed. The intracellular Mg2+ concentration varied synchronously with the ATP level, rising by 16% during the first 3 h postinfection and then progressively falling to lower values in the late period of the infectious cycle. After an initial slight enhancement, the putrescine, spermidine, and spermine concentrations declined at about 1.5 h postinfection. Wherease the intracellular K+ concentration increased by 17% during the first hour postinfection, the Na+ concentration diminished by the same value within the same time period, leaving the internal ionic strength unchanged early in infection. Three hours after the beginning of virus infection, there was a rapid decline of K+ and enhancement of Na+ within the cell. These alterations of the intracellular energetic and ionic conditions seem to be, at least in part, responsible for the cessation of virus-specific protein synthesis in mengovirus-infected Ehrlich ascites tumor cells commencing 3 to 3.5 h postinfection.
Full text
PDF

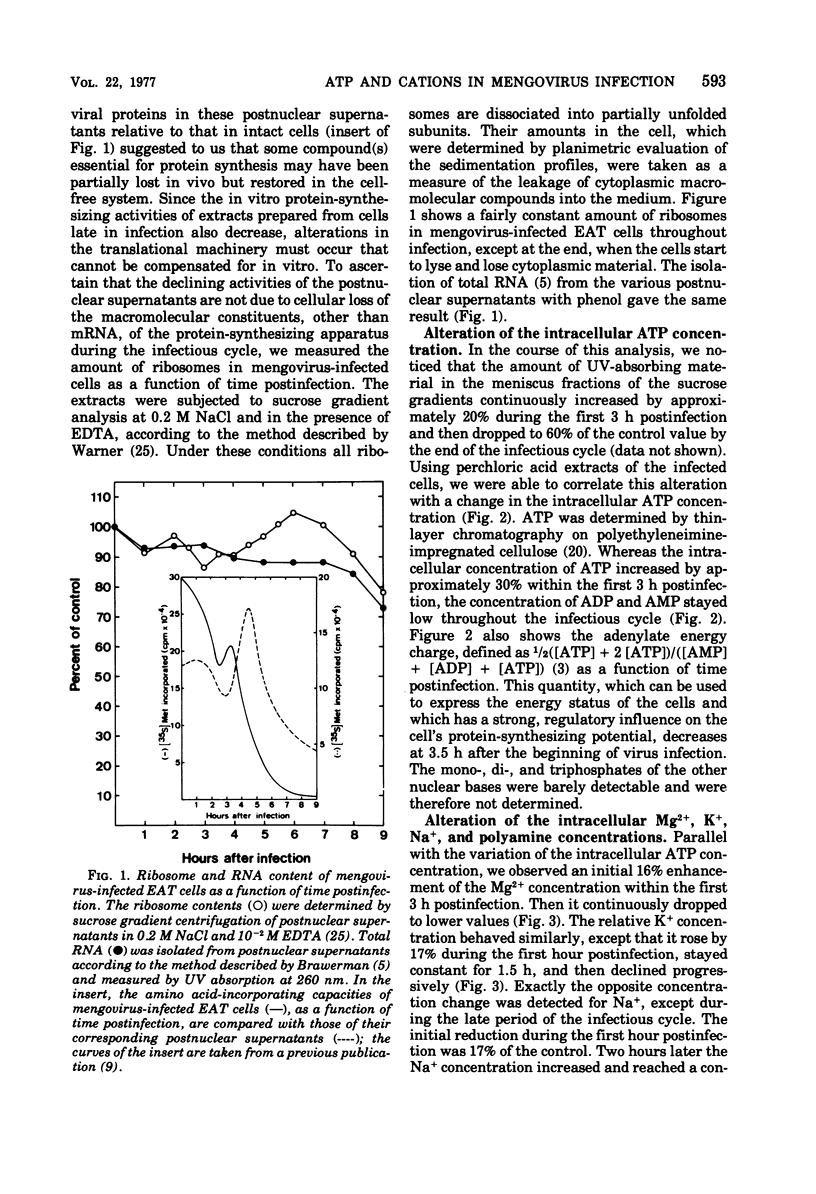


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Dales S. Cytopathology of Mengovirus infection. I. Relationship between cellular disintegration and virulence. Virology. 1967 Jun;32(2):184–200. doi: 10.1016/0042-6822(67)90269-3. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Lewis J. B., Anderson C. W., Gesteland R. F. Enhanced differential synthesis of proteins in a mammalian cell-free system by addition of polyamines. J Biol Chem. 1975 Jul 25;250(14):5688–5695. [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
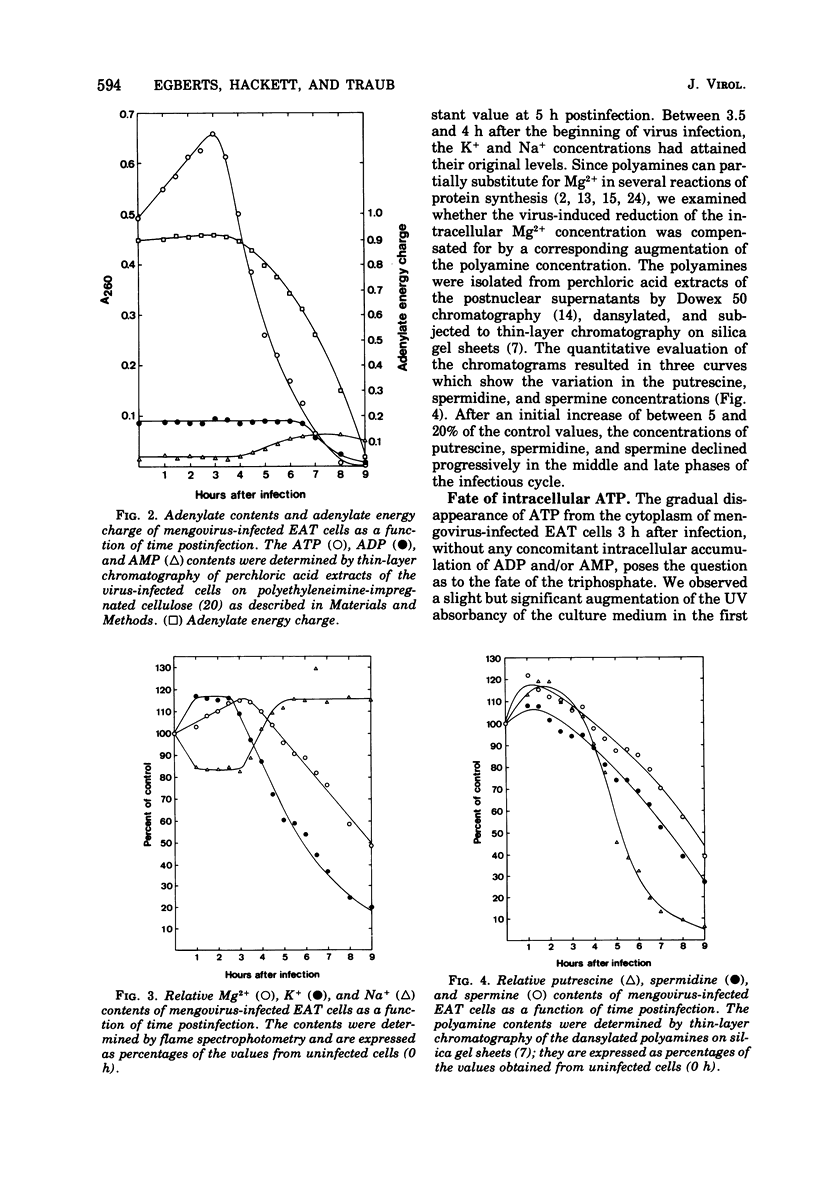
- Brawerman G. The isolation of messenger RNA from mammalian cells. Methods Enzymol. 1974;30:605–612. doi: 10.1016/0076-6879(74)30058-4. [DOI] [PubMed] [Google Scholar]
- Carrasco L., Smith A. E. Sodium ions and the shut-off of host cell protein synthesis by picornaviruses. Nature. 1976 Dec 23;264(5588):807–809. doi: 10.1038/264807a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. F. Hydrolytic enzymes in KB cells infected with poliovirus and herpes simplex virus. J Bacteriol. 1966 Feb;91(2):789–797. doi: 10.1128/jb.91.2.789-797.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotham-Iglewski B., Ludwig E. H. Effect of cortisone on activation of lysosomal enzymes resulting from mengovirus infection of L-929 cells. Biochem Biophys Res Commun. 1966 Jan 24;22(2):181–186. doi: 10.1016/0006-291x(66)90429-3. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hikami K., Sugawara K., Hirose S. Effect of polyamines on polypeptide synthesis in rat liver cell-free system. Biochim Biophys Acta. 1973 Mar 19;299(2):325–330. doi: 10.1016/0005-2787(73)90356-0. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Takahashi K., Hirose S. Necessity of polyamines for maximum isoleucyl-tRNA formation in a rat liver cell-free system. Biochem Biophys Res Commun. 1974 Sep 9;60(1):234–240. doi: 10.1016/0006-291x(74)90196-x. [DOI] [PubMed] [Google Scholar]
- Inoue H., Mizutani A. A new method for isolation of polyamines from animal tissues. Anal Biochem. 1973 Dec;56(2):408–416. doi: 10.1016/0003-2697(73)90206-6. [DOI] [PubMed] [Google Scholar]
- Konecki D., Kramer G., Pinphanichakarn P., Hardesty B. Polyamines are necessary for maximum in vitro synthesis of globin peptides and play a role in chain initiation. Arch Biochem Biophys. 1975 Jul;169(1):192–198. doi: 10.1016/0003-9861(75)90332-x. [DOI] [PubMed] [Google Scholar]
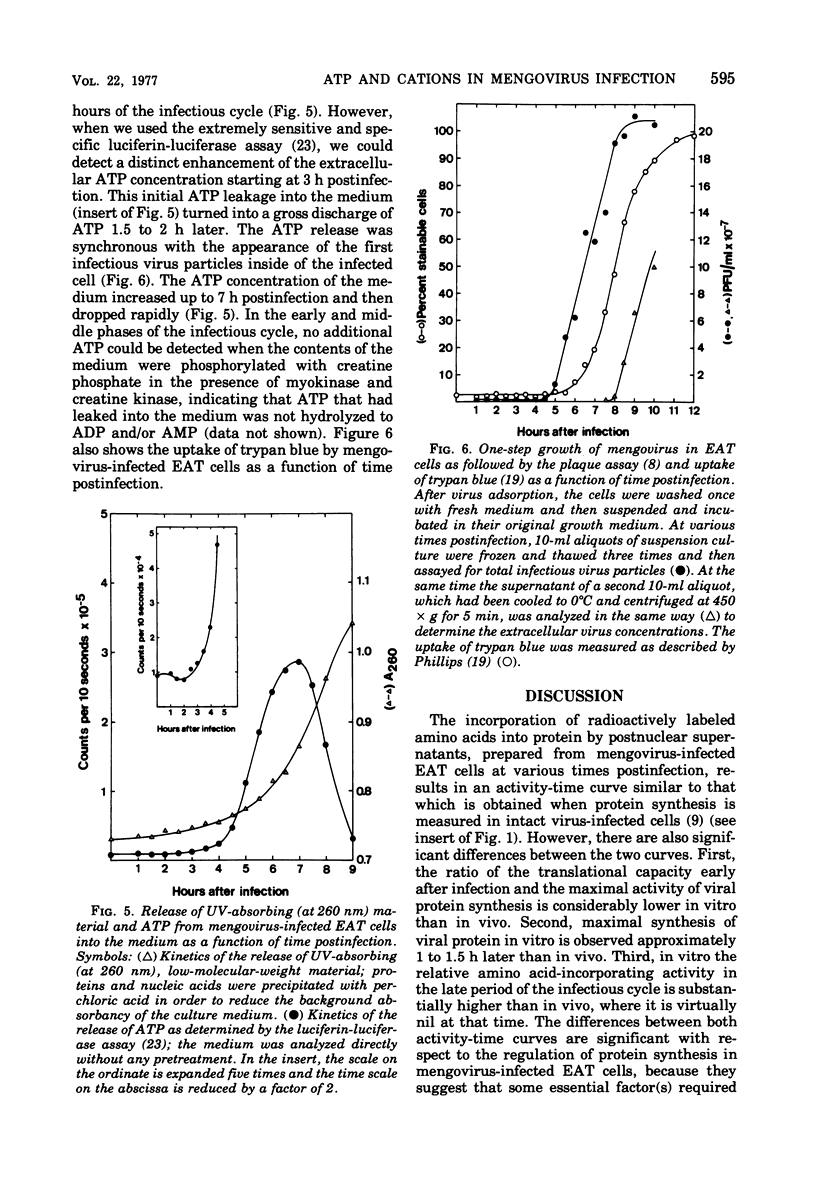
- Live T. R., Kaminskas E. Changes in adenylate energy charge in Ehrlich ascites tumor cells deprived of serum, glucose, or amino acids. J Biol Chem. 1975 Mar 10;250(5):1786–1789. [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Scheid A. S., Tamm I. Chemical and enzymatic characteristics of cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):30–38. doi: 10.1016/0042-6822(72)90235-8. [DOI] [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Tamm I. Incorporation of lipid precursors into cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):39–47. doi: 10.1016/0042-6822(72)90236-x. [DOI] [PubMed] [Google Scholar]
- Salden M., Bloemendal H. Polyamines can replace the dialyzable component from crude reticulocyte initiation factors. Biochem Biophys Res Commun. 1976 Jan 12;68(1):157–161. doi: 10.1016/0006-291x(76)90023-1. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr The decrease in size and synthetic activity of poliovirus polysomes late in the infectious cycle. Virology. 1967 Mar;31(3):427–435. doi: 10.1016/0042-6822(67)90222-x. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- WOLFF D. A., BUBEL H. C. THE DISPOSITION OF LYSOSOMAL ENZYMES AS RELATED TO SPECIFIC VIRAL CYTOPATHIC EFFECTS. Virology. 1964 Nov;24:502–505. doi: 10.1016/0042-6822(64)90196-5. [DOI] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]



