Abstract
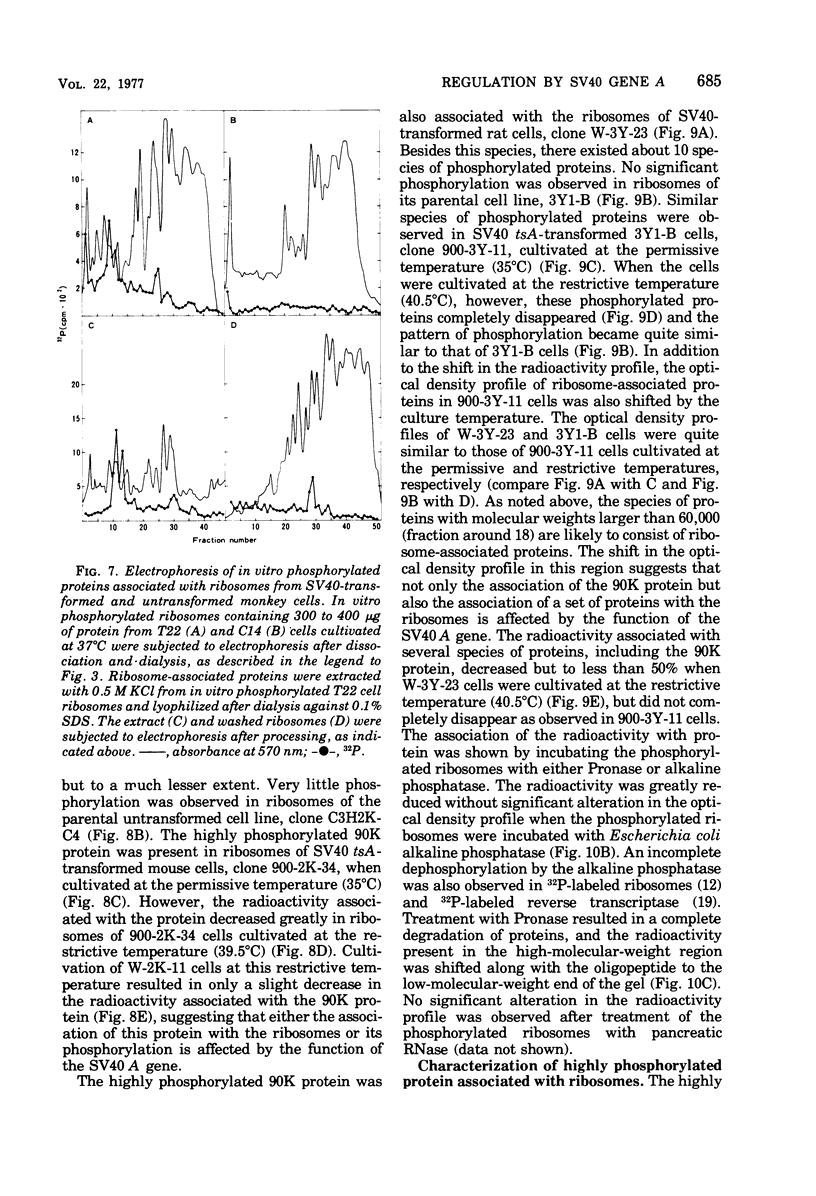
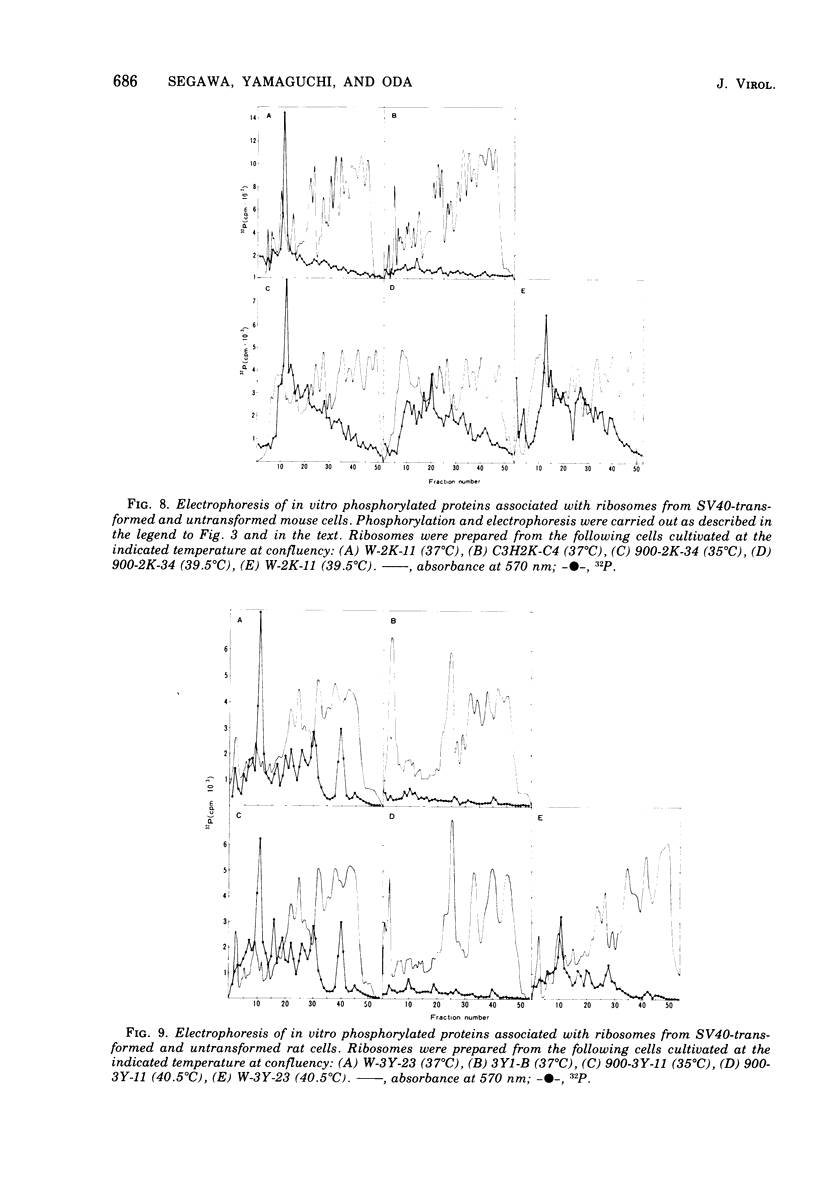
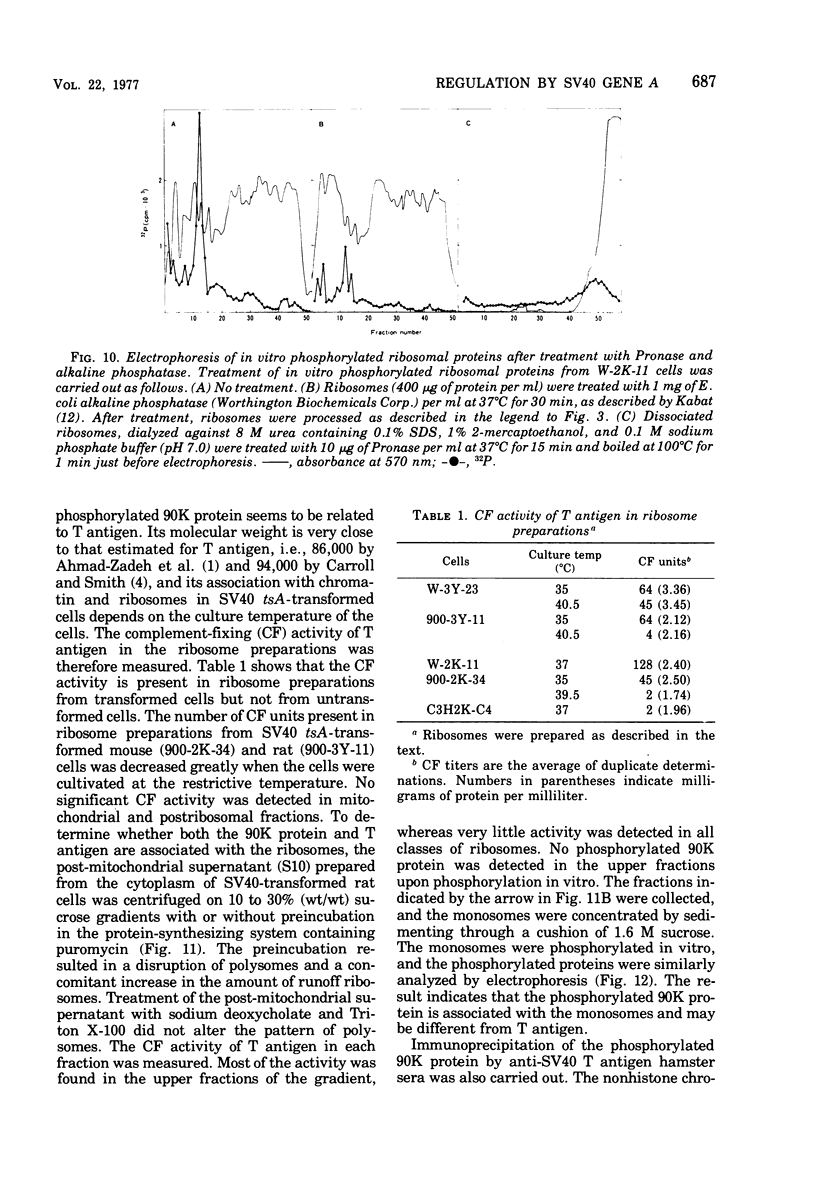
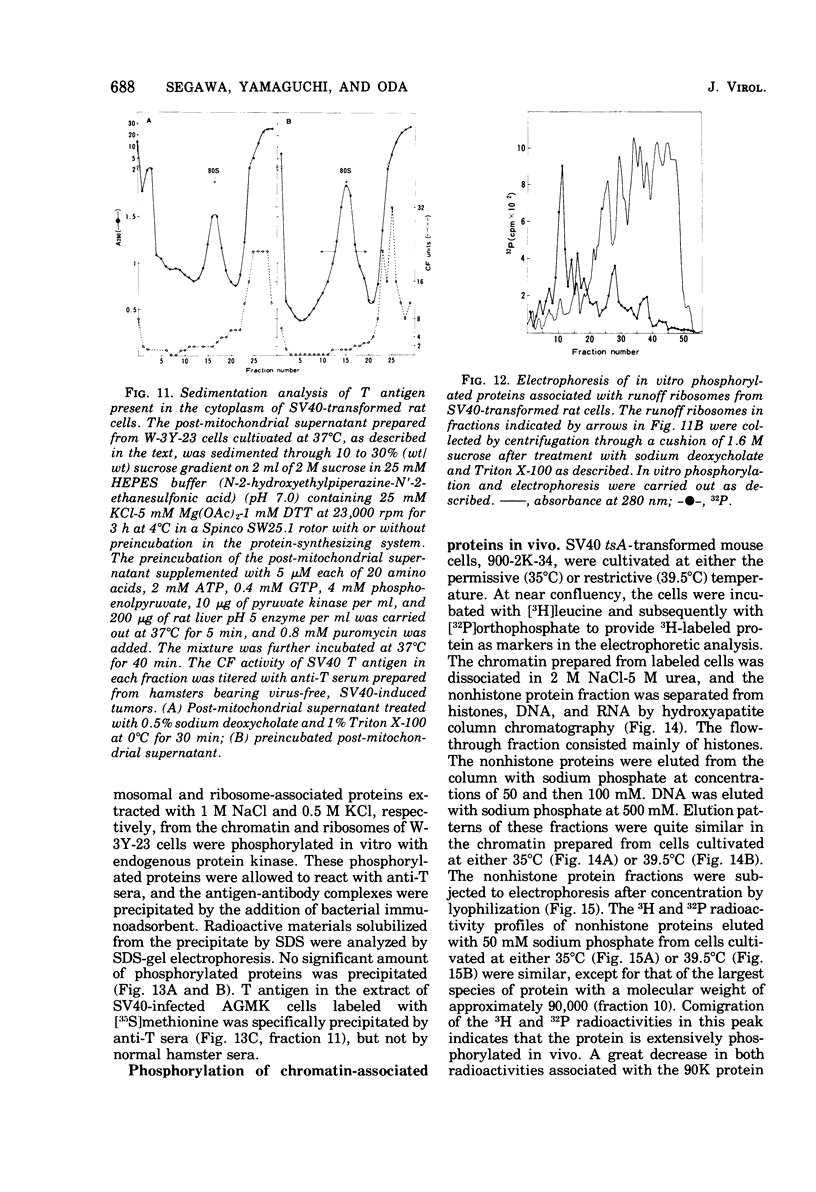
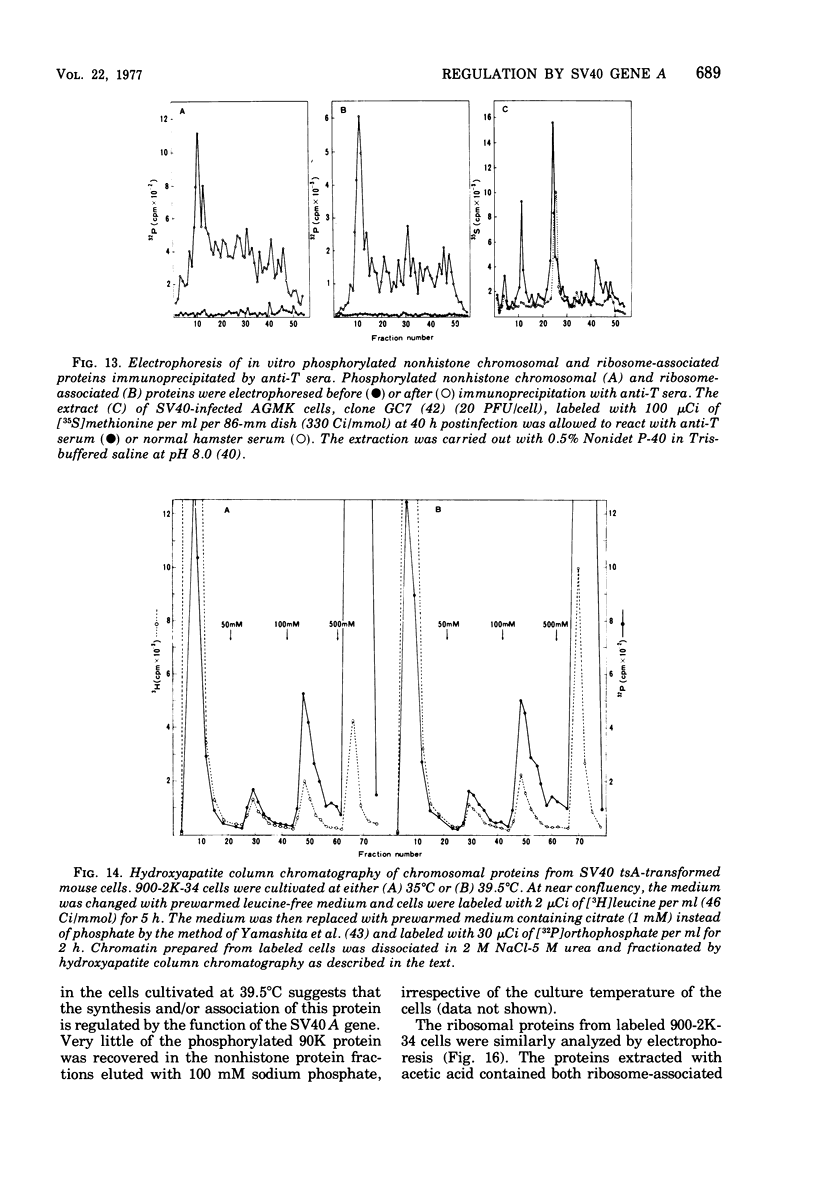
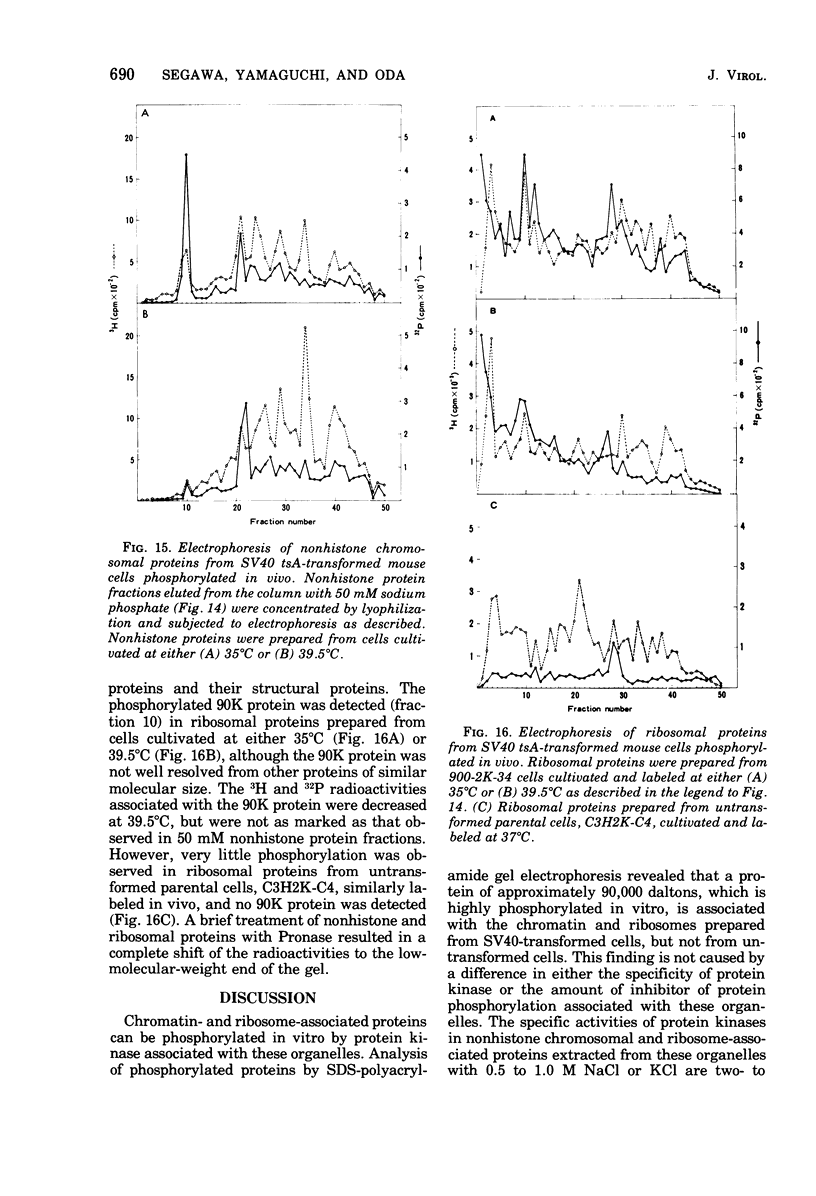
The species of proteins associated with chromatin and ribosomes of simian virus 40 (SV40)-transformed and untransformed monkey, mouse, and rat cells have been compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis after phosphorylation of these proteins either in vivo or in vitro. In vitro phosphorylation was carried out by protein kinase associated with these organelles and [γ-32 P]ATP as the phosphoryl donor. The reaction products contained both phosphoserine and phosphothreonine in approximately equal amounts. The electrophoretic analysis of the phosphorylated proteins revealed that the highly phosphorylated protein with a molecular weight of approximately 90,000 (90K protein) was associated with chromatin and ribosomes from transformed cells but not from untransformed cells. The 90K protein could be extracted from chromatin and ribosomes with 0.5 to 1.0 M NaCl or KCl. The 90K protein was still associated with the runoff ribosomes prepared by the puromycin reaction of the post-mitochondrial supernatant in the protein-synthesizing system. In vitro phosphorylation of chromatin and ribosomes from SV40 tsA-transformed mouse and rat cells indicated that the amounts of 90K protein associated with these organelles decreased greatly when the cells were cultivated at the restrictive temperature. A similar temperature-dependent decrease in the amount of 32P-labeled 90K protein was observed in nonhistone chromosomal and ribosome-associated protein fractions prepared from SV40 tsA-transformed cells labeled with [3H]leucine and [32P]orthophosphate in vivo. In vitro phosphorylated 90K protein in nonhistone chromosomal and ribosome-associated proteins extracted with high salt was not immunoprecipitated with anti-SV40 T sera.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Allet B., Greenblatt J., Weil R. Two forms of simian-virus-40-specific T-antigen in abortive and lytic infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1097–1101. doi: 10.1073/pnas.73.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eil C., Wool I. G. Phosphorylation of rat liver ribosomal subunits: partial purification of two cyclic AMP activated protein kinases. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1001–1009. doi: 10.1016/0006-291x(71)90561-4. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Sierra J. M., Nombela C., Ochoa S., Merrick W. C., Anderson W. F. Polypeptide chain initiation in eukaryotes: initiation factor requirements for translation of natural messengers. Proc Natl Acad Sci U S A. 1976 Jan;73(1):44–48. doi: 10.1073/pnas.73.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Iida H., Oda K. Stimulation of non-histone chromosomal protein synthesis in simian virus 40-infected simian cells. J Virol. 1975 Mar;15(3):471–478. doi: 10.1128/jvi.15.3.471-478.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issinger O. G., Benne R., Hershey J. W., Traut R. R. Phosphorylation in vitro of eukaryotic initiation factors IF-E2 and IF-E3 by protein kinases. J Biol Chem. 1976 Oct 25;251(20):6471–6474. [PubMed] [Google Scholar]
- Jergil B. Protein kinase from rainbow-trout-testis ribosomes. Partial purification and characterization. Eur J Biochem. 1972 Aug 4;28(4):546–554. doi: 10.1111/j.1432-1033.1972.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Kabat D. Turnover of phosphoryl groups in reticulocyte ribosomal phosphoproteins. J Biol Chem. 1972 Sep 10;247(17):5338–5344. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Kleinsmith L. J. Nuclear protein kinases. Evidence for their heterogeneity, tissue specificity, substrate specificities, and differential responses to cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1974 Feb 10;249(3):750–760. [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino T., Yamaguchi N. Characterization of T antigen in cells infected with a temperature-sensitive mutant of simian virus 40. J Virol. 1975 Jun;15(6):1302–1307. doi: 10.1128/jvi.15.6.1302-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. G., Miceli M. V., Jungmann R. A., Hung P. P. Protein kinase and its regulatory effect on reverse transcriptase activity of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2945–2949. doi: 10.1073/pnas.72.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Ranu R. S., Ernst V., Fifer M. A., London L. M. Association of a cyclic AMP-dependent protein kinase with a purified translational inhibitor isolated from hemin-deficient rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4849–4853. doi: 10.1073/pnas.72.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. C., Amos H. Alteration of phosphorylation of ribosomal proteins as a function of variation of growth conditions of primary cells. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1398–1407. doi: 10.1016/0006-291x(71)90176-8. [DOI] [PubMed] [Google Scholar]
- MacGillivray A. J., Carroll Dana, Paul J. The heterogeneity of the non-histone chromatin proteins from mouse tissues. FEBS Lett. 1971 Mar 16;13(4):204–208. doi: 10.1016/0014-5793(71)80536-7. [DOI] [PubMed] [Google Scholar]
- Macgillivray A. J., Paul J., Threlfall G. Transcriptional regulation in eukaryotic cells. Adv Cancer Res. 1972;15:93–162. doi: 10.1016/s0065-230x(08)60373-5. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Ishitsuka H., Oda K. An SV40-induced initiation factor for protein synthesis concerned with the regulation of permissiveness. Nature. 1974 Dec 20;252(5485):649–653. doi: 10.1038/252649a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Honjo T., Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968 Jul 10;243(13):3765–3767. [PubMed] [Google Scholar]
- Osborn M., Weber K. SV40: T antigen, the A function and transformation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):267–276. doi: 10.1101/sqb.1974.039.01.035. [DOI] [PubMed] [Google Scholar]
- Pestana A., Pitot H. C. Acetylation of ribosome-associated proteins in vitro by an acetyltransferanse bound to rat liver ribosomes. Biochemistry. 1975 Apr 8;14(7):1397–1403. doi: 10.1021/bi00678a009. [DOI] [PubMed] [Google Scholar]
- Quirin-Stricker C., Schmitt M., Egly J. M., Kempf J. A plasmocytoma ribosome-associated protein kinase which phosphorylates a specific protein of the ribosomal KCl wash. Eur J Biochem. 1976 Feb 2;62(1):199–209. doi: 10.1111/j.1432-1033.1976.tb10114.x. [DOI] [PubMed] [Google Scholar]
- Racey L. A., Byvoet P. Histone acetyltransferase in chromatin. Evidence for in vitro enzymatic transfer of acetate from acetyl-coenzyme A to histones. Exp Cell Res. 1971 Feb;64(2):366–370. doi: 10.1016/0014-4827(71)90089-9. [DOI] [PubMed] [Google Scholar]
- Roberts J. H., Stard P., Giri C. P., Smulson M. Cytoplasmic poly(ADP-ribose) polymerase during the HeLa cell cycle. Arch Biochem Biophys. 1975 Nov;171(1):305–315. doi: 10.1016/0003-9861(75)90037-5. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Rupp R. G., Humphrey R. M., Shaeffer J. R. Phosphorylation of ribosome-associated proteins during the mammalian cell cycle. Unique phosphorylation of a specific protein during mitosis. Biochim Biophys Acta. 1976 Jan 5;418(1):81–92. doi: 10.1016/0005-2787(76)90329-4. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Transformation of green monkey kidney cells by SV40 genome: the establishment of transformed cell lines and the replication of human adenoviruses and SV40 in transformed cells. Virology. 1971 Jul;45(1):163–171. doi: 10.1016/0042-6822(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Sugimura T. Poly(adenosine diphosphate ribose). Prog Nucleic Acid Res Mol Biol. 1973;13:127–151. doi: 10.1016/s0079-6603(08)60102-6. [DOI] [PubMed] [Google Scholar]
- Takeda M., Yamamura H., Oga Y. Phosphoprotein kinases associated with rat liver chromatin. Biochem Biophys Res Commun. 1971 Jan 8;42(1):103–110. doi: 10.1016/0006-291x(71)90368-8. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Kuchino T. Temperature-sensitive mutants of simian virus 40 selected by transforming ability. J Virol. 1975 Jun;15(6):1297–1301. doi: 10.1128/jvi.15.6.1297-1301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Moritsugu Y., Shimojo H. Suppression of cellular DNA synthesis in growing cells by ultraviolet-irradiated adenoviruses. Virology. 1971 Sep;45(3):687–696. doi: 10.1016/0042-6822(71)90182-6. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y. Induction of cell replication. Exp Cell Res. 1968 Oct;52(2):439–444. doi: 10.1016/0014-4827(68)90485-0. [DOI] [PubMed] [Google Scholar]
- Zardi L., Lin J. C., Baserga R. Immunospecificity to non-histone chromosomal proteins of anti-chromatin antibodies. Nat New Biol. 1973 Oct 17;245(146):211–213. doi: 10.1038/newbio245211a0. [DOI] [PubMed] [Google Scholar]



