Abstract
Advancements in tissue engineering require the development of new technologies to study cell behavior in vitro. This study focuses on stem cell behavior within various miniaturized three-dimensional (3D) culture conditions of alginate biomaterials modified with the Arg-Gly-Asp (RGD) peptide known for its role in cell adhesion/attachment. Human embryonic palatal mesenchyme (HEPM) cells, bone marrow derived mesenchymal stem cells (MSCs), and human adipose derived stem cells (ADSCs) were cultured on a flat hydrogel of different concentrations of alginate-RGD, and in the miniaturized 3D core of microcapsules with either a 2% alginate or 2% alginate-RGD shell. The core was made of 0%, 0.5%, or 2% alginate-RGD. Cell spreading was observed in all systems containing the RGD peptide, and the cell morphology was quantified by measuring the cell surface area and circularity. In all types of stem cells, there was a significant increase in the cell surface area (p < 0.05) and a significant decrease in cell circularity (p < 0.01) in alginate-RGD conditions, indicating that cells spread much more readily in environments containing the peptide. This control over the cell spreading within a 3D microenvironment can help to create the ideal biomimetic condition in which to conduct further studies on cell behavior.
Keywords: Core-shell microcapsules, biomimetic, 3D culture, stem cell
Introduction
In the field of biomedical engineering, the study of the engineering and regeneration of new tissues and organs is becoming increasingly important.1–3 In order to construct these tissues and organs, the behavior of the cells that make up these structures must be better understood. Many in vitro studies include the culture of cells on or within a biomaterial scaffold to mimic the extracellular matrix (ECM), which is comprised of the insoluble materials that surround the cells in tissue.1–3 The ECM is necessary to provide the cell with the proper chemical and mechanical signals to help direct the cell proliferation and differentiation.1–3 Well-defined biomaterials play a large role in the study of cellular processes and of the interaction of the cells with the surrounding matrix. This knowledge can be crucial to the development of new approaches in the field of regenerative medicine.
Alginate is one such biomaterial that is found in nature as a polysaccharide of brown algae, and it has been previously used in many applications, from a staple in the food industry to a shear-thinning agent in the textile/paper industry.3,4 As a biomaterial, alginate is incredibly favorable due to its biocompatibility, non-immunogenicity, low toxicity, easy reversible gelation with divalent ions, and affordability.2–5 With its gelling capabilities, alginate has been used as a vehicle for drug delivery and cell studies.2–5 As the degree of alginate gelling can be controlled depending on the concentration of the alginate or the divalent cation gelling agent, often Ca2+, it is believed that the stiffness of the environment in which the cells are cultured can also be controlled.6,7
While alginate is very advantageous as a biomaterial for cell studies, it does have some disadvantages, such as cells being unable to naturally adhere to it. However, the incorporation of an arginine-glycine-aspartic acid (RGD) peptide that contains cell-adhesion ligands to the alginate polymer backbone through aqueous carbodiimide chemistry has been shown to increase cell adherence to a substrate.5 With the use of 1-ethyl-(dimethylaminopropyl) carbodiimide (EDC), an intermediate is formed that is then stabilized with the addition of N-hydroxy-sulfosuccinimide (Sulfo-NHS). Then, the RGD peptide is added to the reaction to be incorporated into the alginate backbone via the N-terminal amine of the peptide.5 Multiple studies have been conducted demonstrating its success and its influence on various cell lines. In one study, hyaluronan (HA) was modified with the RGD peptide and was shown to successfully improve the adhesion and spreading of mice fibroblast cells on flat two-dimensional (2D) surface of the hydrogel.8 Additionally, another study that involves RGD modified alginate has shown that chondrocyte attachment was 10–20 fold higher on the flat 2D alginate-RGD surface than to the flat 2D surface of unmodified alginate.9 Other studies have indicated that the RGD peptide has an effect on the adhesion and proliferation of pre-osteoblasts, as well as on their osteogenic differentiation.10–15
Human embryonic palatal mesenchyme (HEPM) cells are pre-osteoblasts that have been previously used in osteogenic studies.16,17 These cells exhibit a fibroblast-like morphology and were studied to observe the effect of RGD peptide on their adhesion and morphology. Additionally, mesenchymal stem cells (MSCs) share the same morphology and were also used in this study to see if the effect of RGD peptide on cell attachment would vary between cell types. MSCs have been used in multiple studies in which it was found that the RGD peptide had an effect on the MSC viability, adhesion, proliferation, and spreading on flat 2D surface.18 Furthermore, past research with human adipose derived stem (or stromal) cells (ADSCs) and RGD modified alginate has shown that while the cells spread on a 2D hydrogel, they remained rounded when cultured in a macroscale three-dimensional (3D) hydrogel disk.19 Other studies have also shown that the presence of the RGD peptide has an effect on the fate of ADSCs cultured on micropatterned 2D hydrogel surfaces with different crosslinking densities.20
In addition to the advancement of biomaterials used for regenerative studies, cellular processes and behaviors have also been studied extensively under both 2D and 3D conditions. A 3D environment is native to most cells in human body while 2D systems have been shown to induce different protein and gene expression within cells from 3D systems.20–27 While various 3D environments have been developed, microencapsulation of cells within a matrix of a biocompatible hydrogel has emerged as a technology that can overcome some of the challenges previously faced, primarily the diffusion limit of oxygen and nutrient in highly cellularized constructs and large-scale production needed for clinical applications.28–34 Microcapsules are able to provide a miniaturized culture condition that allows efficient transport of nutrients, oxygen, and metabolites to/from the cells to maintain viability.35 Additionally, the hydrogel provides a selective permeability that protects the cells from factors that could harm them during delivery or transplantation, such as a host’s immune system.35 For these reasons, microencapsulation of cells within biocompatible polymers has been attracting more and more attention in recent years.
Several approaches have been used to generate microcapsules including emulsification, electrospray, and planar microfluidics.28,34,36–39 However, most of the microcapsules produced are homogeneous hydrogel microbeads that are difficult to use for controlling the size and shape of cell aggregates formed in them.28,34,40–42 The capability of producing microcapsules with a hydrogel shell and a core of aqueous liquid or a different hydrogel has emerged as a way to create an environment in which the cells can proliferate and grow in a more biomimetic and controllable fashion.35,43–46 Past practices of producing an aqueous liquid core microcapsule involves extensive procedure that often causes damage to the encapsulated cells.41,47,48 Through the use of coaxial electrospray or non-planar microfluidic flow-focusing device, a simple one-step generation of microcapsules with an aqueous liquid core and hydrogel shell has been achieved.35,43 Moreover, microcapsules with a collagen core containing mouse embryonic stem cells or ovarian follicles semi-enclosed in alginate hydrogel shell have been produced for miniaturized biomimetic 3D cell and tissue culture.44,45
Enabled by the aforementioned capability of RGD modification of alginate and non-planar microfluidic technology for generating cell-laden core-shell microcapsules, this study explores the effect of RGD peptide on the spreading/morphology of three different types of stem cells (HEPM cells, MSCs, and ADSCs) when they are cultured on the conventional 2D flat surface, miniaturized 3D curved surface, and miniaturized 3D matrices of alginate hydrogel with and without the RGD peptide.
Methods and Materials
Culture of stem cells
Human embryonic palatal mesenchyme (HEPM) cells (ATCC) were cultured in EMEM medium (Millipore) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin; medium was changed every other day. C3H10T1/2 mesenchymal stem cells (MSCs) were cultured in DMEM (Millipore) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin; medium was changed every few days. Human adipose derived stem cells (ADSCs) (ATCC) were cultured in ADSC basal medium (Millipore) supplemented with 10% FBS, 5mL L-glutamine and 0.5 mL gentamicin-amphotericin; medium was changed every other day. All cells were cultured in a T-75 culture flask at 37°C in a humidified 5% CO2 incubator. Cells were passaged with trypsin/EDTA (Invitrogen) once 70% confluence was reached and used for encapsulation studies or passaged for further use.
Alginate-RGD modification and x-ray photoelectron spectroscopy analysis
Alginate-RGD was prepared using the same procedure as previously described.5 Briefly, purified and lyophilized alginate was dissolved in 0.1 M, pH 6.5, 0.3 M NaCl, MES buffer (Thermo Scientific) to make a 1% solution. Modification was done with carbodiimide chemistry. N-hydroxy-sulfosuccinimide (Sulfo-NHS)(Sigma) and 1-ethyl-(dimethylaminopropyl) carbodiimide (EDC)(Sigma) were added to the alginate and the solution was set on a rocking plate for 15 min, after which H-Gly-Gly-Gly-Gly-Arg-Gly-Asp-Ser-Pro-OH peptide (RGD) (Peptide International) was added. The molar ratio of EDC:Sulfo-NHS:RGD used was 50:25:3.4. After 24 hours, the solution was dialyzed against DI water in Spectra/Por dialysis tubing (MWCO 50 kDa) for 36 hours with three water changes. The solution was then charcoal treated for 4 hours before being filtered through a 0.45 μm filter and lyophilized. Lyophilized alginate-RGD was stored at −20°C until use.
X-ray photoelectron spectroscopy (XPS) was conducted with a Kratos Axis Ultra XPS on alginate and RGD modified alginate samples to determine the success of the incorporation of the RGD peptide. Samples of lyophilized purified alginate and alginate-RGD were used for analysis, and the samples were mounted in dry-box to be analyzed in an environment free of oxygen and vapor.
Cell culture on flat hydrogels
To further evaluate the effect of the RGD peptide incorporation, HEPM cells, MSCs, and ADSCs were seeded onto flat hydrogels to study the cell attachment and proliferation. Many studies have indicated that the cell behavior, such as adhesion and spreading, is noticeably different when cells are cultured on alginate hydrogels that contain the RGD peptide.12–14,18,20,26,27,49 Hydrogels of 0.5% alginate-RGD, 2% alginate-RGD, and 2% unmodified alginate were used as the conditions. Hydrogels were made by first dissolving the respective lyophilized alginate in 0.25 M D-mannitol buffered with 10 mM HEPES to get the desired concentration; all solutions were syringe filtered through a 0.45 μm filter. Then, in a 24-well plate, 500 μL of the alginate solution were pipetted into separate wells. 100 mM CaCl2 was slowly added to the well to gel the alginate for 20 min. Then, once the hydrogels were formed, the CaCl2 was removed, gels were washed with 0.25 M D-mannitol, and cells were seeded onto the hydrogels at a concentration of 5x104 cells/mL and cultured in their respective medium under typical culture conditions. Live-dead staining was conducted on day 6 to show cell viability.
Fabrication of microfluidic device
All encapsulations in this report were conducted within a non-planar microfluidic device created through a fabrication method detailed elsewhere.35,44 Briefly, a silicon master was made through photolithography, and polydimethylsiloxane (PDMS) was poured onto the master and cured at 65 °C for 3 hours. The PDMS slab with the microchannel pattern was removed, and holes were punched at the inlets for fluid flow. At the flow-focusing junction, as seen in Figure S1, the oil channel was 400 μm x 400 μm, the shell channel was 300 μm x 300 μm, and the core channel was 200 μm x 200 μm.44 The channel sides were treated with oxygen plasma and then pressed together and aligned under a microscope. Methanol was used to prevent the channel sides from binding together prior to proper alignment. These devices were then placed in an oven at 65°C to allow the surfaces to bind and become hydrophobic.
Preparation of microencapsulation solutions
To examine the behavior of cells in response to the RGD peptide presence on a curved surface as well as within a 3D hydrogel microenvironment, we conducted microencapsulation of cells in the core of core-shell structured microcapsules. The cell-laden microcapsules were produced using the aforementioned microfluidic device, for which a “core solution” and a “shell solution” are needed to form the core and shell of the microcapsules, respectively. Two separate shell solutions were studied: 2% alginate-RGD and 2% alginate. Shell solutions were made by dissolving the respective lyophilized alginate in 0.25 M D-mannitol buffered with 10 mM HEPES to maintain a pH of 7.2. The three core solutions were characterized as containing 0%, 0.5%, and 2% alginate-RGD. The 0% alginate-RGD condition consisted of solely 2% carboxymethyl cellulose solution made by dissolving a 1:1 ratio of high viscosity and medium viscosity carboxymethyl cellulose in 0.25 M D-mannitol buffered with 10 mM HEPES. The 0.5% alginate-RGD core was made by dissolving lyophilized alginate-RGD in the 2% carboxymethyl cellulose solution to a concentration of 0.5%, and a 2% alginate-RGD core was made dissolving lyophilized alginate-RGD to a concentration of 2% in a 1% carboxymethyl cellulose solution of high viscosity carboxymethyl cellulose, to maintain similar viscosities between the core solutions. All solutions were syringe filtered through a 0.45 μm filter. Alginate and alginate-RGD were chosen as the shells to examine the difference in cell behavior in the presence of the RGD peptide, as cells typically do not adhere to the plain or unmodified alginate hydrogel. The 0% alginate-RGD condition was chosen because it consists of only 2% carboxymethyl cellulose to allow for the formation of a liquid core in the microcapsules. With this condition, any cell spreading due to the RGD peptide content in the shell can be determined. Moreover, this provides a 3D curved surface that can be used to examine any difference in the morphology of cells adhered to a 3D curved versus 2D flat surface. The 0.5% alginate-RGD core was used to study the effect of a softer 3D matrix on the cells, to see if they would prefer the soft 3D matrix or adhere to the stiffer alginate-RGD shell. The 2% alginate-RGD core was chosen to study the effect of a homogenous micro-matrix of 2% alginate-RGD on the cellular behavior.
Rheological characterization of core and shell hydrogels
Rheological characterization was conducted with a TA instrument AR-1000N rheometer to determine the difference in the storage (G′) and loss (G″) moduli between the shell and core hydrogels. 40 mm parallel plate geometry was used, and frequency sweeps were conducted to determine the values of G′ and G″. Each hydrogel was made by dissolving lyophilized purified alginate in 0.25 M D-mannitol solution to reach the desired concentration. Figure S2 shows a schematic illustration on how the samples were made for the rheological measurements. Gels were made separately by pipetting the respective alginate solution into a PDMS circular mold with a diameter of 40 mm; the alginate was then gelled with 75 mM CaCl2 for 20 min to obtain a flat gel 500 μm thick. The gels were washed with mannitol solution and then each gel was separately placed on the rheometer plate for analysis. The 0% alginate-RGD core condition, or 2% carboxymethyl cellulose, was pipetted directly onto the rheometer pedestal and cone-plate geometry was used for rheological characterization.
Encapsulation of HEPM cells, MSCs, and ADSCs
HEPM cells, MSCs, and ADSCs were studied to examine the cell morphology in alginate-RGD and alginate microcapsules. Cells were detached with trypsin/EDTA at 70% confluence for encapsulation and mixed in each of the core solutions (0%, 0.5%, and 2% alginate-RGD) at a density of 5x106 cells/mL by gently pipetting. The oil, shell, and core flow rates used were 7 mL/hr, 200 μL/hr, and 50 μL/hr respectively, and each solution was introduced into the microfluidic device through the respective inlets/channels, as shown in Figure S1. The oil solution was mineral oil emulsified with 100 mM aqueous solution of CaCl2 to crosslink the alginate in the shell solution as the two solutions came into contact with each other at the flow-focusing junction within the microfluidic device. An extraction solution of carboxymethyl cellulose was used in the extraction channel (Figure S1) to extract/transfer the microcapsules out of the oil and into an aqueous solution for efficient collection and ensure high cell viability.44,45,50,51 Microcapsules were collected at 4°C in a solution of the appropriate cell culture medium and 150 mM CaCl2 solution at a ratio of 1:1. After being centrifuged and washed three times in medium, cells were cultured in normal conditions in a 6-well plate. Medium was changed every other day.
Quantification of cell morphology
To determine the degree to which cells spread, the cell surface area and circularity were measured with NIH ImageJ. Circularity measures the compactness of the cell and is defined by Equation 1 below.
| (1) |
Values were averaged and analyzed with JMP 10 software for statistical significance. Cells from the flat hydrogels were measured in addition to cells cultured in the microcapsules to compare cell behavior on 2D flat surfaces as well as curved surfaces, in addition to culture in a 3D microenvironment. The main conditions studied were cells cultured on the alginate-RGD flat hydrogel (2D-RGD) or alginate flat hydrogel (2D-Alg), and in microcapsules with a 2% alginate-RGD shell with a 0% alginate-RGD core (RGD-0%), 2% alginate shell with a 2% alginate-RGD core (Alg-2%), and 2% alginate shell with a 0% alginate-RGD core (Alg-0%), as the control.
Results
XPS analysis of alginate-RGD
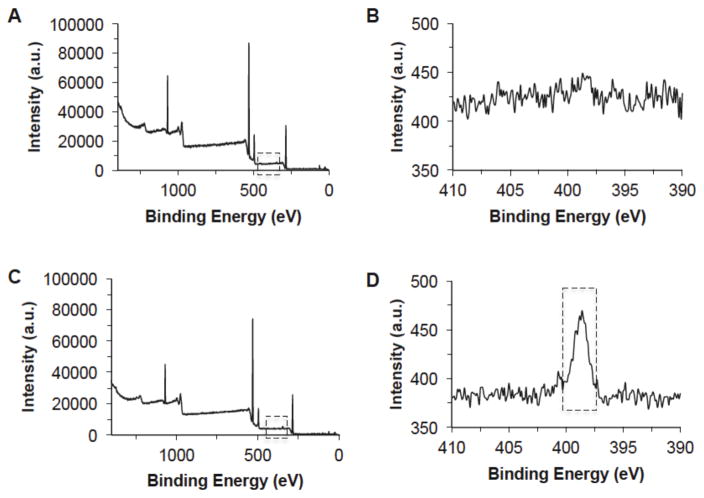
Following the RGD modification, X-ray photoelectron spectroscopy (XPS) was conducted in order to determine the success of the RGD incorporation to the alginate peptide. XPS functions by irradiating the sample’s surface with X-rays, and depending on which elements are present on the surface of the sample, different electron counts, measured as intensity, at element specific binding energy values (eV) are measured. Figure 1 shows that while the majority of the alginate-RGD spectrum remained unchanged (C), a new peak was observed between 395–400 eV (D), indicating the presence of nitrogen in the sample. As alginate alone does not include any nitrogen (B), this peak can be attributed to the successful incorporation of the RGD peptide within the alginate polymer backbone. Using a comparison of the peak intensities (or counts) of the carbon, oxygen, hydrogen, and nitrogen of the XPS spectrum, as well as the known atomic mass of the four elements, it was determined that the resultant alginate-RGD had a nitrogen composition of 0.55 ± 0.02% wt. This is equivalent to approximately one RGD peptide per 168 monosaccharide-units (i.e., ~30 kDa) of chain length in alginate on average. In other words, if the molecular weight of alginate were ~30 kDa, there would be one RGD on each alginate molecule. However, the molecular weight of alginate typically varies from a few tens to a few hundreds of kilodaltons.
Figure 1.
X-ray photoelectron spectroscopy (XPS) spectra showing the successful modification of alginate with RGD: (A) alginate, (B) nitrogen peak of alginate, (C) alginate-RGD, and (D) nitrogen peak of alginate-RGD. Boxed rectangle shows a higher nitrogen peak due to RGD modification.
Cell culture on flat hydrogels
Additionally, the effect of the RGD peptide modification was studied via cell attachment to flat alginate-RGD hydrogels, which was also used in comparison with the morphology of cells cultured in the 3D microenvironment. HEPM cells, MSCs and ADSCs were seeded onto flat hydrogels of 0.5% alginate-RGD, 2% alginate-RGD, and 2% unmodified alginate, and the day 7 images are shown in Figure 3.
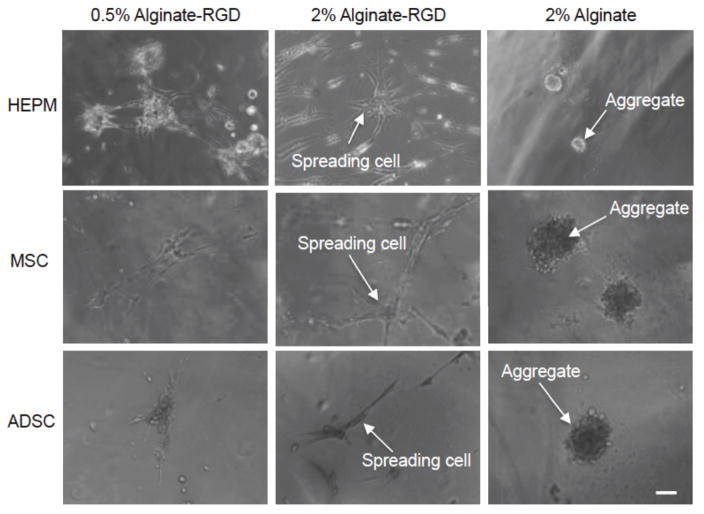
Figure 3.
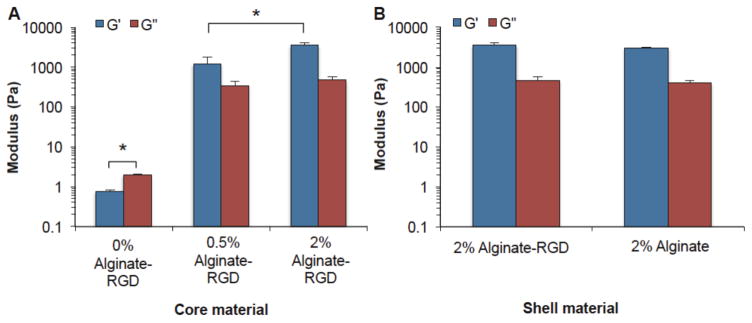
Storage modulus (G′) and loss modulus (G″) of the (A) core and (B) shell hydrogels. Solutions were gelled with 75 mM CaCl2 for 20 minutes prior to measurements. Significant differences were observed between the 0% alginate-RGD core and other conditions, as well as between the G′ of 0.5% and 2% alginate-RGD cores (p < 0.05). The 0% alginate-RGD core refers to a 2% carboxymethyl cellulose solution that contained no alginate-RGD. It is the solution in which alginate-RGD was dissolved in at the concentrations 2% and 0.5%, for making the 2% alginate-RGD and 0.5% alginate-RGD core, respectively.
As seen in Figure S3, by Day 4, HEPM cells spread on the 0.5% alginate-RGD hydrogel and the 2% alginate-RGD hydrogel. Spreading had not occurred on the 2% alginate, and there was very little attachment and proliferation. Figure 2 shows that by day 7, the majority of the HEPM cells had adhered to and spread on the 0.5% and 2% alginate-RGD hydrogels. The 2% unmodified alginate still did not have any cell spreading, but rather cells begun to form aggregates.
Figure 2.
Human embryonic palatal mesenchyme (HEPM) cells, mesenchymal stem cells (MSCs), and adipose derived stem cells (ADSCs) cultured on flat hydrogels of 0.5% alginate-RGD, 2% alginate-RGD, and 2% unmodified alginate on day 7. The initial cell density was 5x104 cells per well. Scale bar is 100 μm.
Compared to the HEPM cell morphology on the flat hydrogels, MSCs and ADSCs behaved in a similar manner. That is, as seen in Figure 2, cells adhered to and spread readily on both 0.5% and 2% alginate-RGD hydrogels, and instead of adhering, formed aggregates over the 2% alginate gels. All cell types were found to spread better on the 2% alginate-RGD hydrogels than 2% alginate without RGD or 0.5% alginate-RGD hydrogels. Images for days 0 and 4 can be found in the supplementary information (Figures S4–S6). Additionally, live-dead viability staining shows that cells were viable whether spread on the alginate-RGD hydrogels or aggregated on the alginate hydrogels (Figure S7), indicating that this difference in cell morphology is a result of the cells adherence in the presence of the RGD peptide.
Rheological characterization of core and shell hydrogels
Others have shown that cell spreading on top of a flat surface is dependent on the materials stiffness.52 To determine whether differences in the stiffness of the hydrogels could potentially be impacting cell spreading, rheology was used to determine the storage (G′) and loss (G″) moduli of the shell and core hydrogels. As seen in Figure 3, the 2% alginate-RGD core condition had the highest G′ and G″ moduli, followed by the 0.5% alginate-RGD condition, and then the 0% alginate-RGD condition (which was simply 2% carboxymethyl cellulose solution without any alginate-RGD). It was found that there was a significant difference (p < 0.05) for both the G′ and G″ of the 0% alginate-RGD core and the G′ and G″ for the other two core conditions. Furthermore, there was also a significant difference in the G′ of the 0.5% and 2% alginate-RGD core (p < 0.05).
Additionally, rheology was conducted on the shell conditions; this was done to determine if there was a significant difference in mechanical properties that may be effecting the cell spreading rather than an effect solely from the presence of the RGD peptide. Figure 3 also shows that while the alginate-RGD has a slightly higher loss and storage moduli than the unmodified alginate, the difference is not statistically significantly higher than the unmodified alginate. Therefore, any cell attachment to the alginate-RGD surface was not due to changes in the hydrogel stiffness but likely due to the presence of the RGD peptide.
Encapsulation of HEPM cells, MSCs, and ADSCs
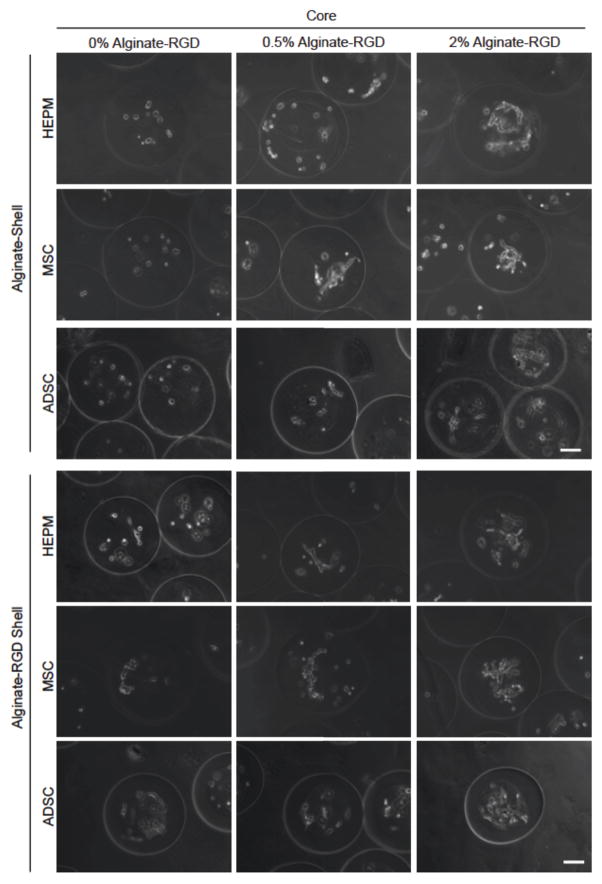
Encapsulation of the HEPM cells was conducted to determine if there would be a difference in the cell morphology on a curved surface and within 3D matrix. Figure 4 shows images or all cell types on day 6, while additional image can be found in the supplementary information for days 0 and 4. Figure 4 shows the encapsulation of HEPM cells in the two shell conditions (2% alginate and 2% alginate-RGD) as well as the three core conditions (0%, 0.5%, and 2% alginate-RGD). Microcapsule conditions will be labeled and discussed as shell material-core alginate-RGD concentration (e.g., Alg-2% for an alginate shell with a 2% alginate-RGD core, and RGD-0% for an alginate-RGD shell with a 0% alginate-RGD core). Spreading was observed in all microcapsules that contained the RGD peptide in their system, whether it was in the core or the shell. All HEPM cells within microcapsules of a 2% alginate-RGD shell exhibited attachment and spreading, while HEPM cells within microcapsules of the 2% alginate shell only spread in the 0.5% and 2% alginate-RGD core environments. Cells within the 0% alginate-RGD core remained rounded, indicating that the RGD peptide was required for cell spreading.
Figure 4.
Typical images showing the morphology of HEPM cells, MSCs, and ADSCs cultured in the core of microcapsules with an alginate or alginate-RGD shell on day 6. Three different cores were studied: liquid core (0% alginate-RGD), hydrogel core of 0.5% alginate-RGD, and hydrogel core of 2% alginate-RGD. Scale bar is 100 μm.
As HEPM cells are used to study (epigenetic) osteogenesis, it was hypothesized that they would adhere to and generally prefer to spread on a stiffer substrate. The most pronounced spreading can be seen in the 2% alginate-RGD shell with a 0% alginate-RGD core (RGD-0%), which indicates attachment on the stiff curved surface, as well as in both the 2% alginate-RGD and 2% alginate shelled microcapsules that have a core of 2% alginate-RGD, the stiffest of the cores.
The same encapsulation was done with MSCs, which, like HEPM cells, also exhibit a fibroblast like morphology. As seen in Figure 4, the MSCs spread in all alginate-RGD environments, much like the HEPM cells had. Only the microcapsules with 2% alginate shell and a 0% alginate-RGD core (Alg-0%) had cells that remained rounded.
ADSCs were also encapsulated in the same conditions, to determine if the RGD peptide effect would be different for the ADSCs, as they were derived from softer adipose tissue. As shown in Figure 4, the same peptide effect was observed in that only the microcapsules with the 2% alginate shell and 0% alginate-RGD core had cells that remained unattached and rounded. Again, spreading was not as pronounced in the 0.5% alginate-RGD cores. This continues to support the hypothesis that the presence and concentration of RGD peptide has an effect on the cell attachment within a 3D microenvironment.
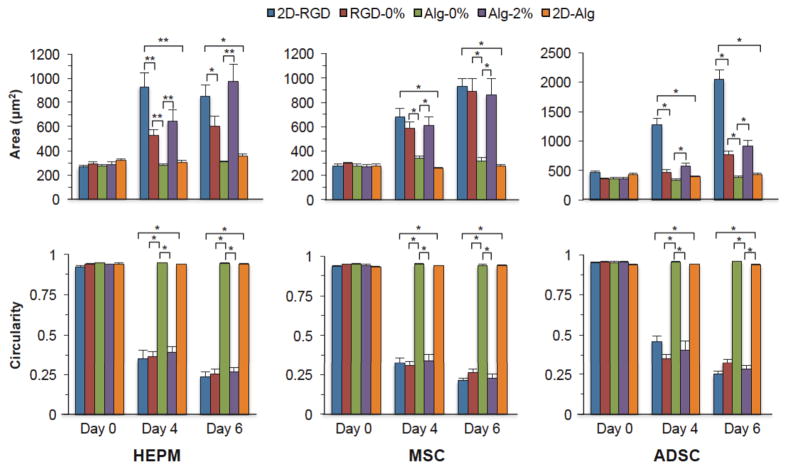
Quantification of cell surface area and circularity
The degree of cell spreading and attachment was quantified by measuring the cell surface area as well as the circularity. Circularity is an index of the compactness of an object; circularity would be one for a circular cell and would decrease as the cell elongated or formed extensions. To focus on the effect of RGD peptide in the shell, the morphology of cells cultured in microcapsules with a 2% alginate-RGD shell:0% alginate-RGD core (RGD-0%) was compared to the morphology of cells cultured in microcapsules with a 2% alginate shell:0% alginate-RGD core (Alg-0%) condition. The morphology of cells cultured in microcapsules with a 2% alginate-RGD shell:0% alginate-RGD core (RGD-0%) was also compared to the morphology of cells cultured on the flat 2% alginate-RGD hydrogel (2D-RGD) to compare the effect of a flat versus curved surface. Finally, to compare the effect of the alginate-RGD in the 3D microenvironment, the morphology of cells cultured in microcapsules with a 2% alginate shell:2% alginate-RGD core (Alg-2%) was compared to the morphology of cells cultured in microcapsules with a 2% alginate shell:0% alginate-RGD core (Alg-0%). The morphology of cells cultured in microcapsules with a 0.5% alginate-RGD core was excluded from this analysis, as not as large of an effect was observed as compared to the morphology of cells cultured in microcapsules within the 2% alginate-RGD cores.
As can be seen in Figure 5, there is a clear increase in HEPM cell surface area on both days 4 and 6 for all conditions that contain the RGD peptide, specifically the flat RGD hydrogel (2D-RGD), the 2% alginate-RGD shell:0% alginate-RGD core (RGD-0%), and the 2% alginate:2% alginate-RGD core (Alg-2%). Significant differences were also seen between the conditions of interest (p < 0.01). The 2D-RGD had a significantly higher surface area than the RGD-0% on day 4 (p < 0.01). There was also a significant increase in cell surface area for the Alg-2% compared to the Alg-0% (p < 0.01). The 2D-RGD also had a larger cell surface area than the flat 2% alginate hydrogel cells (2D-Alg) (p < 0.05).
Figure 5.
Quantitative analysis of the HEPM cell, MSC, and ADSC morphology including surface area and circularity for cells cultured in microcapsules with either alginate or alginate-RGD shell (Alg-, RGD) and different core alginate-RGD concentrations (0%, 2%). Additionally, the cell surface area and circularity for cells cultured on the 2D hydrogels of alginate-RGD (2D-RGD) and alginate (2D-Alg) were also quantified. Modification of alginate with the RGD peptide resulted in significant changes in the cell morphology (**p < 0.01, *p < 0.05).
As expected, there were also significant differences in the HEPM cell circularity. On days 4 and 6 there was a significant decrease in the circularity of all conditions with the RGD peptide (p < 0.01). Additionally, on days 4 and 6 there was a significant decrease (p < 0.01) in both the RGD-0% and Alg-2% circularities compared to the control Alg-0% circularity. However, even though the 2D-RGD had a higher surface area than the RGD-0%, there was no significant difference in their circularities; therefore, while the cells on the flat alginate-RGD surface (2D-RGD) may have spread more, it had a similar degree of spreading as cells on the curved alginate-RGD surface (RGD-0%).
Figure 5 shows the cell surface area and circularity for the MSC microcapsules. On days 4 and 6, there was a significant increase (p < 0.01) in both RGD-0% and Alg-2% surface area compared to Alg-0%. As expected, there was also a significant decrease (p < 0.01) in the circularity on both days 4 and 6 for the RGD-0% and Alg-2% cells compared to the Alg-0% cells. Furthermore, there was a significant difference (p < 0.01) in the cell surface area and the circularity between cells cultured on both flat gels on days 4 and 6. However, there was no significant difference in the cell surface area or circularity between the 2D-RGD and RGD-0% cells on both days, indicating that MSCs will adhere to and spread on a curved surface as well as they will to a flat surface. This is similar to the HEPM cells, which also had no significant difference between the surface area and circularity of 2D-RGD and RGD-0% cells.
The same trends were observed in the ADSCs, shown in Figure 5, in that the conditions containing the RGD peptide had significant increases (p < 0.01) in cell surface area and significant decreases (p < 0.01) in the circularity. As shown, days 4 and 6 show a significant increase (p < 0.01) in cell surface area in the Alg-2% cells compared to those in the Alg-0%. Significant differences were also observed on days 4 and 6 between cells cultured in 2D-RGD and 2D-Alg, as well as between 2D-RGD and RGD-0%. Correspondingly, there was a significant difference (p < 0.01) in the circularity of the Alg-2% cells compared to the circularity of the Alg-0% cells, as well as between the two cells from flat gel conditions and between 2D-RGD and RGD-0% cells. As for the RGD-0%, while there wasn’t a significant difference (p < 0.01) in cell surface area on day 4 between RGD-0% and Alg-0%, there was a significant decrease (p < 0.01) in the circularity that still indicates the RGD peptide effect from the curved surface. The RGD-0% had a significant increase (p < 0.01) in surface area on day 6, as well as a significant decrease (p < 0.01) in circularity compared to Alg-0%.
Discussion
The RGD peptide sequence has been extensively studied and shown to effectively promote various cell attachment to a 2D flat surface. This study confirmed the success of the alginate modification with an RGD peptide through XPS analysis indicating the presence of a nitrogen peak. The functional significance of this modification was demonstrated by the increased spreading of the cells when seeded on the 0.5% alginate-RGD and 2% alginate-RGD hydrogels as opposed to 2% alginate hydrogel surfaces. Cell attachment and spreading was observed on 0.5% and 2% alginate-RGD 2D hydrogel surfaces, with better spreading after day 7. This is consistent with previous studies demonstrating cell attachment in the presence of the RGD peptide and the rounded cell morphology observed on unmodified alginate substrates.8,9,12,14,18,20,26,27,44,49
Next, HEPM cells, as well as MSCs and ADSCs, were encapsulated through the previously described microencapsulation methods to evaluate the impact that the RGD peptide presence in the substrate has on different stem cell types. Two shell conditions were studied: 2% alginate-RGD and 2% unmodified alginate. Additionally, three different core conditions were studied for each shell type: 0%, 0.5%, 2% alginate-RGD hydrogel. Rheology was conducted on all shell and core solutions. It was found that while the 2% alginate-RGD hydrogel had slightly higher loss and storage moduli than the 2% unmodified alginate, it was not significant and so any effect on cell attachment to the shell would be a result of the presence of adhesion ligands from the RGD peptide. As expected, the 2% RGD alginate hydrogel core had the highest moduli, followed by the 0.5% RGD alginate hydrogel core, and then the 0% RGD alginate core (2% carboxymethyl cellulose liquid core).
The different types of stem cells were studied to see if there would be a difference in the cell behavior in favor of a stiffness effect, or if the RGD effect would take precedence. All three stem cell types attached and spread in all systems in which RGD was present. This means that cells remained rounded only in the microcapsules with a 2% alginate shell:0% alginate-RGD core. As all three stem cell types adhered readily to shell of the microcapsules with the 2% alginate-RGD shell:0% alginate-RGD core, it can be determined that all cell types found the curved hydrogel surface favorable for attachment and spreading. This was also supported by the significant decreases in circularity and significant increases in cell surface area that were observed. While the cells did not have as pronounced of spreading in the 0.5% alginate-RGD core, there was still an apparent fibroblast like morphology. Spreading did appear to be slightly better in the 2% alginate-RGD shell:0.5% alginate-RGD core than in the 2% alginate shell:0.5% alginate-RGD core, most likely due to the increased RGD peptide content in that system. Finally, whether the shell was comprised of 2% alginate-RGD or 2% alginate, all cells seemed to attach and spread prominently within the 2% alginate-RGD core. There was even the presence of some aggregate formation from the HEPM cells and MSCs, which is atypical compared to 2D conventional culture. Overall, systems that contained the RGD peptide had significant increases in the cell surface area and significant decreases in the circularity (p < 0.01). It appears that the RGD peptide effect does indeed outweigh the stiffness effect, as it was originally hypothesized that the ADSCs would prefer a microenvironment of a softer matrix, like the 0.5% alginate-RGD hydrogel core.
This study illustrates the simplicity of combining the chemistry of the RGD peptide modification with the technology of the microfluidic encapsulation in order to study the effect that different surface compositions can have on the behavior of various types of stem cells. Future work into studying the gene expression to determine any genotypic differences can be done to shed more light on the extent of the RGD peptide effect on cell behavior when incorporated into the miniaturized 3D culture conditions.
Supplementary Material
Acknowledgments
This work was partially supported by grants from NSF (CBET-1033426) and NIH (R01EB012108). We would like to thank Dr. Lisa Hommel in The Ohio State University Surface Analysis Facility for her assistance in conducting and analyzing the XPS spectra and Anne Shim for her technical help.
Footnotes
Conflicts of Interest:
Jenna Dumbleton, Pranay Agarwal, Haishui Huang, Nathaniel Hogrebe, Renzhi Han, Keith J. Gooch, and Xiaoming He declare that they have no conflicts of interest.
Ethical Standards:
No human studies were carried out by the authors for this article.
No animal studies were carried out by the authors for this article.
References
- 1.Langer RJV. Tissue Engineering. Science. 1993;260:920–26. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Alsberg EKA, Albeiruti A, Rowley JA, Mooney DJ. Engineering Growing Tissues. Proc Natl Acad Sci USA. 2002;99:12025–30. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augst AD, HK, Mooney DJ. Alginate Hydrogels as Biomaterials. Macromol. 2006;6:623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 4.Lee KY, DM Alginate: Properties and Biomedical Applications. Prog Polym Sci. 2012;37:106–26. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowley JA, GM, Mooney DJ. Alginate Hydrogels as Synthetic Materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 6.Ouwerx C, NV, Mestdagh MM, Axelos MAV. Physico-Chemical Properties and Rheology of Alginate Gel Beads Formed with Various Divalent Cations. Poly Gels & Net. 1998;6:393–408. [Google Scholar]
- 7.Harper BA, SB, Lim LT, Marcone MF. Effect of Various Gelling Cations on the Physical Properties of “Wet” Alginate Films. J Food Sci. 2014;79:e562–7. doi: 10.1111/1750-3841.12376. [DOI] [PubMed] [Google Scholar]
- 8.Shu XZ, KG, Liu Y, Palumbo F, Luo Y, Clark R, Prestwich G. Attachment and Spreading of Fibroblasts on an Rgd Peptide-Modified Injectable Hyaluronan Hydrogel. J Biomed Research A. 2004;68:365–75. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 9.Genes NG, JR, Mooney DJ, Bonassar LJ. Effect of Substrate Mechanics on Chondrocyte Adhesion to Modified Alginate Surfaces. Arch Biochem Biophys. 2004;422:161–7. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Hsiong SX, TB, Huebsch N, Mooney DJ. Cyclic Arginine-Glycine-Aspartate Peptides Enhance Three-Dimensional Stem Cell Osteogenic Differentiation. Tissue Eng Part A. 2009;15:263–72. doi: 10.1089/ten.tea.2007.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comisar WA, NK, Mooney DJ, Linderman JJ. Engineering Rgd Nanopatterned Hydrogels to Control Preosteoblast Behavior: A Combined Computational and Experimental Approach. Biomaterials. 2007;28:4409–17. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Alsberg E, AK, Albeiruti A, Franceschi RT, Mooney DJ. Cell Interactive Alginate Hydrogels for Bone Tissue Engineering. J Dent Res. 2001;80:2025–29. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 13.Alsberg E, KH, Hirano Y, Smith MK, Albeiruti A, Mooney DJ. Regulating Bone Formation Via Controlled Scaffold Degradation. Journal of Dental Research. 2003;82:903–8. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, AE, Hsiong S, Comisar W, Linderman J, Ziff R, Mooney DJ. Nanoscale Adhesion Ligand Organization Regulates Osteoblast Proliferation and Differentiation. Nano Lett. 2004;4:1501–6. doi: 10.1021/nl0493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons CA, AE, Hsiong S, Kim WJ, Mooney DJ. Dual Growth Factor Delivery and Controlled Scaffold Degradation Enhance in Vivo Bone Formation by Transplanted Bone Marrow Stromal Cells. Bone. 2004;35:562–9. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Barkhordarian A, JS, Cayabyab R, Mahanian N, Chiappelli F. Epigenetic Regulation of Osteogenesis: Human Embryonic Palatal Mesencymal Cells. Bioinformation. 2011;5:278–81. doi: 10.6026/97320630005278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider GB, RZ, Seabold D, Keller J, Stanford C. Differentiation of Preosteoblasts Is Affected by Implant Surface Microtopographies. J Biomed Mater Res A. 2004;69:462–8. doi: 10.1002/jbm.a.30016. [DOI] [PubMed] [Google Scholar]
- 18.Jeon O, AE Photofunctionalization of Alginate Hydrogels to Promote Adhesion and Proliferation of Human Mesenchymal Stem Cells. Tissue Eng Part A. 2013;19:1424–32. doi: 10.1089/ten.tea.2012.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang SW, BC, Park H, Park KS, Lee KY, Lee SH. The Effect of Conjugating Rgd into 3d Alginate Hydrogels on Adipogenic Differentiation of Human Adipose-Derived Stromal Cells. Macromol Biosci. 2011;11:673–9. doi: 10.1002/mabi.201000479. [DOI] [PubMed] [Google Scholar]
- 20.Jeon O, AE Regulation of Stem Cell Fate in a Three-Dimensional Micropatterned Dual-Crosslinked Hydrogel System. Adv Func Mater. 2013;23:4765–75. doi: 10.1002/adfm.201300529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, JL, Roy K. Effect of 3d Scaffold and Dynamic Culture Condition on the Global Gene Expression Profile of Mouse Embryonic Stem Cells. Biomaterials. 2006;27:5878–89. doi: 10.1016/j.biomaterials.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 22.Cukierman E, RP, Stevens DR, Yamada KM. Taking Cell-Matrix Adhesions to the Third Dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 23.DTS The Stem-Cell Niche as an Entity of Action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 24.Griffith LG, MS Capturing Complex 3d Tissue Physiology in Vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 25.Fluri DA, PT, Song H, Baptista RP, Shakiba N, Shukla S, Clarke G, Nagy A, Zandstra PW. Derivation, Expansion and Differentiation of Induced Pluripotent Stem Cells in Continuous Suspension Cultures. Nat Methods. 2012;9:509–16. doi: 10.1038/nmeth.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon O, AD, Linderman SW, Alsberg E. Biochemical and Physical Signal Gradients in Hydrogels to Control Stem Cell Behavior. Adv Mater. 2013;25:6366–72. doi: 10.1002/adma.201302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samorezov JE, MC, Alsberg E. Dual Ionic and Photo-Crosslinked Alginate Hydrogels for Micropatterned Spatial Control of Material Properties and Cell Behavior. Bioconjugate Chem. 2015;26:1339–47. doi: 10.1021/acs.bioconjchem.5b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson JL, TM Stem Cell Microencapusulation for Phenotypic Control, Bioprocessing, and Transplantation. Biotechnol Bioeng. 2013;110:667–82. doi: 10.1002/bit.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velasco D, ET, Kumacheva E. Microfluidic Encapsulation of Cells in Polymer Microgels. Small. 2012;8:1633–42. doi: 10.1002/smll.201102464. [DOI] [PubMed] [Google Scholar]
- 30.Ma M, AC, Saha G, Doloff JC, Dholakia N, Thakrar R, Cohen J, Vegas A, Chen D, Bratlie KM, Dang T, York RL, Hollister-Lock J, Weir GC, Anderson DG. Core-Shell Hydrogel Microcapsules for Improved Islets Encapsulation. Adv Healtchare Mater. 2013;2:667–72. doi: 10.1002/adhm.201200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, SZ, Rao W, Snyder J, Choi JK, Wang J, Khan IA, Saleh NB, Mohler PJ, Yu J, Hund TJ, Tang C, He X. A Novel Core-Shell Microcapsule for Encapsulation and 3d Culture of Embryonic Stem Cells. J Mater Chem B Mater Biol Medl. 2013;1:1002–9. doi: 10.1039/C2TB00058J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serra M, CC, Malpique R, Brito C, Jensen J, Bjorquist P, Carrondo MJ, Alves PM. Microencapsulation Technology: A Power Tool for Integrating Expansions and Cryopreservation of Human Embryonic Stem Cells. PLoS One. 2011;6:e23212. doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, GY, Zhang L, Xu X, He X. Preferential Vitrification of Water in Small Alginate Microcapsules Significantly Augments Cell Cryopreservation by Vitrification. Biomed Microdevices. 2012;12:89–96. doi: 10.1007/s10544-009-9363-z. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, XH Microencapsulating and Banking Living Cells for Cell-Based Medicine. Healthcare Eng. 2011;2:427–46. doi: 10.1260/2040-2295.2.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal P, Zhao S, Bielecki P, Rao W, Choi JK, Zhao Y, Yu J, Zhang W, He X. OneStep Microfluidic Generation of Pre-Hatching Embryo-Like Core-Shell Microcapsules for Miniaturized 3d Culture of Pluripotent Stem Cells. Lab Chip. 2013;13:4525–33. doi: 10.1039/c3lc50678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang KS, KL, Yeh CS, Chung SR, Lin CH, Dong YS. Microfluidic Controlling Monodisperse Microdroplet for 5-Fluorouracil Loaded Genipin-Gelatin Microcapsules. J Controlled Release. 2009;137:15–9. doi: 10.1016/j.jconrel.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Overhauser J. Encapsulation of Cells in Agarose Beads. Methods Mol Biol. 1992;12:129–34. doi: 10.1385/0-89603-229-9:129. [DOI] [PubMed] [Google Scholar]
- 38.Chayosumrit M, BT, Sidhu K. Alginate Microcapsule for Propagation and Directed Differentiation of Hescs to Definitive Endoderm. Biomaterials. 2010;31:505–14. doi: 10.1016/j.biomaterials.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, XH Encapsulation of Living Cells in Small (Approximately 100 Microm) Alginate Microcapsules by Electrostatic Spraying: A Parametric Study. J Biomech Eng. 2009;131:074515. doi: 10.1115/1.3153326. [DOI] [PubMed] [Google Scholar]
- 40.Watt FM, BH Out of Eden: Stem Cells and Their Niches. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, WW, Ma J, Guo X, Yu Z, Ma X. Proliferation and Differentiation of Mouse Embryonic Stem Cells in Apa Microcapsule: A Model for Studying the Interaction between Stem Cells and Their Niche. Biotechnol Prog. 2006;22:791–800. doi: 10.1021/bp050386n. [DOI] [PubMed] [Google Scholar]
- 42.Maguire T, EN, Schloss R, Yarmush M. Alginate-Pll Microencapsulation: Effect on the Dfiferentation of Embryonic Stem Cells into Hepatocytes. Biotechnol Bioeng. 2006;93:581–91. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, Agarwal P, Rao W, Huang H, Zhang R, Liu Z, Yu J, Weisleder N, Zhang W, He X. Coaxial Electrospray of Liquid Core-Hydrogel Shell Microcapsules for Encapsulation and Miniaturized 3d Culture of Pluripotent Stem Cells. Integrative Biology. 2014;6:874–884. doi: 10.1039/c4ib00100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal P, Choi JK, Huang H, Zhao S, Dumbleton J, Li J, He X. A Biomimetic Core-Shell Platform for Miniaturized 3d Cell and Tissue Engineering. Particle & Particle Systems Characterization. 2015;32:809–816. doi: 10.1002/ppsc.201500025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi JK, Agarwal P, Huang H, Zhao S, He X. The Crucial Role of Mechanical Heterogeneity in Regulating Follicle Development and Ovulation with Engineered Ovarian Microtissue. Biomaterials. 2014;35:5122–8. doi: 10.1016/j.biomaterials.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, He X. Fluid Displacement During Droplet Formation at Microfluidic Flow-Focusing Junction. Lab Chip. 2015;15:4197–4205. doi: 10.1039/c5lc00730e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakai S, IH, Kawakami K. Production of Cell-Enclosing Hollow-Core Agarose Microcapsules Via Jetting in Water-Immiscible Liquid Paraffin and Formation of Embryoid Body-Like Spherical Tissues from Mouse Es Cells Enclosed within These Microcapsules. Biotechnol Bioeng. 2008;99:235–43. doi: 10.1002/bit.21624. [DOI] [PubMed] [Google Scholar]
- 48.Sakai S, SI, Kawakami K. Calcium Alginate Microcapsules with Spherical Liquid Cores Templated by Gelatin Microparticles for Mass Production of Multicellular Spheroids. Acta Biomater. 2010;6:3132–7. doi: 10.1016/j.actbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Jeon O, PC, Ahmed SM, Alsberg E. Biodegradable, Photocrosslinked Alginate Hydrogels with Independently Tailorable Physical Properties and Cell Adhesivity. Tissue Eng Part A. 2010;16:2915–25. doi: 10.1089/ten.TEA.2010.0096. [DOI] [PubMed] [Google Scholar]
- 50.Huang H, He X. Interfacial Tension Based on-Chip Extraction of Microparticles Confined in Microfluidic Stokes Flows. Applied Physics Letters. 2014;105:143704. doi: 10.1063/1.4898040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang H, Sun M, Heisler-Taylor T, Kiourti A, Volakis J, Lafyatis G, He X. Stiffness-Independent Highly Efficient on-Chip Extraction of Cell-Laden Hydrogel Microcapsules from Oil Emulsion into Aqueous Solution by Dielectrophoresis. Small. 2015;11:5369–5374. doi: 10.1002/smll.201501388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engler ALB, Newman C, Hategan A, Griffin M, Discher D. Substrate Compliance Versus Ligand Density in Cell on Gel Responses. Biophys J. 2004;86 doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.