Abstract
Objective
To determine the relationships between American style football (ASF) participation, acquired left ventricular (LV) hypertrophy, and LV systolic function as assessed using contemporary echocardiographic parameters.
Background
Participation in ASF has been associated with the development of hypertension and LV hypertrophy. To what degree these processes impact LV function is unknown.
Methods
This is a prospective, longitudinal, cohort study evaluating NCAA Division I collegiate football athletes stratified by field position (linemen, n=30 vs. non-linemen, n=57) before and after a single competitive season with transthoracic echocardiography. LV systolic function was measured using complementary parameters of global longitudinal strain (GLS, 2D speckle-tracking) and ejection fraction (EF, 2D biplane).
Results
ASF participation was associated with field position-specific increases in systolic blood pressure (linemen Δ SBP = 10±8 mmHg vs. non-linemen Δ SBP = 3±7 mmHg, p<0.001) and an overall increase in incident LV hypertrophy (pre = 8% vs. post = 25%, p<0.05). Linemen who developed LV hypertrophy had concentric geometry (9/11, 82%) with decreased GLS (Δ = −1.1%, p<0.001) while non-linemen demonstrated eccentric LV hypertrophy (8/10, 80%) with increased GLS (Δ = +1.4%, p<0.001). In contrast, LV ejection fraction in the total cohort and when stratified by field position was not significantly affected by ASF participation. Among the total cohort, lineman field position, postseason weight, systolic blood pressure, average LV wall thickness, and relative wall thickness were all independent predictors of postseason GLS.
Conclusions
ASF participation at a lineman field position may lead to a form of sport-related myocardial remodeling that is pathologic rather than adaptive. Future study will be required to determine if targeted efforts to control blood pressure, minimize weight gain, and to include an element of aerobic conditioning in this subset of athletes may attenuate this process and translate into tangible downstream health benefits.
Keywords: Hypertension, Athlete’s Heart Syndrome: Left Ventricular Hypertrophy, Left Ventricular Function
Introduction
Hypertension affects approximately 30% of adults in the United States and is associated with premature cardiovascular disease including myocardial infarction, stroke, arrhythmias, and heart failure (1,2). Recently, it has been demonstrated that early life hypertension is a strong independent predictor of later life cardiovascular disease and premature mortality (3,4). Additionally, elevated blood pressure in adolescent athletes predicts sustained hypertension one year later (5). As such, the identification and management of hypertension in at-risk younger people may improve long-term cardiovascular health.
American style football (ASF) is a popular sport with more than 5 million annual participants in the United States (6,7). Prior cross-sectional studies suggest an association between ASF participation and hypertension (8–11). Recent longitudinal work from our group demonstrated that participation in a single season of ASF is associated with increased systolic blood pressure among linemen and that increases in blood pressure are associated with the development of concentric left ventricular (LV) hypertrophy (12). These findings are relevant in the context of prior data from the National Institute of Occupational Health and Safety (NIOHS) which demonstrated increased risk of cardiovascular mortality among former professional linemen compared to the general population but provided no insights regarding causal mechanisms (13).
Sustained exposure to elevated blood pressure results in end-organ damage including hypertensive heart disease. Structurally, hypertensive heart disease is characterized by concentric LV hypertrophy which is defined by an increase in LV mass and wall thickness without concomitant enlargement in LV cavity size (14). Functionally, patients with early-stage hypertensive concentric LV hypertrophy typically demonstrate preserved LV ejection fraction (15–17), but often display subclinical systolic dysfunction as measured by global longitudinal strain (GLS), a well-validated echocardiographic parameter of myocardial systolic function with attendant prognostic implications (18,19).
We therefore undertook the present study with the following goals. First, we aimed to validate our prior observation that ASF participation is accompanied by an increase in blood pressure and LV hypertrophy. Second, we aimed to examine the functional correlates of ASF-induced LV hypertrophy in an attempt to delineate the functional implications of this form of “athlete’s heart”.
Methods
Study Design
We utilized a prospective, longitudinal design to examine blood pressure, LV remodeling, and LV systolic function among competitive ASF participants at an NCAA Division 1 University. We enrolled varsity-level ASF athletes as part of the Harvard Athlete Initiative, an ongoing research program designed to address issues relevant to athlete health and exercise physiology. Clinical and anthropometric data related to blood pressure including age (years), height (cm), weight (kg), ethnicity (black versus non-black), personal and family history of hypertension were obtained (20). Body surface area (BSA) was calculated with the Mosteller formula (21), and body mass index (BMI) was calculated as follows: [weight (kg)/height (m)2]. Resting blood pressure, heart rate, LV structural parameters, and indices of LV systolic function were assessed before and after a single season of ASF participation as detailed below. Each participant provided written consent prior to enrollment and all aspects of the study were approved by the Partners Human Research Committee.
Study Population
ASF athletes were eligible to participate if they were ≥ 18 years of age and recruited members of the Harvard University varsity football team. For this study, we enrolled first-year university athletes to ensure data capture of their initial season of college-level participation. The study period began at the time of enrollment and lasted for the entire ASF season (~90 days). Field position for each participant was classified as either lineman or non-lineman. Linemen included players at tackle, guard, center, or defensive end positions while non-linemen included quarterbacks, running backs, wide receivers, tight ends, linebackers, cornerbacks, safeties, kickers, and punters (22). Each participant was subject to testing for performance-enhancing drugs as dictated by NCAA standards. Subjects were excluded from the final analysis if they undertook training breaks of ≥ 3 days for any reason or if their echocardiographic images were unsuitable for GLS analysis.
Blood Pressure Measurement
A cardiologist (J.L., A.B., R.W., M.W.) measured blood pressures at enrollment (preseason) and during postseason assessment with a manual sphygmomanometer and an appropriately sized arm cuff. Participants were supine for at least 10 minutes of quiet rest for this process. Reported values represent the average of triplicate measurements. Blood pressure was classified according to Joint National Commission-7 (JNC-7) guidelines into the following categories: normal (SBP<120 mmHg and SBP<80 mmHg), pre-hypertension (SBP=120 to 139 mmHg or DBP=80–89 mmHg), and stage 1 or greater hypertension (SBP≥140 mmHg or DBP≥90 mmHg).23 Joint National Commission-8 (JNC-8) guidelines were not used as they do not define blood pressure thresholds for pre-hypertension. The JNC-8 report does recommend pharmacologic therapy at a blood pressure of 140/90 (24), which is consistent with the definition of hypertension used in this study.
Transthoracic Echocardiography
Cardiac imaging was performed with a commercially available echocardiography system (Vivid-I, GE Healthcare, Milwaukee, WI) with a 1.9- to 3.8-mHz phased-array transducer. Images were obtained by a sonographer credentialed in cardiac ultrasound or a cardiologist trained in echocardiography. Participants were imaged at rest ≥ 12 hours after the most recent training session at Harvard University during the preseason and postseason. The postseason images were acquired within 5 days of the completion of the final game of the season. Two-dimensional imaging was performed from standard parasternal and apical transducer positions with frame rates confined to 60–100 Hz as determined on an individual basis for image optimization. All data were stored digitally and two cardiologists (J.L., A.B) blinded to study time point performed all off-line analyses (EchoPac, version 7.0, GE Healthcare).
Cardiac structural measurements were made in accordance with current guidelines (25). LV volumes and ejection fraction were measured and calculated with the modified Simpson biplane technique. LV mass was calculated using the area-length method, which was chosen because it accounts for LV morphology in both short- and long-axis dimensions, and LV hypertrophy was defined as LV mass index > 102 g/m2 (25). Average LV wall thickness was calculated as follows: [interventricular septal thickness (mm)+posterior wall thickness (mm)]/2. Relative wall thickness was calculated as: [interventricular septal thickness (mm)+posterior wall thickness (mm)]/LV end-diastolic diameter (mm). Concentric LV hypertrophy was defined by relative wall thickness > 0.42 with LV mass index > 102 g/m2; eccentric LV hypertrophy was defined by relative wall thickness ≤ 0.42 and LV mass index > 102 g/m2 (25).
GLS measurements were made using commercially available speckle-tracking analysis software (EchoPac, Version 7.0, GE Healthcare) as previously reported.26–28 In brief, the highest-quality digital 2D apical 4-chamber view was selected for analysis. The endocardium was traced and a full thickness myocardial region of interest was selected. The software then automatically partitioned the LV into 6 segments including apical (n=2), septal (n=2), and lateral (n=2) territories and selected suitable speckles for tracking. The reliability of tracking was confirmed by the software’s reliability parameter (V = valid tracking; X = unacceptable tracking). When the software signaled poor tracking efficiency, the observer readjusted the endocardial trace and/or region of interest width until an acceptable tracking score was obtained. By convention, GLS values are presented as negative numbers with lower (i.e. more negative) values representing greater systolic shortening. Measurements were obtained on 3 consecutive cardiac cycles and reported values represent a 3-cycle average. Participants in whom full 6-segment strain data over 3 consecutive cardiac cycles could not be obtained were excluded from final analyses.
Measurement Variability
The intraobserver and interobserver variability for LV mass and GLS were examined. Intraobserver variability was performed by a single investigator through blinded assessment of 10 randomly selected subjects on 2 separate occasions. Interobserver variability was assessed in a group of 10 randomly selected subjects by 2 investigators blinded to each other’s measurements and to study time point. Correlation coefficients for each measurement, derived from simple linear regression, were used to quantify variability with the following results: intraobserver LV mass (R2= 0.946), intraobserver GLS (R2= 0.968), interobserver LV mass (R2= 0.921), and interobserver GLS (R2= 0.972).
Statistical Analysis
Categorical variables are presented as proportions and continuous variables as mean±SD. Paired data were compared using paired t-testing or McNemar’s exact test while unpaired data were compared using Student’s independent sample t-test or Fisher’s exact test as appropriate for data distribution. Correlation analyses were performed using the Spearman or Pearson’s technique as appropriate for data distribution. Univariate linear regression analyses were used to identify factors associated with postseason GLS. Parameters with a univariate p-value of <0.10 were included in a forward stepwise multivariate linear regression model designed to identify factors independently associated with postseason GLS. All analyses were performed with SPSS (Version 22, IBM Corp ©).
Results
Study Population
Among 190 eligible ASF participants enrolled from 2008–2014, 87 (all men, age=18.8±0.8 years) were included in this analysis. Subject attrition (n=103) was due to intraseason injuries (25 of 103 athletes, 24%) and our strict a priori imaging quality exclusion criteria (78 of 103 athletes, 76%). Of note, there were no significant differences in field position (34% linemen and 66% non-linemen), blood pressure trends, or LV structural parameters between those excluded and those retained. The final cohort included 30 linemen (height 191±5 cm; weight 111±12 kg) and 57 non-linemen (height 185±6 cm; weight 90±9). Linemen had significantly higher BMI than non-linemen (30.6±3.5 vs. 26.4±2.0 kg/m2, p<0.001). Twenty-four athletes (27%) were of black ethnicity and 22 athletes (25%) had a family history of hypertension. Weight (111±12 to 114 ±12 kg, p=0.002), BMI (30.6±3.5 to 31.2±3.5 kg/m2, p=0.007), and BSA (2.42±0.13 to 2.44±0.13 m2, p=0.01) all increased significantly in the linemen group from baseline to postseason while no significant changes occurred among the non-linemen (Table 1).
Table 1.
Anthropometric and clinical measurements between linemen and non-linemen before and after 90 days of American style football participation.
| Linemen (n=30) | Non-Linemen (n=57) | |||||
|---|---|---|---|---|---|---|
| Preseason | Postseason | P-value | Preseason | Postseason | P-value | |
| Height (cm) | 191±5 | ----- | N/A | 185±6 | ----- | N/A |
| Weight (kg) | 111±12 | 114±12 | 0.002 | 90±9* | 90±9 | 0.77 |
| BMI (kg/m2) | 30.6±3.5 | 31.2±3.4 | 0.007 | 26.4±2.0 | 26.4±2.1 | 0.68 |
| BSA (m2) | 2.42±0.13 | 2.44±0.13 | 0.01 | 2.14±0.14 | 2.15±0.13 | 0.76 |
| Heart Rate (bpm) | 66±10 | 66±10 | 0.72 | 64±7* | 59±7† | <0.001 |
| Systolic BP (mmHg) | 122±8 | 132±10 | <0.001 | 117±11 | 120±13 | <0.001 |
| Diastolic BP (mmHg) | 62±8 | 64±7 | 0.12 | 65±7 | 66±8† | 0.36 |
| Systolic BP < 120 | 13/30 (43%) | 3/30 (10%) | <0.001 | 28/57 (49%) | 29/57 (51%)† | 0.16 |
| Systolic BP 120–139 | 17/30 (57%) | 18/30 (60%) | 0.16 | 29/57 (51%) | 24/57 (42%) | 0.003 |
| Systolic BP ≥ 140 | 0/30 (0%) | 9/30 (30%) | N/A | 0/57 (0%) | 4/57 (7%)† | N/A |
BMI = body mass index, BSA = body surface area, BP = blood pressure
p<0.05 for comparison with preseason measurement in linemen.
p<0.05 for comparison with postseason measurement in linemen.
Blood Pressure
ASF participation was associated with an increase in systolic blood pressure in the total cohort (119±10 to 124±12 mmHg, p<0.001) but no significant change in diastolic blood pressure. Although both linemen (pre: 122±8 vs. post: 132±10 mmHg, p<0.001; Table 1) and non-linemen (pre: 117±11 vs. post: 120±13 mmHg, p<0.001) had increases in systolic blood pressure, the magnitude of increase was significantly greater among linemen (linemen: Δ = 10±8 vs. non-linemen: Δ = 3±7 mmHg, p<0.001) (Figure 1A). At preseason, the prevalence of pre-hypertension was similar between linemen (17/30, 57%) and non-linemen (29/57, 51%, p=0.31) and no athletes met criteria for overt hypertension. At postseason assessment, 90% of linemen met criteria for pre-hypertension (18/30, 60%) or Stage 1 hypertension (9/30, 30%) while only 49% (28/57) of non-linemen, a proportion similar to the preseason, had blood pressure exceeding upper limits of normal (Figure 1B).
Figure 1. Blood Pressure Among ASF Participants.
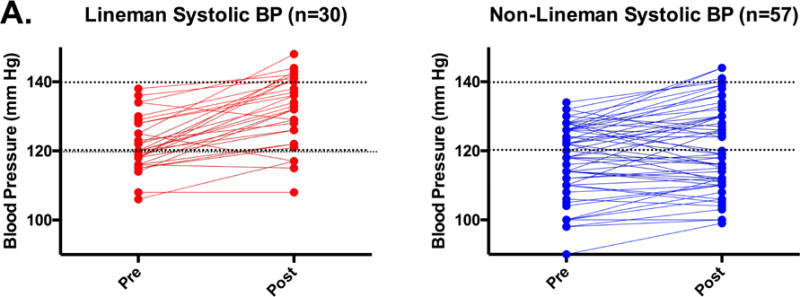
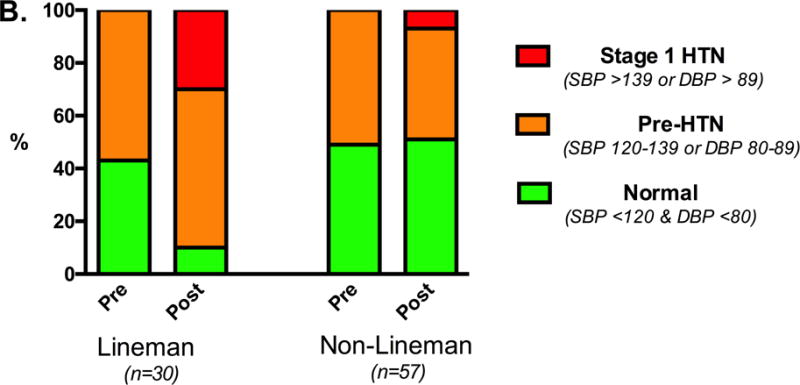
Individual blood pressure (A) and categorical distribution of blood pressure status by Joint National Commission (seventh report) stage (B) stratified by field position before and after 90 days of American Style Football participation.
Left Ventricular Structure
LV mass index did not differ significantly at preseason as a function of field position (linemen: 97±15 vs. non-linemen: 94±12 g/m2, p=0.10; Table 2). Among the total cohort, ASF participation lead to a significant increase in LV mass index (pre: 95±13 vs. post: 104±15 g/m2, p<0.001) but LV mass index increased more among linemen (Δ = linemen: 13±15 vs. non-linemen: Δ = 7±10 g/m2, p=0.02). In the total cohort, the prevalence of LV hypertrophy increased from 8% (7/87) at preseason to 24% (21/87, p<0.001) at postseason. Among linemen with LV hypertrophy at postseason, 9/11 (82%) demonstrated concentric geometry while in contrast, 8/10 (80%) non-lineman with LV hypertrophy demonstrated eccentric geometry.
Table 2.
Structural cardiac parameters among linemen and non-linemen before and after 90 days of American style football participation.
| Linemen (n=30) | Non-Linemen (n=57) | |||||
|---|---|---|---|---|---|---|
| Preseason | Postseason | P-value | Preseason | Postseason | P-value | |
| LV Mass (gm) | 235±36 | 265±43 | <0.001 | 200±27* | 217±35† | <0.001 |
| LV Mass Index (gm/m2) | 97±15 | 109±15 | <0.001 | 94±12 | 101±15† | <0.001 |
| IVS (mm) | 9.6±0.9 | 10.6±1.2 | <0.001 | 9.3±1.3 | 9.8±1.4† | 0.004 |
| PWT (mm) | 9.9±1.0 | 11.0±1.2 | <0.001 | 9.9±1.0 | 10.4±1.1† | 0.01 |
| LVIDd (mm) | 54±4.6 | 53.8±3.9 | 0.72 | 52±3.9* | 52.7±4 | 0.02 |
| Relative Wall Thickness | 0.36±0.05 | 0.40±0.06 | <0.001 | 0.37±0.05 | 0.39±0.06 | <0.07 |
| LVH, n/N (%) | 4/30 (13) | 11/30 (37) | <0.001 | 3/57 (5) | 10/57 (18)† | <0.001 |
| Eccentric, n/N (%) | 4/30 (13) | 2/30 (7) | 0.62 | 3/57 (7) | 8/57 (18) | 0.003 |
| Concentric, n/N (%) | 0/30 (0) | 9/30 (30) | ----- | 0/57 (0) | 2/57 (5)† | ----- |
IVS = interventricular septum, LV = left ventricular, LVH = left ventricular hypertrophy, LVIDd = LV internal diameter at end-diastole, PWT = posterior wall thickness.
p<0.05 for comparison with preseason measurement in linemen.
p<0.05 for comparison with postseason measurement in linemen.
Left Ventricular Systolic Function
LV ejection fraction was similar among linemen (59 ± 6 %, range: 49–69%) and non-linemen (59 ± 6 %, range: 46–79%) at preseason (p=0.93) and was not significantly different at postseason. GLS was also similar between field position groups at preseason (linemen = −21.2 ± 2.4% vs. non-linemen = −20.8 ± 2.7%, p=0.26). However, GLS was significantly changed at postseason in both position groups with changes occurring in opposite directions. Specifically, linemen demonstrated a significant decrement in GLS (Δ = 1.1%, p<0.001) while non-linemen experienced a significant increase (Δ = 1.4%, p<0.001; Figure 2). The observed GLS decrement among linemen correlated indirectly with changes in systolic blood pressure (R= −0.47, p=0.009) and LV mass index (R= −0.52, p=0.003) while no such relationships were observed among non-linemen (R = 0.01, p=0.49; R = 0.09, p=0.93 respectively). Univariate and multivariate predictors of postseason GLS are shown in Table 3. In a multivariate linear regression model, lineman position (β=0.402, p<0.001), postseason weight (β=0.375, p<0.001), systolic blood pressure (β=0.325, p<0.001), average LV wall thickness (β=0.234, p<0.001), and LV relative wall thickness (β=0.218, p=0.003) were independent predictors of postseason GLS.
Figure 2. Left Ventricular Systolic Function Among ASF Participants.
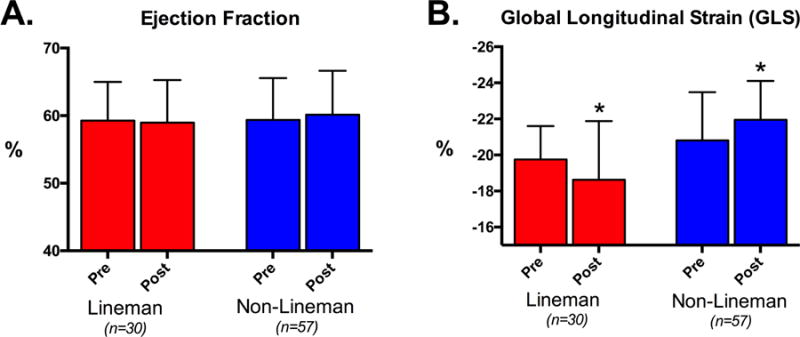
Left ventricular systolic function as measured by ejection fraction (A) and global longitudinal strain (B) stratified by field position before and after 90 days of American Style Football participation. * = p<0.05 compared to preseason values.
Table 3.
Univariate and multivariate predictors of postseason global longitudinal strain (GLS) in American style football after 90 days of participation.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| β -Coefficient | P-value | β -Coefficient | P-value | |
| Black ethnicity | .121 | 0.27 | ----- | ----- |
| Family history of HTN | .095 | 0.38 | ----- | ----- |
| Linemen field position | .458 | <0.001 | .402 | <0.001 |
| Preseason Data: | ||||
| Weight (kg) | .420 | <0.001 | ----- | ----- |
| Height (cm) | .335 | 0.002 | ----- | ----- |
| BMI (kg/m2) | .313 | 0.003 | ----- | ----- |
| BSA (m2) | .426 | <0.001 | ----- | ----- |
| Heart rate (bpm) | .215 | 0.04 | ----- | ----- |
| SBP (mmHg) | .323 | 0.002 | ----- | ----- |
| DBP (mmHg) | −0.45 | 0.68 | ----- | ----- |
| GLS (%) | .418 | <0.001 | ----- | ----- |
| Intra-season Data (Δ’s): | .183 | 0.09 | ----- | ----- |
| Weight Δ (kg) | ||||
| BMI Δ (kg/m2) | .169 | 0.012 | ||
| BSA Δ (m2) | .271 | 0.04 | ||
| Heart rate Δ (bpm) | .072 | 0.510 | ----- | ----- |
| SBP Δ (mmHg) | .202 | 0.06 | ----- | ----- |
| DBP Δ (mmHg) | .166 | 0.13 | ----- | ----- |
| GLS Δ (%) | .465 | <0.001 | ----- | ----- |
| Postseason Data: | ||||
| Weight (kg) | .447 | <0.001 | .375 | <0.001 |
| BMI (kg/m2) | .347 | <0.001 | ----- | ----- |
| BSA (m2) | .452 | <0.001 | ----- | ----- |
| Heart rate (bpm) | .246 | 0.02 | ----- | ----- |
| SBP (mmHg) | .376 | <0.001 | .325 | <0.001 |
| DBP (mmHg) | .106 | 0.329 | ----- | ----- |
| LV Mass (g) | .258 | 0.02 | ----- | ----- |
| LV Wall Thickness (mm) | .389 | <0.001 | .234 | <0.001 |
| Relative Wall Thickness | .342 | <0.001 | .218 | 0.003 |
BMI = body mass index, BSA = body surface area, DBP = diastolic blood pressure, GLS = global longitudinal strain, HTN = hypertension, LV = left ventricular, SBP = systolic blood pressure
Discussion
Key findings from this study are summarized as follows. First, our data confirm previously reported associations between ASF participation, increases in systolic blood pressure, and the development of concentric LV hypertrophy. These observations appear particularly relevant to ASF linemen. Second, we demonstrate that the development of concentric LV hypertrophy among linemen is characterized by concomitant reductions in LV longitudinal strain, a well-validated marker of subclinical LV dysfunction with attendant adverse prognosis. In aggregate, these findings advance our understanding of cardiac remodeling among athletes and challenge the pervasive notion that all sport-related cardiovascular changes are adaptive rather than pathologic.
A 1994 report from the NIOSH evaluated the medical status of 6848 retired NFL players (13). While the study documented a 46% decreased rate of death among former NFL players compared to a general population of age-matched men, it showed a 52% increase in cardiovascular mortality among linemen. Subsequent studies have confirmed a similar increase in heart disease mortality in active and former ASF linemen as well as a higher risk of metabolic syndrome and subclinical carotid atherosclerosis (11,29,30). While elevated body mass has been proposed as a key explanatory factor, this parameter unlikely explains the complete mechanistic story (10,31). Data from the current study introduce a more comprehensive mechanistic explanation in the form of acquired, early-life hypertension with resultant target organ damage in the form of concentric LV hypertrophy with subclinical LV systolic dysfunction.
Traditional assessment of LV systolic function relies on measurement of LV ejection fraction, a relatively crude volumetric technique that is exquisitely load dependent, highly reliant on image quality, and based on key geometric assumptions that may not be valid in the setting of significant cardiac remodeling (32). Speckle-tracking echocardiography minimizes these limitations and more accurately captures subtle yet clinically relevant changes in cardiac function. Global longitudinal strain (GLS), the systolic function parameter utilized in this study, has emerged as a highly reproducible metric with powerful prognostic implications in numerous clinical populations. Among patients with hypertensive heart disease and preserved ejection fraction, mean GLS is uniformly decreased and inversely correlated with systolic blood pressure, LV mass, and the presence of concentric remodeling or hypertrophy (33–35).
Prior studies of GLS among athletic cohorts, almost exclusively confined to athletes in endurance sports, have demonstrated preservation or increase in contractile function highlighting the generally adaptive nature of exercise-induced cardiac remodeling. In a study evaluating GLS in rowers, age-matched hypertensive subjects on no therapy, and normotensive sedentary controls, the hypertensive group had significantly lower GLS (17.5±2.8%) compared to rowers (−22.2±2.7%) and controls (21.1±2.0%) (36). In addition, prior work by our group documented increases in GLS after 90-days of endurance training among competitive rowers (26). The present study builds on this work in two principal ways. First, the finding of LV hypertrophy with GLS augmentation among non-lineman ASF athletes, a group exposed to a mixture of isometric and isotonic physiology (i.e. skills training and aerobic conditioning), suggests an adaptive form of remodeling similar to that seen in other athlete cohorts. Second, in stark comparison, LV hypertrophy with relative impairment of GLS among ASF lineman, a group exposed to predominantly isometric physiology (i.e. blocking and tackling drills), suggests maladaptive remodeling. To our knowledge, this study is the first to document a relative impairment of GLS among athletes and provides plausible physiologic and anatomic correlates in the forms of acquired hypertension and concentric LV hypertrophy, respectively.
There are discrete clinical and scientific implications of the data presented. First, our data confirm the notion that ASF linemen are at substantial risk for the development of hypertension. Clinicians who care for ASF athletes should be encouraged to screen for hypertension and to implement lifestyle and pharmacologic therapies as suggested by current guidelines. Additionally, this study clearly documents position-specific changes in blood pressure and cardiac structure and function in response to ASF participation. This finding reinforces the notion that not all forms of athletic participation result in similar cardiovascular remodeling and for the first time, identifies an athletic population that appears to remodel with maladaptive attributes. The cardiovascular changes seen in linemen - concentric LVH with reduced GLS -should be viewed as a unique entity from traditional “athlete’s heart” with pathologic rather than adaptive characteristics.
Several limitations of this study set the stage for future work. First, we employed a longitudinal, repeated measures study design in an effort to establish temporal and causal relationships between ASF participation, systolic blood pressure increase, and myocardial remodeling. This approach does not however permit definitive determination of underlying mechanisms. Future studies examining and controlling for factors including inflammation, peripheral vascular dysfunction, distinct cellular pathway activation, and diffuse myocardial fibrosis represent logical next steps. Second, we acknowledge that possible confounding factors known to impact blood pressure including non-steroidal anti-inflammatory drug use, psychosocial stress level, and dietary intake were not standardized. Whether these factors impacted our data and to what degree efforts to control these factors will benefit ASF athletes remains uncertain. Third, our study duration was relatively brief as many athletes accrue numerous years of ASF exposure. Carefully designed longer term studies that capture blood pressure and cardiac parameters during more lengthy ASF careers and in the post-ASF years are warranted. Finally, we acknowledge the potential impact of subject attrition due to the unavoidably high rate of participant exclusion from the final analysis (103 of 190 athletes, 54%) due to injury and technically inadequate echocardiographic images for strain analysis. Although there were no differences in baseline parameters, blood pressure trends, or prevalent LV hypertrophy among those excluded, we cannot eliminate the possibility of some attrition bias.
In conclusion, data from the present study advance our understanding of the cardiovascular response to participation in American style football. ASF participants at the lineman field position appear to be at risk for incident hypertension and secondary maladaptive cardiac remodeling. The development of concentric LV hypertrophy with relative impairment of GLS, a phenotype unequivocally associated with adverse prognoses in other clinical populations, is concerning in this population of young, otherwise healthy athletes. These findings underscore the need for future work designed to confirm the scope and long-term clinical implications of this problem which may affect millions of young people.
Perspectives.
Competency in Medical Knowledge
The cardiovascular care of American style football athletes requires specific consideration of the unique cardiovascular demands during training and competition in this sport. Understanding the effects of football participation on cardiovascular remodeling using echocardiography represents an important step in providing more specialized long-term care for this group of athletes.
Translational Outlook
Our findings demonstrate that American style football athletes, especially those at a lineman position, are at increased risk for early life hypertension and associated secondary cardiac remodeling including a decrement in left ventricular systolic function as measured by global longitudinal strain. This decrease in function appears to be mediated in part by acquired resting hypertension and weight gain highlighting the importance of the modifiable factors in mitigating the risk of later life heart disease in football linemen.
Acknowledgments
The authors wish to thank the Harvard University coaching staff, athletic department administration, and student athletes for their continued support of the Harvard Athlete Initiative.
Sources of Funding: This work was supported by research grants from the National Institutes of Health/National Heart Lung & Blood Institute (HL RO1 125869, A. Baggish) and the American Heart Association (FTF220328, A. Baggish).
Abbreviations
- ASF
American Style Football
- LV
Left ventricular
- NIOHS
National Institute of Occupational Health and Safety
- GLS
Global Longitudinal Strain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures: None
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. Jama. 2010;303:2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet (London, England) 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study) Journal of the American College of Cardiology. 2011;58:2396–403. doi: 10.1016/j.jacc.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yano Y, Stamler J, Garside DB, et al. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. Journal of the American College of Cardiology. 2015;65:327–35. doi: 10.1016/j.jacc.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanji JL. Tracking of elevated blood pressure values in adolescent athletes at 1-year follow-up. American journal of diseases of children (1960) 1991;145:665–7. doi: 10.1001/archpedi.1991.02160060083026. [DOI] [PubMed] [Google Scholar]
- 6.Participation in Selected Sports Activities: 2009. US Census Bureau. 2012 [Google Scholar]
- 7.Irick E. NCAA Sports Sponsorship and Participation Rates Report 1981–82 – 2013–14. 2014 [Google Scholar]
- 8.Dobrosielski DA, Rosenbaum D, Wooster BM, et al. Assessment of cardiovascular risk in collegiate football players and nonathletes. J Am Coll Health. 2010;59:224–7. doi: 10.1080/07448481.2010.483719. [DOI] [PubMed] [Google Scholar]
- 9.Karpinos AR, Roumie CL, Nian H, Diamond AB, Rothman RL. High prevalence of hypertension among collegiate football athletes. Circ Cardiovasc Qual Outcomes. 2013;6:716–23. doi: 10.1161/CIRCOUTCOMES.113.000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucker AM, Vogel RA, Lincoln AE, et al. Prevalence of cardiovascular disease risk factors among National Football League players. Jama. 2009;301:2111–9. doi: 10.1001/jama.2009.716. [DOI] [PubMed] [Google Scholar]
- 11.Selden MA, Helzberg JH, Waeckerle JF, et al. Cardiometabolic abnormalities in current National Football League players. The American journal of cardiology. 2009;103:969–71. doi: 10.1016/j.amjcard.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 12.Weiner RB, Wang F, Isaacs SK, et al. Blood pressure and left ventricular hypertrophy during American-style football participation. Circulation. 2013;128:524–31. doi: 10.1161/CIRCULATIONAHA.113.003522. [DOI] [PubMed] [Google Scholar]
- 13.Baron S, Rinsky R. Rate and Causes of Death of National Football League Players. National Institute of Occupational Safety and Health, Letter. 1994 Jan 10; [Google Scholar]
- 14.Radulescu D, Stoicescu L, Buzdugan E, Donca V. Patterns of left ventricular remodeling among patients with essential and secondary hypertension. Revista medica de Chile. 2013;141:1520–7. doi: 10.4067/S0034-98872013001200004. [DOI] [PubMed] [Google Scholar]
- 15.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–34. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 16.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Annals of internal medicine. 1991;114:345–52. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 17.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. The New England journal of medicine. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 18.Mentz RJ, Khouri MG. Longitudinal Strain in Heart Failure With Preserved Ejection Fraction: Is There a Role for Prognostication? Circulation. 2015;132:368–70. doi: 10.1161/CIRCULATIONAHA.115.017683. [DOI] [PubMed] [Google Scholar]
- 19.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1716–7. doi: 10.1093/eurheartj/ehs124. [DOI] [PubMed] [Google Scholar]
- 20.Baggish AL, Weiner RB, Yared K, et al. Impact of family hypertension history on exercise-induced cardiac remodeling. The American journal of cardiology. 2009;104:101–6. doi: 10.1016/j.amjcard.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 21.Mosteller RD. Simplified calculation of body-surface area. The New England journal of medicine. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 22.Croft LB, Belanger A, Miller MA, Roberts A, Goldman ME. Comparison of National Football League linemen versus nonlinemen of left ventricular mass and left atrial size. The American journal of cardiology. 2008;102:343–7. doi: 10.1016/j.amjcard.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 24.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Baggish AL, Yared K, Wang F, et al. The impact of endurance exercise training on left ventricular systolic mechanics. American journal of physiology Heart and circulatory physiology. 2008;295:H1109–1116. doi: 10.1152/ajpheart.00395.2008. [DOI] [PubMed] [Google Scholar]
- 27.Wasfy M, Weiner RB, Wang F, et al. Endurance Exercise-Induced Cardiac Remodling: Not All Sports Are Created Equal. J Am Soc Echocardiogr. 2015;28(12):1434–40. doi: 10.1016/j.echo.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Weiner RB, Hutter AM, Jr, Wang F, et al. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 2010;3:1001–9. doi: 10.1016/j.jcmg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Baron SL, Hein MJ, Lehman E, Gersic CM. Body mass index, playing position, race, and the cardiovascular mortality of retired professional football players. The American journal of cardiology. 2012;109:889–96. doi: 10.1016/j.amjcard.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Hurst RT, Burke RF, Wissner E, et al. Incidence of subclinical atherosclerosis as a marker of cardiovascular risk in retired professional football players. The American journal of cardiology. 2010;105:1107–11. doi: 10.1016/j.amjcard.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy CG, Webb RC. The Toll of the Gridiron: damage-associated molecular patterns and hypertension in American football. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2016;30:34–40. doi: 10.1096/fj.15-279588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biering-Sorensen T, Solomon SD. Assessing Contractile Function When Ejection Fraction Is Normal: A Case for Strain Imaging. Circ Cardiovasc Imaging. 2015;8:e004181. doi: 10.1161/CIRCIMAGING.115.004181. [DOI] [PubMed] [Google Scholar]
- 33.Goncalves S, Cortez-Dias N, Nunes A, et al. Left ventricular systolic dysfunction detected by speckle tracking in hypertensive patients with preserved ejection fraction. Rev Port Cardiol. 2014;33:27–37. doi: 10.1016/j.repc.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2:382–90. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 36.Galderisi M, Lomoriello VS, Santoro A, et al. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2010;23:1190–8. doi: 10.1016/j.echo.2010.07.010. [DOI] [PubMed] [Google Scholar]


