Abstract
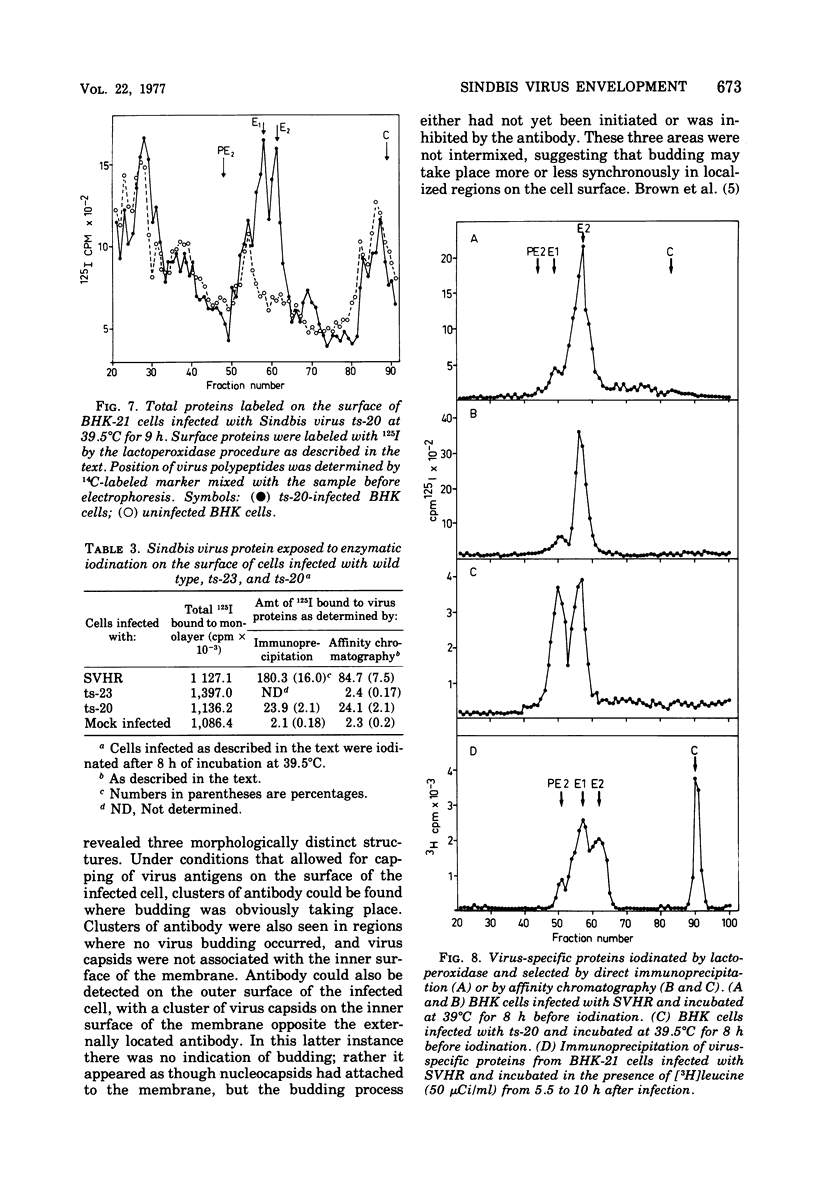
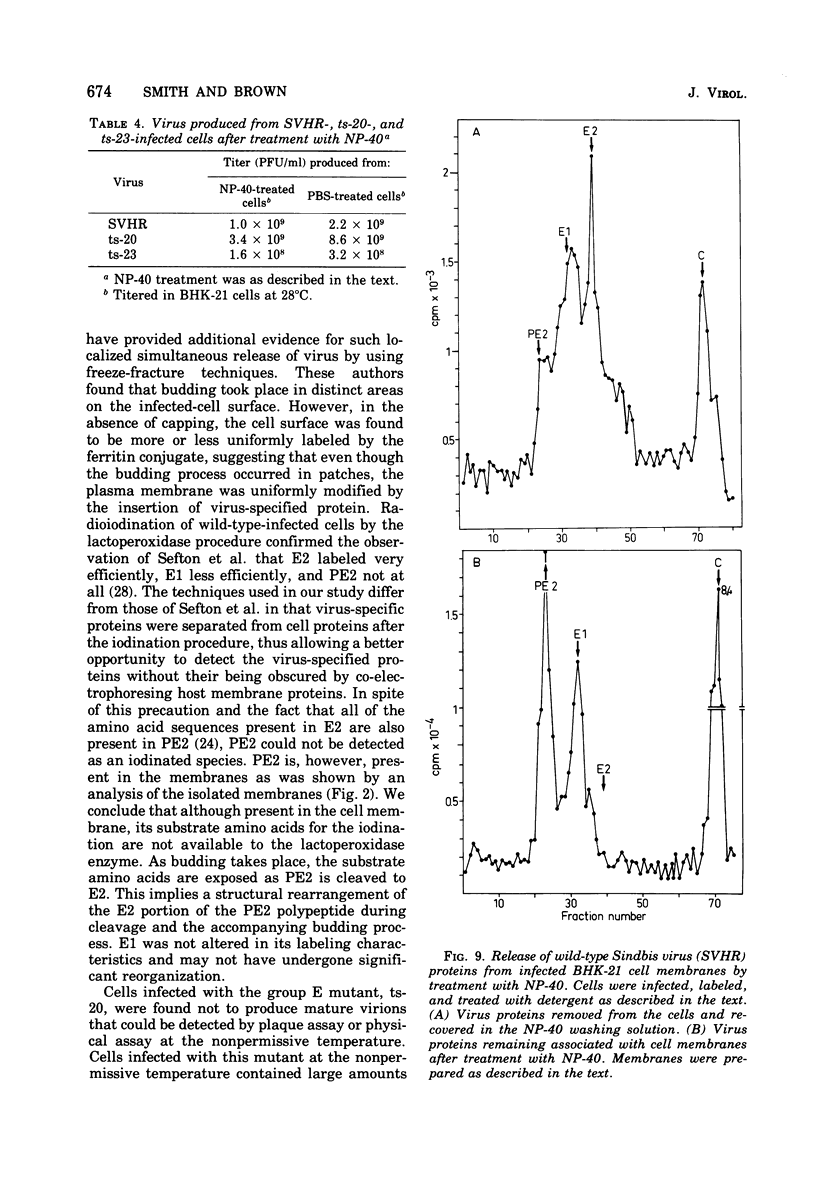
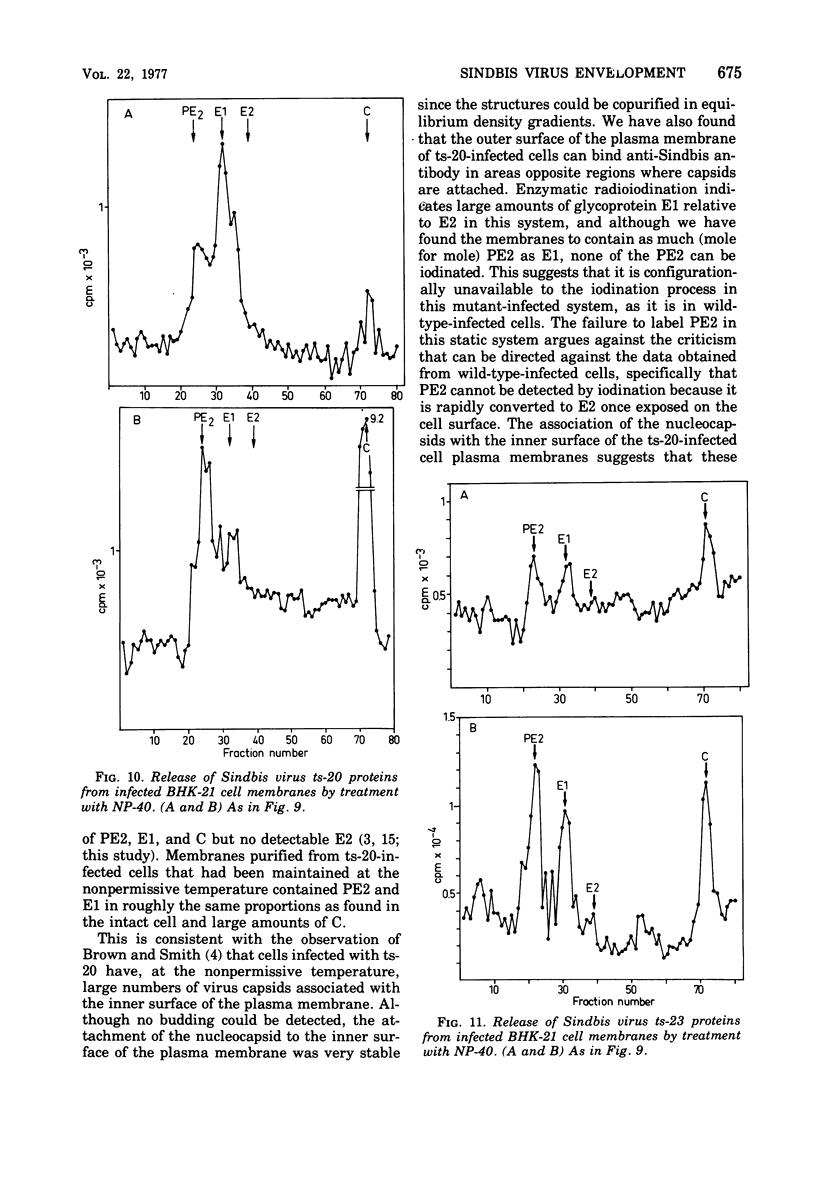
The synthesis and organization of Sindbis virus structural proteins was investigated in BHK cells infected with wild-type virus (SVHR) or temperature-sensitive (ts) mutants defective in maturation. Cells infected with ts-23 or ts-20 (complementation groups D and E) were similar in the polypeptides synthesized at the nonpermissive temperature and differed from SVHR-infected cells in that the envelope protein E2 was not cleaved from the PE2 precursor. Data from experiments utilizing pulse-chase procedures or protein synthesis inhibitors indicated that although infectious virions were released from cells infected with these mutants in shift-down experiments, the particles were produced almost exclusively from proteins synthesized after the return to permissive temperature. This suggests that a stable complex may be formed among the structural proteins before budding. A membrane fraction isolated from cells infected with either ts mutants or SVHR contained the PE2, E1, and C polypeptides, whereas E2 was restricted to fractions obtained from SVHR-infected cells. Although equivalent amounts of virus-specific protein were synthesized in cells infected with either mutant and the cells contained qualitatively the same proteins in the isolated membranes, cells infected with ts-23 did not have virus-specific proteins exposed on their surface that could be detected by ferritin-conjugated antibody-labeling procedures or lactoperoxidase-mediated iodination. In contrast, ts-20-infected cells had significant amounts of viral protein, mainly E1, that could be detected on the plasma membrane by either procedure. Iodine was incorporated into E1 and E2 on the surface of SVHR-infected cells in the same relative amounts as seen in iodinated virions. PE2, however, although present in membranes, could not be iodinated on the surface of infected cells under any of the conditions used in this study. We also monitored the relative efficiency with which these viral proteins could be removed from intact cells by dilute solutions of nonionic detergents. The results indicated that E2 was most efficiently removed, followed by E1. PE2 (the precursor to E2) and C remained associated with the cell and could be subsequently isolated in the membrane fraction.
Full text
PDF



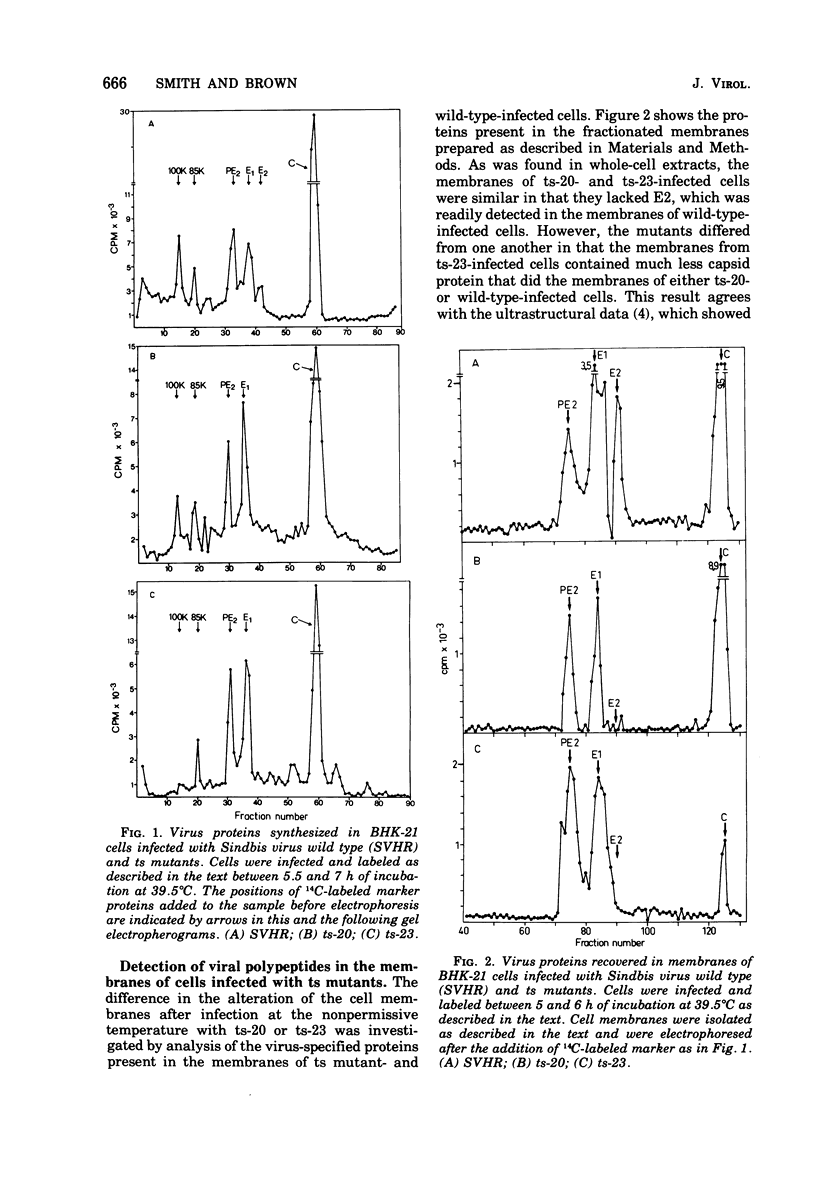
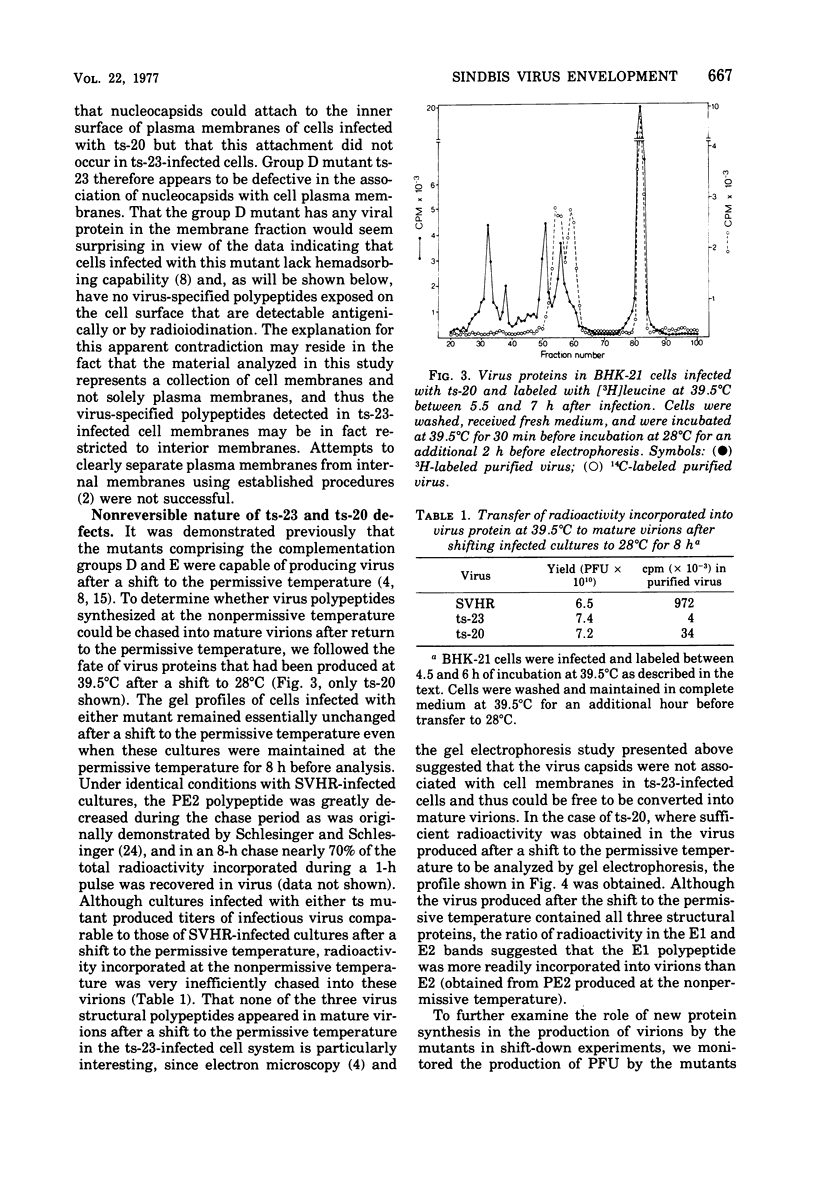
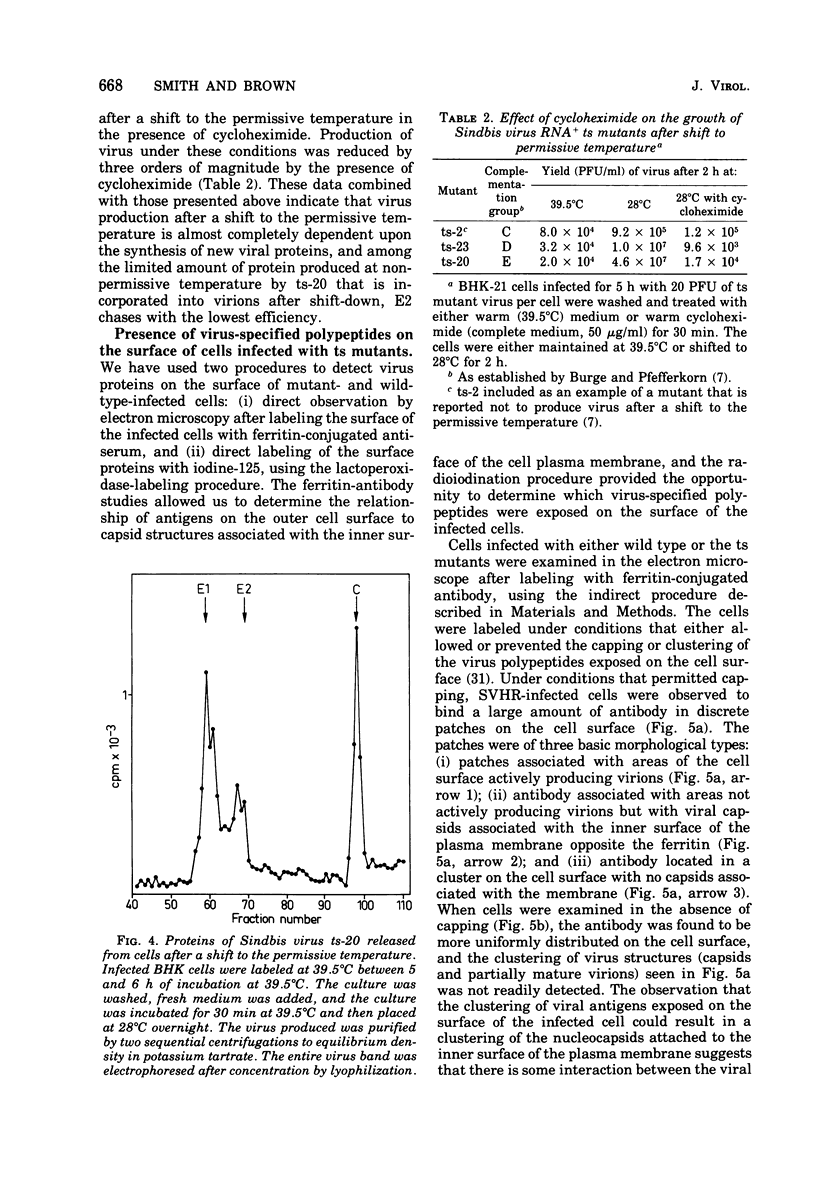





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdwell C. R., Strauss J. H. Agglutination of Sindbis virus and of cells infected with Sindbis virus by plant lectins. J Virol. 1973 Apr;11(4):502–507. doi: 10.1128/jvi.11.4.502-507.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
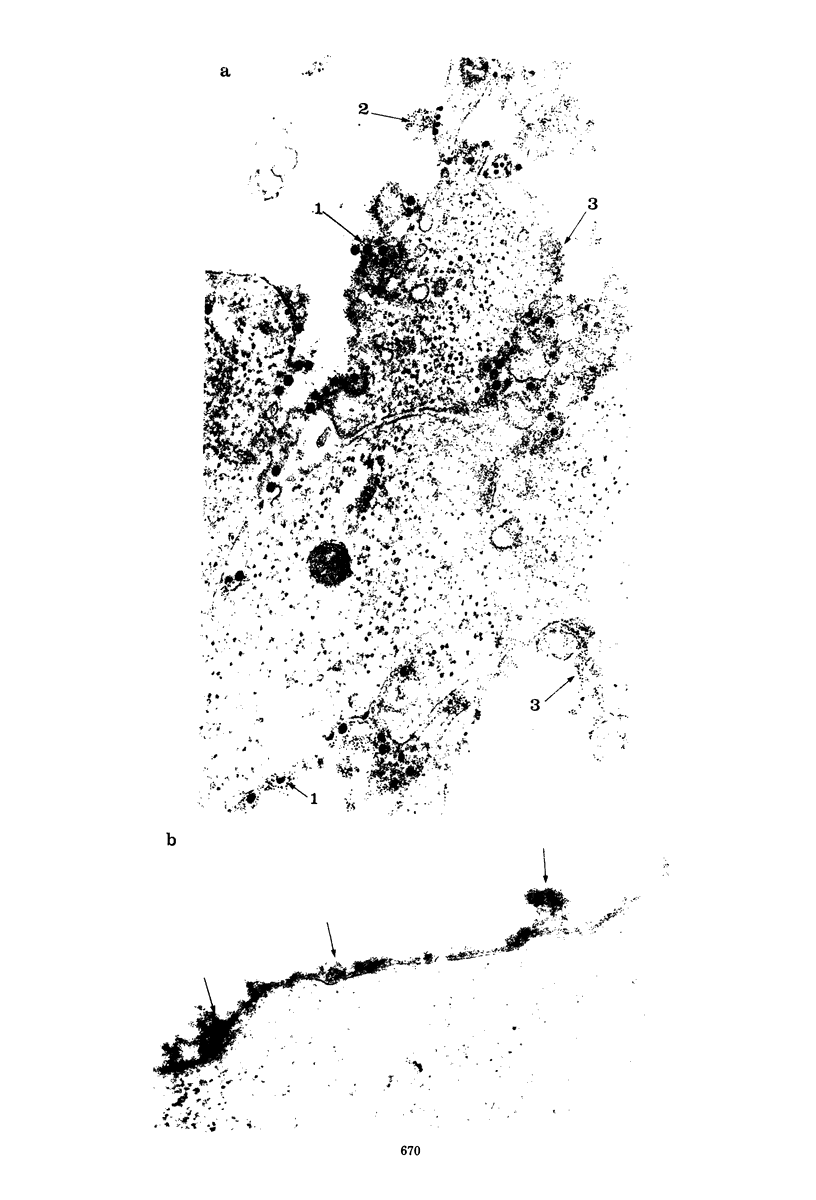
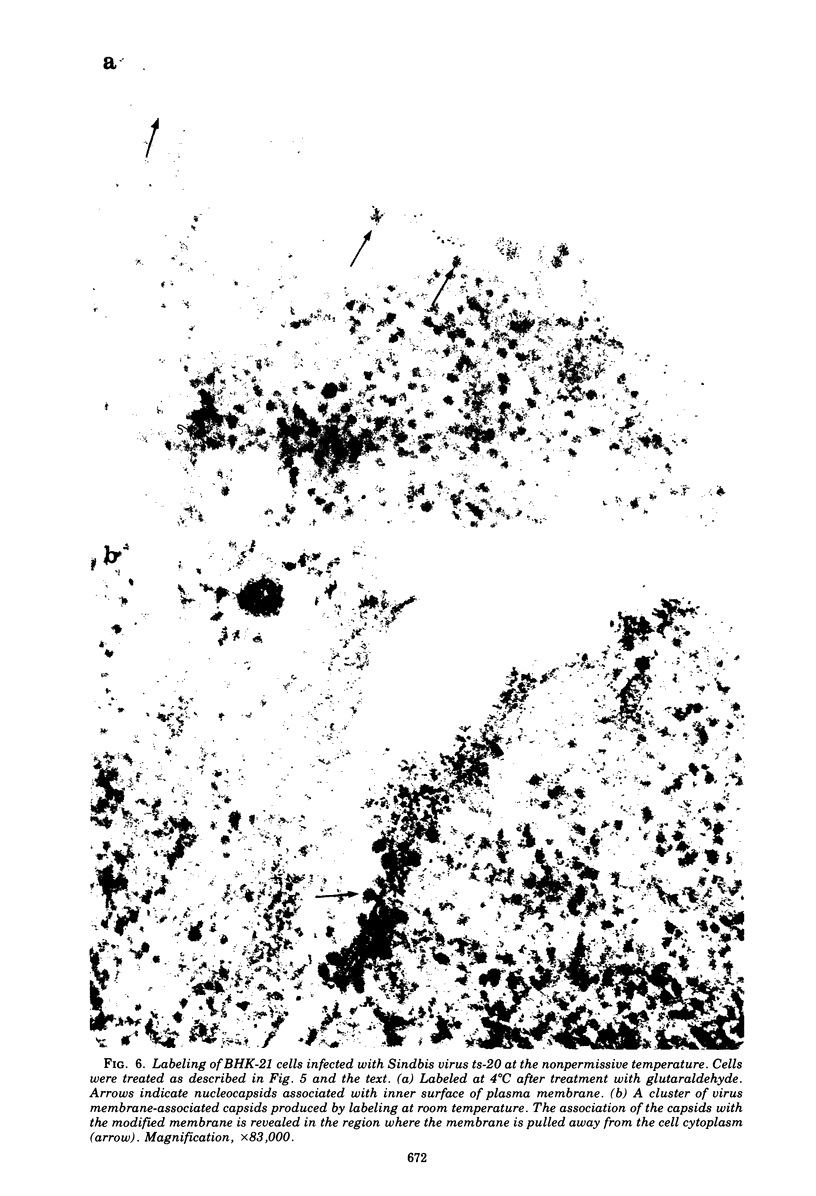
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J., Burge B. W. Modification of Sindbis virus glycoprotein by host-specified glycosyl transferases. J Virol. 1971 Mar;7(3):309–313. doi: 10.1128/jvi.7.3.309-313.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G. Early synthesis of Semliki Forest virus-specific proteins in infected chicken cells. J Virol. 1976 Jul;19(1):1–12. doi: 10.1128/jvi.19.1.1-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. THE ORIGIN OF THE PROTEIN OF SINDBIS VIRUS. Virology. 1964 Jun;23:217–223. doi: 10.1016/0042-6822(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Seaver J. Non-lytic, non-ionic detergent extraction of plasma membrane constituents from normal and transformed fibroblasts. Biochim Biophys Acta. 1976 Apr 5;426(4):589–597. doi: 10.1016/0005-2736(76)90123-1. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Virus-specific proteins synthesized in cells infected with RNA+ temperature-sensitive mutants of Sindbis virus. J Virol. 1970 Mar;5(3):329–337. doi: 10.1128/jvi.5.3.329-337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Gaffney B. J. Effect of the viral proteins on the fluidity of the membrane lipids in Sindbis virus. J Mol Biol. 1974 Dec 5;90(2):343–358. doi: 10.1016/0022-2836(74)90378-7. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Simons K., Helenius A., Garoff H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J Mol Biol. 1973 Oct 15;80(1):119–133. doi: 10.1016/0022-2836(73)90236-2. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]