Abstract
CD4+Foxp3+ regulatory T cells (Tregs) are key immune suppressors that regulate immunity in diverse tissues. The tissue and/or inflammatory signals that influence the magnitude of the Treg response remain unclear. To define signals that promote Treg accumulation we developed a simple system of skin inflammation using defined antigens and adjuvants that induce distinct cytokine milieus: OVA protein in CFA, Aluminum salts (Alum) and Schistosoma mansoni Eggs (Sm Egg). Polyclonal and antigen-specific Treg accumulation in the skin differed significantly between adjuvants. CFA and Alum led to robust Treg accumulation with over 50% of all skin CD4+ T cells being Foxp3+. In contrast, Tregs accumulated poorly in the Sm Egg inflamed skin. Surprisingly, we found no evidence of inflammation-specific changes to the Treg gene program between adjuvant-inflamed skin types suggesting a lack of selective recruitment or adaptation to the inflammatory milieu. Instead, Treg accumulation patterns were linked to differences in CD80/CD86 expression by APC and the regulation of CD25 expression, specifically in the inflamed skin. Inflammatory cues alone, without cognate antigen, differentially supported CD25 upregulation (CFA and Alum > Sm Eggs). Only in inflammatory milieus that upregulated CD25 did the provision of antigen enhance local Treg proliferation. Reduced IL-33 in the Sm Egg inflamed environment was shown to contribute to the failure to upregulate CD25. Thus, the magnitude of the Treg response in inflamed tissues is controlled at two inter-dependent levels: inflammatory signals that support the upregulation of the important Treg survival factor, CD25, and antigen signals that drive local expansion.
Introduction
CD4+ Forkhead boxP3 (Foxp3+) regulatory T cells (Tregs) are important immune sentinels that orchestrate the control of homeostatic and infectious immune activation. Studies have elegantly defined Treg differentiation, homeostasis, phenotype and suppressive function using Tregs within lymphoid tissues (1). However, recent observations suggest that there is a substantial degree of peripheral reshaping of Treg phenotype and function depending on the tissue location and inflammatory milieu (2–4). Tregs in distinct peripheral tissues express specific gene programs that appear to facilitate tailored local function (5, 6) while the type of inflammation can also drive Treg functional specialization (7–11). The relative contribution of location and inflammation to Treg dynamics in peripheral tissues remains unclear. Defining the factors controlling the accumulation, size and function of the Treg compartment in specific tissues will be important as strategies for therapeutic manipulation of Tregs advance.
Increases in Treg number are seen in a variety of infected and autoimmune tissues (12–17). Local Treg proliferation in non-lymphoid tissues has been observed (15, 18, 19) and can be linked in part to cognate antigen expression(13, 20). Co-stimulatory signals from CD28 and CTLA-4, important for Treg development and homeostasis (21, 22), also tune antigen-induced Treg proliferation and function in the periphery (23–27). IL-2 provides a potent survival signal to Tregs (28–31) and has recently become a primary target for regulating Treg number in vivo (32–34). A deficiency in IL-2 within target tissues has been linked to the negative regulation of Tregs in autoimmune settings through downregulation of CD25 expression (19). Less well understood are the positive signals that support CD25 expression by Tregs in peripheral tissues and promote local Treg accumulation. Recent studies suggest IL-33 may be an important factor in promoting Treg expansion in tissues via upregulation of local IL-2 (35, 36) and can be counter-regulated by IL-23 (37).
To study the signals that modulate Treg accumulation at sites of inflammation we focused our studies on the skin and assessed Treg accumulation to ovalbumin (OVA) immunization in the context of different types of induced inflammation, using distinct Th1 (Complete Freunds Adjuvant, CFA) or Th2-inducing (Aluminum Salts, Alum or Schistosome mansoni Eggs, Sm Egg) adjuvants. We demonstrate distinct variations in Treg accumulation in the skin between inflammatory milieus with robust accumulation of Tregs in CFA and Alum-induced skin inflammation but not in Sm Egg inflamed skin. This was tissue specific, observed in the inflamed skin but not the draining lymph node (dLN), and cell type specific, seen for polyclonal and antigen-specific Tregs but not conventional CD4+ T cells (Tconv). The differences were not dependent on the primary effector cytokines in the skin, nor did they reflect the selection of particular milieu-specific Treg subsets. Rather, we found the distinct inflammatory milieus differentially regulated the expression of CD25 by Tregs in the skin, independent of antigen. CFA and Alum potently upregulated CD25 on Tregs while the Sm-Egg milieu failed to support CD25 upregulation. The upregulation of CD25 correlated with differences in extent of CD80/CD86 expression by APCs in the skin. However, CD25 upregulation by the inflammatory milieu was not sufficient to drive local Treg proliferation, which required cognate antigen. Unexpectedly, the effect of antigen was only seen in inflammatory settings that supported CD25 upregulation in the absence of antigen, suggesting that distinct inflammatory cues pre-condition Tregs for receptivity to antigen in inflamed tissues.
Materials and Methods
Mice
Female WT, DO11.10 TCR Tg+, Foxp3-GFP and Thy1.1 BALB/c mice were purchased from The Jackson Laboratory. 4get BALB/c mice were provided by R. Locksley (UCSF). All mouse experimentation was reviewed and approved by the University Committee for Animal Resources at the University of Rochester Medical Center.
Cell Purification
CD4+T cells were enriched from SPN and LN by complement lysis of cells bearing CD8 (3.155), MHC class II (M5/114), and heat-stable Ag (J11D) as described(38) (ATCC). CD25+ and CD25− cells were enriched using MACS column (Miltenyi Biotech) and sorted (FACS Aria, BD) for KJ126+ Foxp3-GFP+ CD62L+ CD44− naïve Tregs and KJ126+ Foxp3GFP− CD62L+ CD44− naïve Tconv cells.
TCR Tg Cell Adoptive Transfer
3–3.5×105 naïve Thy1.1+ DO11.10+ Tregs and 3–3.5×105 naïve Thy1.1+/1.2+ DO11.10+ Tconv cells were injected i.v. together, or alone, into congenic Thy1.2+ WT BALB/c mice 24 h prior to immunization. Day 7 post immunization, single cell suspensions from ear and LN were assessed by flow cytometry.
Immunizations
WT or 4get mice were immunized intradermally in the ear with OVA protein (20μg/ear) and the adjuvants CFA, Alum or Sm Eggs (750 eggs/ear). In some experiments, the contralateral ear was immunized with Keyhole Limpet Hemocyanin (KLH) protein (5μg per ear) and adjuvants. For the peptide pulse, mice were treated with 40μg OVA peptide i.v. in 200μL of PBS 6 h prior to harvest.
Flow Cytometry
Ears were treated for 30 m at 37°C with 1μg of activated Collagenase Dispase (Roche) and deactivated using EDTA. Single cell suspensions of skin and LN were stained with Abs from eBioscience unless otherwise stated: LiveDead Aqua (Lifetech), CD45 (BD clone 30-F11), CD4 (BD clone GK1.5), Thy1.1 (clone H1S51), Thy1.2 (BD clone 53-2.1), CD25 (clone PC61.5), CD127(clone A7R34), FcER1 (clone MAR-1), T1/ST2 (MBBiosystems clone DJ8), CD49b (clone DX5), Gr-1 (Biolegend clone RB6-8C5), CD11b (Biolegend clone M1/70) and Foxp3 (clone FJK-16S) using Foxp3 fix/perm reagents. For 5-bromo-2-deoxyuridine (BrdU), mice received 1mg of BrdU i.p. in PBS 4 hours prior to harvest. Cells were stained with BrdU APC Staining kit (BD).
Cytokine measurements
Whole ear homogenate or 1×106 LN cells were stimulated O/N with 2μM of OVA peptide in Elispot plates coated with Ab for IFNy or IL-4 as described (39). For cytokines in the ear, the ear was treated for 30 m at 37°C with Collagenase Dispase and homogenized in 500μL PBS. Ear homogenate supernatants were assayed for cytokines with the Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead Panel kit. For cytokine blockade, 1mg of anti-IFNy Ab (XMG 1.2) or anti-IL-4 Ab (11B11) or PBS was given i.p. on d6, 7, 8, 9 post-immunization and harvested on d10. For IL-23 cytokine blockade, 100μg of anti-IL23 p19 (Biolegend, Clone MMp19B2) or IgG control was administered i.p. d4, d5, d6 post immunization and harvested d7.
Taqman Low density Array (TLDA)
cDNA from purified Tregs from ear and LN was isolated using Cells-To-CT kit (Ambion Life Technologies). Samples were pre-amplified using primer pools specific to custom TLDA’s (Supplementary Figure 1). Amplified samples were loaded onto TLDA cards and RT-PCR performed using 7900HT Fast Real-Time PCR System (Applied Biosystems) at the University of Rochester Genomics Center. HPRT and GAPDH were used as endogenous controls, and Treg samples from non-draining LN were used as the calibrator.
Anti-IL-23 blockade and IL-33 administration
Anti-IL-23 Ab (Biolegend) or control IgG was administered to immunized mice on days 4, 5 and 6 post-immunization, 100μg/mouse i.p. (40). Recombinant IL-33 (Biolegend) or PBS was administered to immunized mice on days 4, 5 and 6 post-immunization, 2μg/mouse i.p. (41). Treg accumulation in the inflamed ear was assessed day 7 post-immunization.
Statistical Analysis
All statistical analysis was done in graphpad PRISM. Where indicated, Mann-Whitney, Wilcoxon matched pairs or Two-way ANOVA tests were performed for the data. *P< 0.05, **P< 0.005, ***P< 0.0005.
Results
Differential Treg accumulation in the skin with distinct adjuvant-induced inflammation
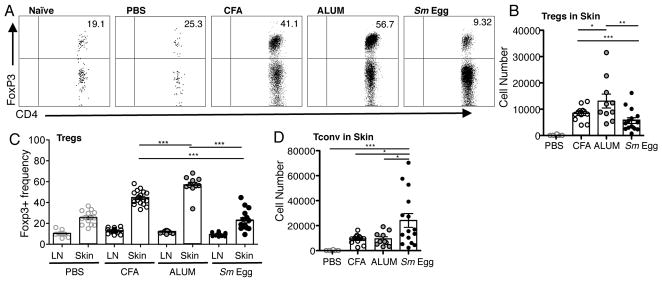
To study the effect of inflammation on Tregs accumulation in the skin, we used adjuvants previously reported to induce distinct Type 1 or 2 cytokine responses (42–46). Foxp3-GFP mice were immunized intradermally with OVA protein in PBS, CFA, Alum or with Schistosome mansoni Eggs (Sm Egg). Seven days post-immunization, cells in the skin and draining lymph nodes (dLN) were assayed for CD4+ Tconv and CD4+ Foxp3+ Treg populations by flow cytometry. Inflammation led to significant increases in the number and frequency of Foxp3+ Tregs in the skin, representing more than half of all CD4+ T cells under certain inflammatory conditions (Fig. 1A–C). The Treg magnitude differed with the adjuvant: CFA and Alum driving strong Treg numbers and Sm Egg-induced inflammation supporting significantly lower Treg numbers (Fig. 1A, 1B). Treg frequencies were consistently elevated in the inflamed tissues compared to their respective dLNs (Fig 1C). Indeed, adjuvant-induced changes in Treg frequency were not seen in the dLN (Fig. 1C), consistent with the local cutaneous milieu modulating Treg accumulation. Adjuvant effects on T cell accumulation in the skin appear to be Treg-specific because the number of Tconv cells were not compromised in the Sm Egg-inflamed conditions (Fig. 1D).
FIGURE 1.
Distinct polyclonal Treg accumulation in the skin with different types of adjuvant-induced inflammation. (A) Representative FACS plots of CD4+ Foxp3+ Tregs cells in inflamed skin, d7 after immunization of BALB/c mice with OVA protein in CFA, Alum or Sm Egg. Numbers in quadrant are % of CD4+ T cells. (B) Number of Foxp3+CD4+ Tregs in the skin on d7. Each symbol represents an individual mouse. (C) Frequency of Foxp3+ Tregs within the CD4 compartment in dLN (LN) and skin as in (A). (D) Number of Foxp3− CD4+ Tconv cells in skin on d7. 4 independent experiments, 3–4 mice per group. Statistics by Mann-Whitney.
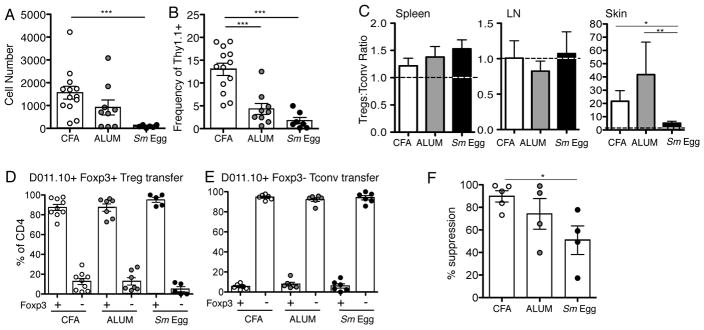
To determine if this accumulation pattern was seen with antigen-specific Tregs, we transferred naïve OVA-specific Foxp3-GFP DO11.10 Tregs (CD4+CD62L+CD44− Foxp3+) and Tconv cells (CD4+CD62L+CD44− Foxp3−) into WT hosts 24 h prior to immunization. DO11.10+ Tregs accumulated to high numbers in the CFA and Alum-inflamed skin, with a lower number in Sm Egg-inflamed skin (Fig. 2A, 2B), leading to a striking dominance of antigen-specific Tregs in the inflamed skin. The input ratio of Treg to Tconv cells (1:1) was maintained in the dLN and SPN but markedly elevated in the skin with CFA and Alum-induced inflammation (Fig. 2C). As seen with the polyclonal response, the Sm Egg-induced inflammation failed to support DO11.10+ Treg accumulation in the skin (Fig 2A–C). Thus, Treg accumulation in the inflamed skin is robust and the magnitude of this response is inflammatory-context dependent.
FIGURE 2.
Antigen-specific Treg accumulation in different types of skin inflammation. Sorted Treg (Thy1.1) and Tconv (Thy1.1/1.2) DO11.10+ T cells were adoptively transferred to BALB/c mice (Thy1.2) (A) Number and (B) frequency of Thy1.1+ DO11.10+ Tregs in the CD4+ compartment in the skin on d7 after immunization with OVA/CFA, OVA/Alum, or OVA/Sm Eggs. (C) Ratio of transferred Tregs to Tconv in SPN, dLN and skin. Input ratio (1:1) marked with dotted line. 5 independent experiments, statistics by Mann-Whitney. (D, E) Sorted Foxp3-GFP+ Treg (Thy1.1) and Foxp3-GFP− Tconv (Thy1.1/1.2) DO11.10+ T cells were transferred into BALB/c mice (Thy1.2) and the frequency of Foxp3-GFP expression in Tregs (D) and Tconv (E) populations assessed in the skin d7 after immunization with OVA and distinct adjuvants. (F) Relative suppression of DO11.10+ Tconv cell accumulation in the inflamed ear with and without co-transfer of DO11.10+ Tregs (Tconv cell number with Treg co-transfer/Tconv cell number in absence of Treg co-transfer X 100). 2–3 independent experiments, statistics by Mann-Whitney.
The differences in accumulation of Tregs in the skin could come from the differential recruitment or survival of natural Tregs, differential loss of Foxp3 in natural Tregs and/or differential de novo generation of Foxp3+ induced-Tregs from Tconv precursors. To test these possibilities, we used sorted naïve DO11.10 T cell transfers from Foxp3-GFP reporter mice to determine inflammation-induced acquisition of Foxp3 expression by Tconv cells and the potential loss of Foxp3 by Tregs in the inflamed skin. In all inflammatory settings the Tregs largely remained Foxp3 positive and the Tconv cells largely remained Foxp3 negative (Fig 2D, Fig 2E). We found no evidence for greater loss of Foxp3 in the transferred Tregs in the Sm Egg-inflamed skin, where Treg numbers had been seen to be lower, nor a greater gain in Foxp3 expression by Tconv cells in CFA and Alum, where Treg numbers were higher (Fig 2D, 2E). The data are in support of the differential accumulation of natural Tregs rather than differential induction of Tregs from Tconv cells.
To assess the physiological consequence of the differences in Treg numbers in the skin, we determined the relative change in DO11.10 Foxp3− Tconv cell accumulation with and without co-transfer of DO11.10 Foxp3+ Tregs. Consistent with there being fewer Tregs in the Sm Egg-inflamed ear, suppression of the Tconv cell response in the ear was attenuated in the Sm Egg-inflamed ear (Fig 2F). Attempts to determine suppressive Treg function from the different milieus on a per cell basis using in vitro suppression assays were technically unsuccessful due to the low Treg numbers recovered from the inflamed ear, particularly the Sm-Egg inflamed tissue (Fig 2A).
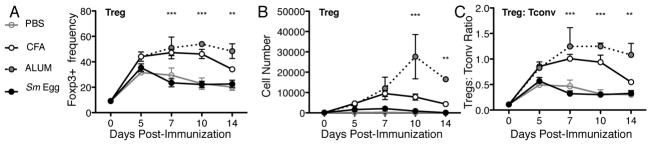
Kinetics of adjuvant-induced differences in Treg accumulation in the skin
Kinetic analysis was performed to examine the stability of Treg accumulation post-immunization. No significant differences between adjuvants were seen in polyclonal Treg skin accumulation early, d5 post-immunization, suggesting initial Treg seeding of the inflamed skin is independent of the type of inflammation (Fig. 3). In contrast, maintenance of elevated Tregs in the inflamed skin was highly context-dependent. CFA and Alum-inflammation supported high frequencies of Tregs in the skin for at least 14 days (Fig. 3A, 3B), while the Sm Egg environment failed to enhance and/or maintain Tregs past d5 (Fig. 3A, 3B). These changes resulted in sustained differences in the Treg:Tconv ratio in the skin (Fig. 3C) and prompted investigation into the signals that regulate Treg accumulation in the inflamed skin.
FIGURE 3.
Differential Treg frequencies sustained over time. BALB/c mice were immunized with OVA/CFA, OVA/Alum, or OVA/Sm Eggs and cells isolated from the inflamed skin on days 5, 7, 10 and 14. (A) Foxp3+ frequency of CD4+ cells and (B) cell number of polyclonal Foxp3+ Tregs in the skin. (C) Ratio of polyclonal Treg:Tconv cell number in skin. Data is representative of two independent experiments with 3–4 mice per group. Statistics by Two-Way ANOVA, p=<0.001 for adjuvant comparison, and bonferroni post test shown for Alum versus Sm Egg. Additional statistics: (A) *** CFA versus Sm Eggs d7, d10; (C) ** CFA versus Sm Eggs d7, d10.
Effector cytokines and Treg accumulation
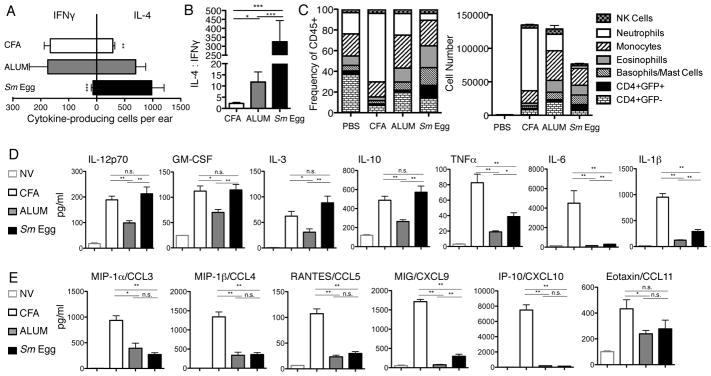
Effector cytokines have recently been shown to influence Treg homing and functional capacity (2, 47). Therefore, we characterized IFNγ and IL-4 effector cytokines and immune subsets present in our inflammatory models using IL-4-reporter mice, 4-get (48), d7 post-immunization when distinct Treg numbers were evident (Fig. 3). The number of OVA-specific cells producing IFNγ and IL-4 in the skin were determined by ELISPOT (Fig. 4A, 4B). CFA induced a potent Type 1 inflammation, with the lowest IL-4 to IFNy ratio and the recruitment of distinct innate subsets dominated by neutrophils (Fig 4C). Alum-inflammation had a greater frequency of IL-4-producing cells and more Type 2 innate subsets such as eosinophils, basophils and mast cells (Fig. 4A–C) (44). Consistent with its potent Type 2 adjuvant effects (45), Sm Egg inflammation was heavily skewed towards a Type 2 response with the highest IL-4:IFNγ ratio and greatest number of CD4+ IL-4-eGFP+ Th2 cells and Type 2 innate effectors eosinophils, basophils and mast cells (Fig. 4A–C). However, Ab blockade of IFNγ or IL-4 after the inflammation was established (d6-9 post-immunization) did not change the pattern of Treg accumulation in the skin (data not shown). Therefore, IFNγ and IL-4 play no role in the maintenance of Tregs in the inflamed skin.
FIGURE 4.
Signature cytokines induced by the different adjuvants. (A) Number and (B) ratio of IFNy and IL-4 producers in skin d7 post-immunization by OVA-specific ELISPOT. (C) Frequency and number of CD45+ immune cell subsets from immunized IL-4-GFP reported mice, d7: CD4 T cell effectors CD4+GFP+ or GFP−; basophills/mast cells CD4−GFP+FcERI+DX5+; eosinophils CD4−GFP+Gr-1+CD11bhi; neutrophils CD4−GFP−GR-1hiCD11b+; monocytes CD4−GFP−GR-1intCD11b+; NK cells CD4−GFP−DX5+CD11b+. Combined results from 3 independent experiments. Statistics by Two Way ANOVA: p= <0.001 for Interaction and p= <0.001 for cell type. (D) and (E) Cytokine and chemokine levels in skin homogenates from naïve skin (NV) or d7 OVA immunized skin with CFA, Alum or Sm eggs. Combined two independent experiments, 4 mice per group. Statistics by Mann Whitney.
Cytokines can directly effect Treg numbers in the steady state and during microbial or autoimmune inflammation (49). To further characterize the inflammatory milieu, we assayed for cytokine and chemokine induction in the inflamed ear tissue (Fig. 4D, 4E). All inflamed environments upregulated cytokines in comparison to naïve, un-immunized, ear tissue, with similarities between the adjuvants in the availability of IL-12p70, GM-CSF, IL-3 and IL-10 (Fig. 4D) and the chemokine CCL11 (Fig. 4E). CFA clearly induced a potent proinflammatory cytokine and chemokine response with strong upregulation of TNFα, IL-6, IL-1β (Fig. 4D) and all chemokines tested including CCL5, CXCL9 and CXCL10 (Fig. 4E). Alum and Sm Egg inflammation shared many similarities consistent with reduced IFNγ and enhanced IL-4 (Fig. 4D, 4E). The pattern of cytokine and chemokine expression in the Sm Egg inflamed tissue was either comparable to both CFA and Alum, or comparable to Alum but not CFA, despite differences in the accumulation of Tregs between Sm Egg and CFA/Alum. Thus, we did not find a cytokine or chemokine pattern that was consistent with the differential Treg numbers in the inflamed dermis.
Gene Expression changes in Foxp3+ Tregs reflect changes in anatomical location but not changes between types of inflammation
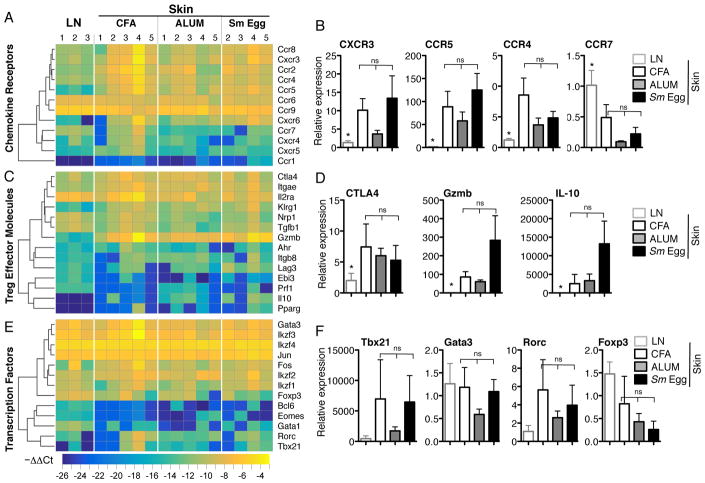
Transcriptional studies have revealed site-specific Tregs profiles that point to tissue regulation of Treg homing and function (50, 51). To determine if Tregs from the skin display molecular characteristics that may support distinct tissue accumulation, we analyzed Treg gene expression from the inflamed skin (d7) using custom gene array micro-fluidics cards containing 186 inflammatory and Treg signature genes (Supplementary Fig. 1) (51, 52). Genes associated with Th1 or Th2-type inflammation (chemokine receptors, effector molecules and CD4 transcription factors), previously associated with ‘inflammatory’ Tregs (4), were not significantly different between types of dermal inflammation (Fig. 5A–F), despite major differences in the local cytokine milieu (Fig. 4). Instead, Treg genes were significantly different by anatomical location, between the skin and LN (50). Chemokine receptors such as CXCR3, CCR5 and CCR4 were upregulated in all inflammatory conditions relative to the LN (Fig. 5A, 5B) and CCR7, important in tissue exit (53), was downregulated (Fig. 5B). Similarly, markers of Treg activation such as CTLA-4, granzyme B and IL-10 were all elevated in the skin, even in the Sm egg-induced inflammatory setting where the number of Tregs was significantly reduced (Fig. 5C, 5D). Indeed, for a number of activation markers (granzyme B, IL-10) there was a trend towards higher expression in the Tregs in the Sm Egg environment suggesting that the Tregs that make it to the Sm Egg inflamed skin retain functional capacity. Thus, differences in Treg accumulation in the skin appear not to be controlled by differential recruitment or activation of inflammation-specific Treg subsets.
FIGURE 5.
Treg phenotype in the distinct inflammatory milieus. (A, C, D) Gene expression analysis by TLDA of Foxp3-GFP+CD4+7AAD- Tregs isolated from inflamed skin d7. LN calibrator and normalized to GAPDH. Genes clustered on log2 of 2−ΔΔCt (−ΔΔCT) using Euclidean distance and average linkage. Numbered groups represent Tregs isolated from individual experiments. (B, D, F) Relative gene expression of select genes. Statistics by Mann-Whitney * p=<0.05 for LN and at least one of the inflamed skin groups, 3–5 independent experiments.
Treg numbers correlate with skin-restricted changes in antigen presenting cell (APC) expression of CD80/CD86 and Treg expression of CD25
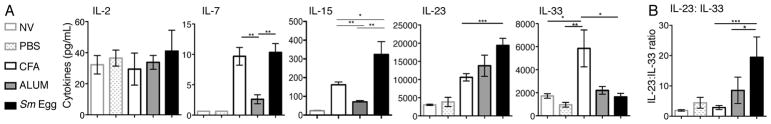
IL-2, IL-7 and IL-15 have all been shown to provide important survival signals to Tregs in tissues (54, 55), IL-2 levels were similar across all types of inflammation (Fig. 6A). IL-7 and IL-15 were also present in both the CFA and Sm Egg inflammation (Fig. 6A). Therefore, Treg numbers were low in the Sm Egg inflammation despite available IL-2, IL-7 and IL-15. In contrast, Treg numbers did correlate with the tissue expression of IL-23 and IL-33; a balance of these cytokines being important in regulating Treg number in the intestine (37). CFA had a low IL-23:IL-33 ratio, driven by high levels of IL-33 in the skin, which correlated with increased Treg accumulation (Fig 6A, 6B). In contrast, the Sm Egg-adjuvant induced limited amounts of IL-33 but high levels of IL-23 resulting in a high IL-23:IL-33 ratio (Fig 6A, 6B) and correlating with poor Treg accumulation in the skin. However, following previously published protocols (40, 41), neither Ab blockade of IL-23, nor addition of recombinant IL-33 was sufficient to boost Treg numbers in the Sm Egg-induced inflamed dermis (Supplemental Figure 2).
FIGURE 6.
Inflammatory milieu and CD25 expression. (A) Cytokine levels in skin homogenates from naïve skin (NV) or d7 OVA immunized skin with PBS, CFA, Alum or Sm eggs. Combined two independent experiments, 4 mice per group. (B) Ratio of IL-23:IL-33 in the inflamed skin. (Statistics by Mann Whitney.
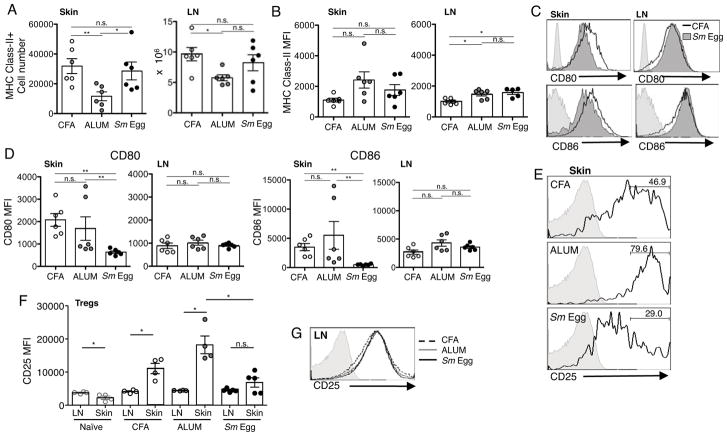
Treg survival is also regulated at the level of CD28 signals (21, 26, 27). Therefore, we next looked at adjuvant-induced changes in APC in the skin and dLN. The number of MHC Class II+ cells (Fig. 7A) and MHC Class II expression levels (Fig. 7B) in the dLN were similar for all adjuvants. MHC Class II+ cell numbers were also similar in the skin for CFA and Sm Eggs (Fig. 7A) despite the marked differences in Treg accumulation. For each type of adjuvant, the MHC Class II+ cells in the skin also expressed both CD11c and CD11b (data not shown). To assess the potential for co-stimulation, we analyzed the expression of the CD28 ligands CD80 and CD86. We chose these particular molecules because of the link between CD28 signaling in the periphery and Treg survival (21, 26, 27). There was a striking difference in the level of CD80 and CD86 on the MHC Class II+ CD11c+ CD11b+ population in the different inflammatory milieus, with APCs in CFA and Alum expressing more CD80 and CD86 than APCs in Sm-Egg-induced inflammation (Fig. 7C, 7D). These differences were not seen in the respective dLNs (Fig 7C, 7D). Thus, skin-restricted changes in expression of CD80 and CD86 by MHC Class II+ CD11c+ CD11b+ cells correlate with the changes in Treg accumulation (Fig. 1).
FIGURE 7.
Differential expression of CD80/CD86 by tissue APCs correlates with differential expression of CD25 by Tregs. Analysis of APCs in inflamed skin, d7 after immunization of BALB/c mice with OVA protein in CFA, Alum or Sm Egg. (A) Number of MHC Class II+ cells in the inflamed ear and dLN. (B) MHC Class II MFI. (C) Representative FACS profiles of CD80 and CD86 expression on MHC Class II+ CD11c+ CD11b+ T cells. (D) CD80 and CD86 MFI. (E) CD25 levels on polyclonal Foxp3+ Tregs in the skin d7 post-immunization. Grey filled histogram, FMO; solid line, CD25 expression; numbers, % of cells in CD25hi gate. (F) CD25 MFI of all Tregs from LN and skin. Statistics by Paired T Test between LN and skin; Mann Whitney between skin groups. (G) Representative histogram of CD25 levels on Foxp3+ Tregs in LN d7 post-immunization.
Consistent with a role for CD28 signals in the regulation of Treg expression of CD25 (27), Tregs displayed higher levels of CD25 in CFA and Alum inflamed skin and lower levels in Sm Egg inflamed skin (Fig 7E, 7F). In contrast, similar CD25 expression levels were seen on Tregs in the dLNs of all inflammatory conditions (Fig 7F, 7G), correlating with the similar CD80/CD86 expression in the LN (Fig 7D). Moreover, we observed a significant upregulation of CD25 expression in the CFA and Alum-inflamed skin compared to the dLN that was not observed with Sm Egg inflammation (Fig 7F). This is in contrast to autoimmune settings where inflamed non-lymphoid tissues fail to support high CD25 expression (19). Interestingly, this phenomenon appears to be dependent on inflammatory cues, as Tregs in naïve skin expressed significantly less CD25 than those in the dLN (Fig 7F) (56). Thus, the ability of Tregs to receive costimulatory signals to maintain CD25 expression in the inflamed skin, and hence respond to IL-2 for survival, appears to be regulated by the type of inflammation and these signals are limited in the Sm Egg environment (19).
CD25 expression is enhanced by inflammation in the absence of cognate-ligand
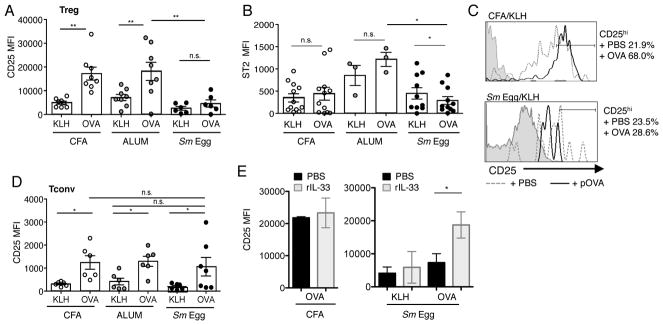
In studies to address the role of antigen recognition in the accumulation of Tregs in the inflamed skin we observed an interesting level of control of CD25 expression independent of antigen. DO11.10 TCR Tg Tregs and Tconv cells were transferred into mice and immunized with non-cognate antigen KLH and the distinct adjuvants. CD25 levels on OVA-specific Tregs were significantly modulated by the type of local inflammation: CFA and Alum inducing high levels of CD25 expression on Tregs in the skin in the absence of cognate antigen (Fig 8A, 8B). Strikingly, the inflamed Sm Egg environment failed to support CD25 upregulation in the skin (Fig. 8A, 8B). This suggests that specific inflammatory millieus can modulate Treg CD25 expression levels locally in the tissue, independent of antigen recognition/TCR signaling. In contrast, while IL-33R (ST2) expression by Tregs was also upregulated in the inflamed tissue independent of cognate antigen, it was not differentially regulated by the distinct adjuvants (Fig. 8C), despite very different IL-23:IL-33 ratios (Fig 6B). IL-23 has been postulated to regulate Treg numbers by inhibiting IL-33 responsiveness via downregulation of ST2 (37). In these studies we did not see a correlation between higher IL-23 and reduced ST2 expression. Thus, rather than IL-23 being a negative regulator of Treg accumulation in our studies, the change in IL-33 levels with the different adjuvants may control Treg accumulation indirectly via changes in paracrine IL-2 production by DC, as previously suggested (35, 36).
FIGURE 8.
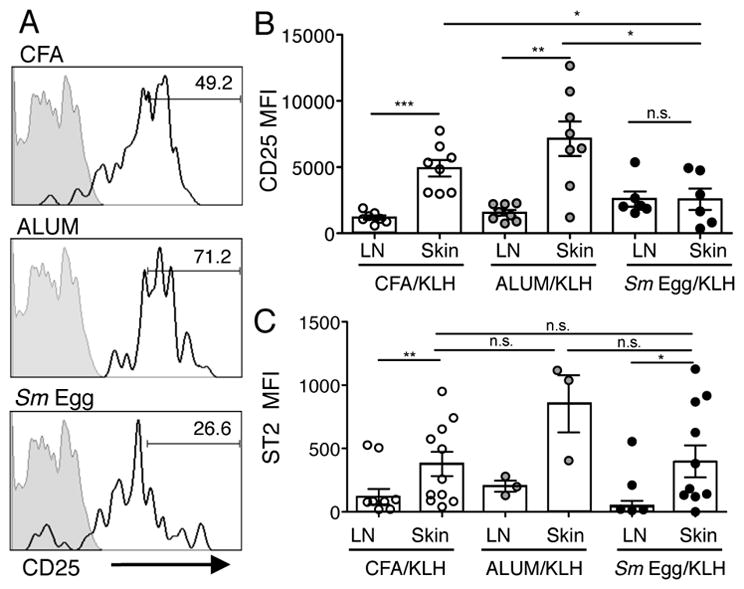
Inflammation drives CD25 expression on skin Tregs. (A) CD25 expression on adoptively transferred DO11.10+ Tregs in the skin d7 post-immunization with KLH and CFA, Alum or Sm Egg. Grey filled histogram, FMO; solid line, CD25 expression; numbers, % of cells in CD25hi gate. (B) CD25 MFI on all DO11.10+ Tregs in skin and LN on d7. (C) ST2 MFI on DO11.10+ Tregs in the skin and LN on d7. Data from 2 independent experiments, 3–5 mice per group. Statistics by Paired T Test between LN and Skin, statistics between groups by Mann-Whitney.
Antigen boosts Treg CD25 expression, if the inflammatory milieu is permissive
Cognate antigen boosted Treg expression of CD25 in CFA and Alum inflammation, but not in Sm Egg inflammation (Fig. 9A). In contrast, cognate antigen did not enhance the level of expression of ST2 by Tregs in any of inflammatory milieus (Fig. 9B), in fact, ST2 levels were moderately but significantly reduced in the presence of cognate-antigen in the Sm Egg milieu which may contribute to the reduced Treg accumulation. To directly test if antigen drives an increased level of CD25 expression in the inflamed skin we pulsed KLH-immunized the mice with OVA peptide (pOVA) 6 h prior to harvest on day 7. Indeed, increased levels of CD25 were observed on Tregs after the pOVA pulse in the KLH/CFA inflamed skin (Fig. 9C). However, CD25 expression was not significantly increased in Sm Egg inflammation with additional pOVA pulse, suggesting that even with an antigen supply the degree of CD25 expression is controlled in part by the inflammatory milieu (Fig 8) or by recognition of antigen in the context of CD28 costimulation (Fig 7). Moreover, Tregs appear specifically sensitive to the local skin inflammatory environment with respect to CD25 expression levels, since Tconv cells upregulated CD25 expression similarly in all inflammatory settings (Fig 9D). Thus, CD25-inducing inflammatory cues may be a necessary pre-conditioning event for Treg antigen-receptivity in inflamed tissues. IL-33 is an interesting candidate for the regulation of CD25 expression on Tregs in inflamed tissues given its links to local induction of IL-2 (35, 36). Indeed, rIL-33 administration was sufficient to boost CD25 expression in the Sm Egg-induced inflamed dermis to levels seen with CFA-inflammation, but only in the context of cognate antigen (Fig 9E). Thus, IL-33 alone is not sufficient to regulate CD25 expression in inflamed tissues (KLH group, Fig 9E) but can synergize with TCR signals to upregulate CD25 (OVA group, Fig 9E).
FIGURE 9.
Cognate-antigen increases Treg accumulation and upregulation of CD25. (A) CD25 MFI and (B) ST2 MFI of all transferred DO11.10+ Tregs in the skin of mice immunized with cognate-Ag (OVA) or non-cognate Ag (KLH). KLH data from Figure 7. (C) CD25 expression on DO11.10+ Tregs in KLH immunized mice with CFA or Sm egg adjuvants. Mice were pulsed with PBS (+PBS) or OVA peptide (+pOVA) i.v. 6 h prior to cell harvest. % of Treg cells in CD25hi gate. (D) DO11.10+ Tconv cells transferred alone, CD25 MFI of all Tconv cells in the skin d7 post-immunization. (E) rIL-33 (2μg/ml) was administered i.p. to CFA or Sm-Egg-inflamed (KLH or OVA) mice on days 4, 5 and 6 post-immunization and tissues analyzed on day 7. Expression levels for CD25 in the presence or absence of rIL-33 and non-cognate Ag (KLH) or cognate Ag (OVA). Data representative of 2–3 independent experiments. Statistics by T Test and Mann-Whitney.
Antigen-driven Treg CD25 expression correlates with enhanced local proliferation
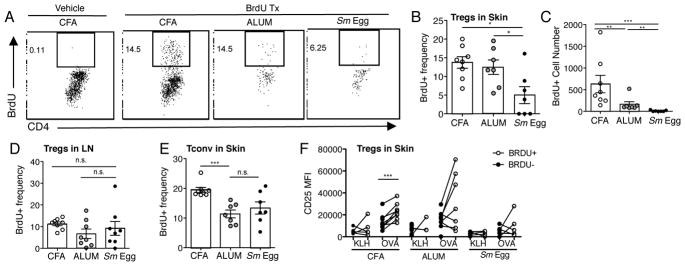
To determine the functional consequence of differential CD25 expression on Tregs in the inflamed skin, we assayed for in situ proliferation using an acute BrdU pulse 4h prior to harvest. Consistent with Treg accumulation patterns, DO11.10+ TCR Tg Tregs displayed robust BrdU-incorporation in CFA and Alum inflammation but limited BrdU staining in the Sm Egg skin (Fig. 10A–C). Treg proliferation appeared to be skin restricted, as Treg expansion in the dLN was not different between adjuvants (Fig. 10D). In contrast, TCR Tg Tconv cells in the skin displayed similar proliferation patterns in all types of inflammation (Fig. 10E), arguing against a major difference in antigen availability in the different adjuvant milieus. Our data suggest that inflammatory signals can modulate CD25 expression levels, but antigen is required for active Treg proliferation in the inflamed skin. Indeed, BrdU+ cells were enriched for higher levels of CD25 (Fig. 10F) suggesting the combination of distinct inflammatory cues and cognate antigen synergize to promote local Treg accumulation in the inflamed tissue.
FIGURE 10.
Inflammation-specific local Treg proliferation. (A) Representative plots of BrdU+ expression in DO11.10+ Tregs from the skin d7 post-immunization. (B) BrdU+ frequency and (C) number of transferred DO11.10+ Tregs in skin d7. (D) BrdU+ frequency of transferred DO11.10+ Tregs in dLN d7. (E) DO11.10 Tconv cells transferred alone, BrdU+ frequency of Tconv in skin on d7. A–E: data representative of 3–4 independent experiments, statistics by Mann Whitney. (F) CD25 MFI of BrdU+ and BrdU− DO11.10+ Tregs in the skin d7. Data representative of 2–3 independent experiments, statistics by Paired T-Test (BrdU+, BrdU−).
Discussion
The data presented in this study highlight an important role for inflammatory signals in the promotion of extensive Treg accumulation locally in the skin. The inflamed microenvironment of the skin can support the upregulation of CD25 in the absence of cognate antigen and appears to facilitate antigen-dependent local Treg proliferation. Distinct inflammatory environments differentially impact the magnitude of the Treg response in this manor. CFA and Alum adjuvants enhance Treg CD25 expression and robustly support prolonged Treg accumulation in the inflamed skin. In contrast, Sm Egg-inflamed skin neither induces CD25 expression on Tregs nor promotes local Treg proliferation and accumulation. Moreover, Tregs appear to be distinctly regulated by the different adjuvant-induced inflammatory milieus because the extent of Tconv cell accumulation was not impacted by the different adjuvants. This raises the possibility that the magnitude of Treg accumulation in inflamed tissues may be therapeutically regulated independent of effects on Tconv cells.
How might Treg accumulation be enhanced by the inflammatory milieu? Our studies explored a number of possible control points. The different patterns of Treg accumulation did not correlate with particular inflammatory or growth factor cytokines that have been implicated in regulating Treg numbers nor did the predominant Tconv cytokines IFNγ and IL-4 play a role. We predicted that the different inflammatory milieus may select the recruitment or retention of distinct Treg subsets based on current models whereby the polarizing cytokines that drive Tconv cell differentiation also modify Treg homing and functional potential (2–4). However, we did not see differential functional specialization in the distinct cytokine milieus in the skin. Instead, the pattern of gene expression appeared anatomically restricted, with an overall increase in Treg effector molecules in the inflamed skin compared to LN. A lack of Treg functional skewing despite very different cytokine environments may be explained by our model being one of acute inflammation and suggests that population based Treg adaptation within tissues may require chronic exposure to distinct inflammatory signals.
Both CFA and Alum-induced inflammation supported the upregulation of CD25 by Tregs, independent of cognate antigen. These adjuvants trigger TLR and NLR-dependent innate immune activation (57) and promote distinct cytokine milieus (Fig. 4). Therefore the signals that regulate Treg CD25 expression in inflamed tissues appear to be induced broadly by disparate microbial and inflammatory signals. Nevertheless, the ability of the inflammatory milieu to support CD25 was selective; with CFA and Alum providing such signals but Sm Egg-induced inflammation lacking. Unexpectedly, the regulation of Treg CD25 expression by inflammation was independent of antigen (Fig. 8). Certain effector functions of conventional CD4 and CD8 T cells have been shown to be induced independent of antigen in response to inflammatory cytokines, such as those of the IL-1 family (58–60). Therefore, similar signals may also regulate Tregs independent of antigen, although IL-1β expression in the inflamed skin did not correlate with its ability to support CD25 (low in both Alum and Sm Egg despite the Alum environment supporting CD25 upregulation, Fig 4). More work will be needed to identify possible cytokines, or combination of cytokines, that may regulate CD25 expression by Tregs independent of antigen. Alternatively, differential availability of costimulatory signals may also promote CD25 expression independent of antigen, as discussed below. Interestingly, for CD8 cells, the inflammatory milieu was shown to enhance CD8 T cell sensitivity to antigen (61), a mechanism that may be consistent with our observations that in the absence of the appropriate inflammatory ‘conditioning’ cues (cytokine and/or costimulation) antigen is not sufficient to rescue Treg accumulation.
The global availability of IL-2 in the tissue is unlikely to be a major driver of the Treg CD25 expression and accumulation in the skin as the adjuvanted milieus all resulted in similar IL-2 tissue levels, although we cannot rule out distinct micro-anatomical differences in available IL-2. Instead, the upregulation of costimulatory ligands such as CD80 and CD86 may be a good mechanism for regulating Treg CD25 expression and/or accumulation in the tissue, given the important role of CD28 signaling in Treg peripheral homeostasis (18, 21, 26, 27). We found that the relative expression levels of CD80/CD86 on APCs in the inflamed skin correlated with the magnitude of Treg accumulation. Strikingly, Sm-Egg-induced inflammation upregulated CD80/CD86 in the dLN but failed to support CD80/CD86 expression levels in the inflamed skin. This pattern correlates with Sm-Egg-inflamed Treg expansion in the dLN, as previously described (62), and the relative inability of the local Sm-Egg microenvironment to support enhanced Treg numbers. Indeed, infection with the Schistosoma mansoni parasite itself has been shown to limit induction of CD28 ligands or upregulate negative regulators such as PD1-L on APC and this may account for the failure of the Sm-Egg-induced inflammatory milieu to support Treg accumulation (63, 64). In an autoimmune setting, such a B7-dependent mechanism may also underlie the marked downregulation of Treg CD25 in autoimmune tissues where chronic non-microbially induced inflammation may fail to support a perceived threshold level of CD28-ligands necessary for Treg CD25 upregulation and/or survival.
In our studies, cognate antigen played a major role in the further upregulation of CD25 and in local Treg proliferation, but the effects of antigen remained inflammatory context-dependent. The simplest explanation for the poor Treg accumulation in the Sm-Egg-inflamed skin would have been that the Sm-Egg-induced inflammation led to reduced antigen presentation and hence an inability to locally expand antigen-specific Tregs. However, antigen-specific Tconv cells robustly accumulated and proliferated locally in the Sm Egg-inflamed skin similar to both CFA and Alum, indicating that antigen was not overtly differentially limited by distinct adjuvants. We suggest that effector Tconv and Tregs may be differentially sensitive to cognate antigen at inflammatory sites or that they may be differentially dependent on costimulatory reinforcement (65). Such a difference in activation thresholds between Treg and Tconv cells could be explained by intrinsic differences in CTLA-4 expression: Tregs express elevated basal levels of CTLA-4 and Treg CTLA-4 is often upregulated in nonlymphoid tissues (Fig 5) (50). This model would enable efficient effector Tconv responses to proceed at the initiation of infection in the absence of strong antigen-specific Treg restraint. Antigen-dependent regulation in this manner may also explain the failure of Treg control as autoimmunity progresses. The more efficient the Tconv cells are at destroying cellular sources of antigen in the target tissue the more compromised, in local accumulation and/or expansion, the Treg response in the tissue may become. It is important to note that our studies have demonstrated an effect of the inflammatory milieu on the differential accumulation of Treg numbers in the skin but there may also be a differential effect of the inflammatory milieu on the intrinsic suppressive capacity of such Tregs in the skin. While our RNA analysis would suggest a number of the key Treg functional molecules are expressed at similar levels in the different settings (CTLA-4, Gzmb, IL-10; Fig 5) it will be interesting to ascertain if, independent of differences in Treg number, there are additional differences in the quality of their suppressive properties influenced by the inflammatory milieu.
There may be additional factors that balance the magnitude of Treg accumulation in the skin. Recent studies in the intestine suggest the balance between IL-23 and IL-33 signals may be critical in the control of local Treg numbers and function (37). IL-33 appears to promote Treg function and accumulation in inflamed tissues, possibly through promoting DC-induced IL-2 production (35), while IL-23 restrains Treg responsiveness to IL-33 (37). Interestingly, the severe hepatic granulomatous inflammation against schistosome eggs is critically dependent on egg-induced IL-23 expression (66, 67). We also see a significant increase in the IL-23: IL-33 ratio in the Sm-Egg milieu compared with CFA or Alum, consistent with the poor Treg accumulation in Sm-Egg-inflamed skin. Indeed, we found an interesting synergy between cognate antigen signals and IL-33 for the upregulation of CD25: rIL-33 boosted CD25 expression on Tregs in the Sm Egg-inflamed dermis if cognate antigen was present (Fig 9E). Thus, reduced IL-33 availability in the Sm Egg-inflamed skin may function to modulate Treg CD25 levels through its role in promoting paracrine IL-2 production from DC (35). Clearly further investigation into this pathway, using genetic manipulation of key components, is warranted given the unchecked schistosome egg-induced immunopathology (68).
We show that adjuvant-induced inflammation strongly supports Treg accumulation in the inflamed skin. Inflammation alone can upregulate Treg CD25 expression locally in the inflamed tissue and this appears to prime cells for additional antigen-dependent signals that drive local antigen-specific Treg expansion. The differences in the magnitude of Treg accumulation in the skin with distinct adjuvants was striking and may have important implications for the control of microbially induced immune pathology as well as the efficacy of vaccination (57). Successful vaccine strategies are currently evaluated based on the magnitude of systemic immune memory. However, given the growing importance of local resident tissue memory (69), the degree of local Treg accumulation induced by specific adjuvants may well modify the establishment of protective immunity.
Supplementary Material
Acknowledgments
Grant support: NIH NIAID P01 AI02851, NIH NIAID R01 AI070826 and NIH NIAID HHSN272201200005C to DJF
We thank the members of the Fowell lab for helpful discussions on the studies. The Schistosome eggs were kindly provided by the labs of Dr. Ed Pearce and Dr. David Artis and originated from B. glabrata snails provided by the NIAID Schistosomiasis Resource Center of the Biomedical Research Institute (Rockville, MD) through NIH-NIAID Contract HHSN272201000005I for distribution through BEI Resources.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 3.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punkosdy GA, Blain M, Glass DD, Lozano MM, O’Mara L, Dudley JP, Ahmed R, Shevach EM. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3677–3682. doi: 10.1073/pnas.1100213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor MD, van der Werf N, Harris A, Graham AL, Bain O, Allen JE, Maizels RM. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. Eur J Immunol. 2009;39:192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor RA, Malpass KH, Anderton SM. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J Immunol. 2007;179:958–966. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 17.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attridge K, Walker LS. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunol Rev. 2014;259:23–39. doi: 10.1111/imr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts RJ, Prabhu N, Ho AW, Lew FC, Hutchinson PE, Rotzschke O, Macary PA, Kemeny DM. Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp3+ regulatory T cell response. J Virol. 2012;86:2817–2825. doi: 10.1128/JVI.05685-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 22.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt EM, Wang CJ, Ryan GA, Clough LE, Qureshi OS, Goodall M, Abbas AK, Sharpe AH, Sansom DM, Walker LS. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 24.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 25.Gogishvili T, Luhder F, Goebbels S, Beer-Hammer S, Pfeffer K, Hunig T. Cell-intrinsic and -extrinsic control of Treg-cell homeostasis and function revealed by induced CD28 deletion. Eur J Immunol. 2013;43:188–193. doi: 10.1002/eji.201242824. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. The Journal of clinical investigation. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 29.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 30.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 31.Cheng G, Yu A, Dee MJ, Malek TR. IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol. 2013;190:1567–1575. doi: 10.4049/jimmunol.1201218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrenshall LE, Platt JL. Regulation of T cell homeostasis by heparan sulfate-bound IL-2. J Immunol. 1999;163:3793–3800. [PubMed] [Google Scholar]
- 33.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 34.Sereti I, Martinez-Wilson H, Metcalf JA, Baseler MW, Hallahan CW, Hahn B, Hengel RL, Davey RT, Kovacs JA, Lane HC. Long-term effects of intermittent interleukin 2 therapy in patients with HIV infection: characterization of a novel subset of CD4(+)/CD25(+) T cells. Blood. 2002;100:2159–2167. [PubMed] [Google Scholar]
- 35.Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, Turnquist HR. IL-33 Is an Unconventional Alarmin That Stimulates IL-2 Secretion by Dendritic Cells To Selectively Expand IL-33R/ST2+ Regulatory T Cells. J Immunol. 2014;193:4010–4020. doi: 10.4049/jimmunol.1400481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, Oboki K, Ohno T, Motomura K, Matsuda A, Yamaguchi S, Narushima S, Kajiwara N, Iikura M, Suto H, McKenzie AN, Takahashi T, Karasuyama H, Okumura K, Azuma M, Moro K, Akdis CA, Galli SJ, Koyasu S, Kubo M, Sudo K, Saito H, Matsumoto K, Nakae S. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43:175–186. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BM, Lohning M, Belkaid Y, Fallon PG, Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowell DJ, Shinkai K, Liao XC, Beebe AM, Coffman RL, Littman DR, Locksley RM. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity. 1999;11:399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 39.Katzman SD, Fowell DJ. Pathogen-imposed skewing of mouse chemokine and cytokine expression at the infected tissue site. The Journal of clinical investigation. 2008;118:801–811. doi: 10.1172/JCI33174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. The Journal of clinical investigation. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell metabolism. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–860. [PubMed] [Google Scholar]
- 43.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, van der Bosch J, Mohrs K, Haas H, Mohrs M, Yazdanbakhsh M, Schramm G. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- 47.Campbell DJ. Control of Regulatory T Cell Migration, Function, and Homeostasis. J Immunol. 2015;195:2507–2513. doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 49.La Cava A. Tregs are regulated by cytokines: implications for autoimmunity. Autoimmun Rev. 2008;8:83–87. doi: 10.1016/j.autrev.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazarski CA, Ford J, Katzman SD, Rosenberg AF, Fowell DJ. IL-4 attenuates Th1-associated chemokine expression and Th1 trafficking to inflamed tissues and limits pathogen clearance. PLoS One. 2013;8:e71949. doi: 10.1371/journal.pone.0071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 55.Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA. The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol. 2008;84:973–980. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol. 2013;190:4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12294–12299. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raue HP, Beadling C, Haun J, Slifka MK. Cytokine-mediated programmed proliferation of virus-specific CD8(+) memory T cells. Immunity. 2013;38:131–139. doi: 10.1016/j.immuni.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O’Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 61.Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity. 2013;38:140–152. doi: 10.1016/j.immuni.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 63.Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, Hunter CA, Pearce EJ. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173:7454–7461. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 64.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, Fallon PG. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173:1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 65.Yan D, Farache J, Mathis D, Benoist C. Imbalanced signal transduction in regulatory T cells expressing the transcription factor FoxP3. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14942–14947. doi: 10.1073/pnas.1520393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL–17. J Immunol. 2008;180:2486–2495. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 67.Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–8567. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.