Abstract
The lck gene encodes a membrane-associated protein tyrosine kinase (p56lck) that is believed to participate in lymphocyte-specific signal transduction pathways. To investigate the function of this molecule, transgenic mice were generated carrying the wild-type lck gene or a mutated lck gene encoding a constitutively activated form of p56lck (p56lckF505). Transgene expression in thymocytes was achieved in each case using the lck proximal promoter element. Mice expressing high levels of either p56lckF505 or p56lckY505 reproducibly developed thymic tumors. The sensitivity of thymocytes to p56lck-induced transformation suggests that disturbances in lck expression may contribute to the pathogenesis of some human neoplastic diseases.
Full text
PDF

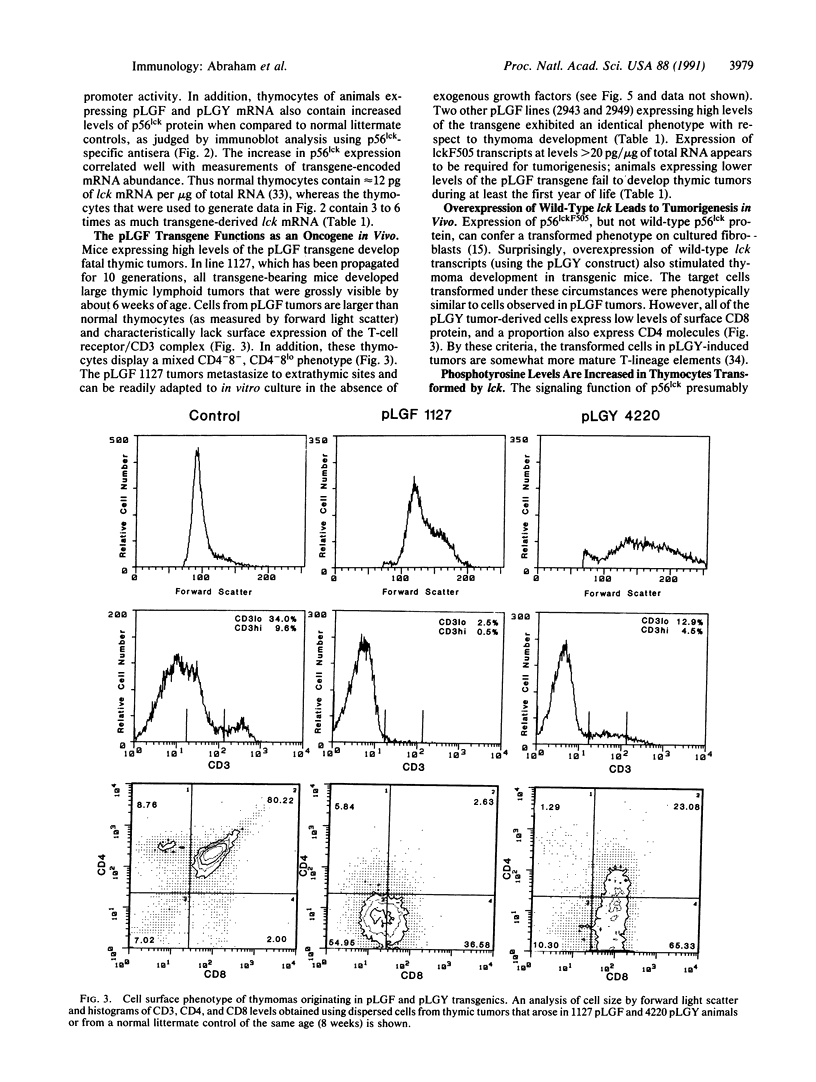
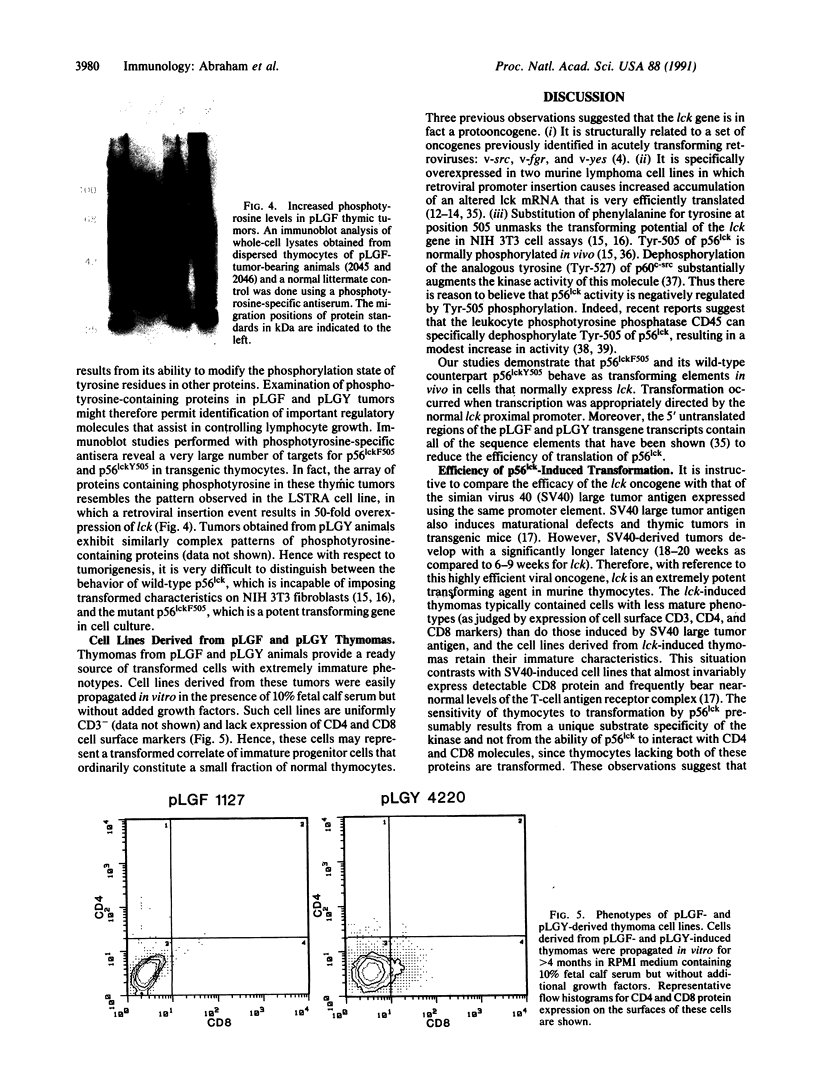

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. T., Reynolds P. J., Kelley C. M., Sefton B. M. Transcriptional activation of lck by retrovirus promoter insertion between two lymphoid-specific promoters. J Virol. 1988 Nov;62(11):4113–4122. doi: 10.1128/jvi.62.11.4113-4122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin K. E., Beals C. R., Wilkie T. M., Forbush K. A., Simon M. I., Perlmutter R. M. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 1990 Dec;9(12):3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- Garvin A. M., Abraham K. M., Forbush K. A., Farr A. G., Davison B. L., Perlmutter R. M. Disruption of thymocyte development and lymphomagenesis induced by SV40 T-antigen. Int Immunol. 1990;2(2):173–180. doi: 10.1093/intimm/2.2.173. [DOI] [PubMed] [Google Scholar]
- Garvin A. M., Pawar S., Marth J. D., Perlmutter R. M. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol Cell Biol. 1988 Aug;8(8):3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L., Poenie M., Kimura J., Tsien R., Weiss A., Allison J. P. Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature. 1987 Nov 12;330(6144):170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hurley T. R., Sefton B. M. Analysis of the activity and phosphorylation of the lck protein in lymphoid cells. Oncogene. 1989 Mar;4(3):265–272. [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Forbush K. A., Carpenter J., Sato T. A., Grossman A., Liggitt D. H., Perlmutter R. M. Interleukin 4 expressed in situ selectively alters thymocyte development. J Exp Med. 1991 Jan 1;173(1):89–100. doi: 10.1084/jem.173.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie R. R., King C. S., MacAuley A., Marth J. D., Perlmutter R. M., Eckhart W., Cooper J. A. p56lck protein-tyrosine kinase is cytoskeletal and does not bind to polyomavirus middle T antigen. J Virol. 1988 Dec;62(12):4673–4679. doi: 10.1128/jvi.62.12.4673-4679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Disteche C., Pravtcheva D., Ruddle F., Krebs E. G., Perlmutter R. M. Localization of a lymphocyte-specific protein tyrosine kinase gene (lck) at a site of frequent chromosomal abnormalities in human lymphomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7400–7404. doi: 10.1073/pnas.83.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Lewis D. B., Wilson C. B., Gearn M. E., Krebs E. G., Perlmutter R. M. Regulation of pp56lck during T-cell activation: functional implications for the src-like protein tyrosine kinases. EMBO J. 1987 Sep;6(9):2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Overell R. W., Meier K. E., Krebs E. G., Perlmutter R. M. Translational activation of the lck proto-oncogene. Nature. 1988 Mar 10;332(6160):171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Shackelford D. A., Hurley T. R., Johnson P., Hyman R., Sefton B. M., Trowbridge I. S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Lewis D. B., Peet R., Ziegler S. F., Wilson C. B. Structure and expression of lck transcripts in human lymphoid cells. J Cell Biochem. 1988 Oct;38(2):117–126. doi: 10.1002/jcb.240380206. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Reynolds P. J., Lesley J., Trotter J., Schulte R., Hyman R., Sefton B. M. Changes in the relative abundance of type I and type II lck mRNA transcripts suggest differential promoter usage during T-cell development. Mol Cell Biol. 1990 Aug;10(8):4266–4270. doi: 10.1128/mcb.10.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor O., Gregory F. S., Templeton N. S., Pawar S., Perlmutter R. M., Rosen N. Selective expression of alternative lck mRNAs in human malignant cell lines. Mol Cell Biol. 1989 Jul;9(7):2983–2988. doi: 10.1128/mcb.9.7.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H. The human growth hormone gene family: nucleotide sequences show recent divergence and predict a new polypeptide hormone. DNA. 1982;1(3):239–249. doi: 10.1089/dna.1.1982.1.239. [DOI] [PubMed] [Google Scholar]
- Shaw A. S., Amrein K. E., Hammond C., Stern D. F., Sefton B. M., Rose J. K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989 Nov 17;59(4):627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Garvin A. M., Forbush K. A., Carlow D. A., Davis C. B., Littman D. R., Perlmutter R. M. Participation of CD4 coreceptor molecules in T-cell repertoire selection. Nature. 1991 Jan 17;349(6306):241–243. doi: 10.1038/349241a0. [DOI] [PubMed] [Google Scholar]
- Turner J. M., Brodsky M. H., Irving B. A., Levin S. D., Perlmutter R. M., Littman D. R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990 Mar 9;60(5):755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Wildin R. S., Garvin A. M., Pawar S., Lewis D. B., Abraham K. M., Forbush K. A., Ziegler S. F., Allen J. M., Perlmutter R. M. Developmental regulation of lck gene expression in T lymphocytes. J Exp Med. 1991 Feb 1;173(2):383–393. doi: 10.1084/jem.173.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willman C. L., Stewart C. C., Longacre T. L., Head D. R., Habbersett R., Ziegler S. F., Perlmutter R. M. Expression of the c-fgr and hck protein-tyrosine kinases in acute myeloid leukemic blasts is associated with early commitment and differentiation events in the monocytic and granulocytic lineages. Blood. 1991 Feb 15;77(4):726–734. [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]